Abstract
Background
Due to the effect of synthetic and commercial insecticides on non-target organisms and the resistance of mosquitoes, non-chemical and environmentally friendly methods have become prevalent in recent years. The present study was to isolate entomopathogenic fungi with toxic effects on mosquitoes in natural larval habitats.
Methods
Larvae of mosquitoes were collected from Central, Qamsar, Niasar, and Barzok Districts in Kashan County, Central Iran by standard dipping method, from April to late December 2019. Dead larvae, live larvae showing signs of infection, and larvae and pupae with a white coating of fungal mycelium on the outer surface of their bodies were isolated from the rest of the larvae and sterilized with 10% sodium hypochlorite for 2 min, then washed twice with distilled water and transferred to potato-dextrose-agar (PDA) and water-agar (WA) media and incubated at 25 ± 2 °C for 3–4 days. Larvae and fungi were identified morphologically based on identification keys.
Results
A total of 9789 larvae were collected from urban and rural areas in Kashan County. Thirteen species were identified which were recognized to belong to three genera, including Anopheles (7.89%), Culiseta (17.42%) and Culex (74.69%). A total of 105 larvae, including Anopheles superpictus sensu lato (s.l), Anopheles maculipennis s.l., Culex deserticola, Culex perexiguus, and Culiseta longiareolata were found to be infected by Nattrassia mangiferae, Aspergillus niger, Aspergillus fumigatus, Trichoderma spp., and Penicillium spp. Of these, Penicillium spp. was the most abundant fungus isolated and identified from the larval habitats, while An. superpictus s.l. was the most infected mosquito species.
Conclusions
Based on the observations and results obtained of the study, isolated fungi had the potential efficacy for pathogenicity on mosquito larvae. It is suggested that their effects on mosquito larvae should be investigated in the laboratory. The most important point, however, is the proper way of exploiting these biocontrol agents to maximize their effect on reducing the population of vector mosquito larvae without any negative effect on non-target organisms.
Similar content being viewed by others
Background
Transmission of malaria, filariasis, Japanese encephalitis, dengue fever, and other arboviral diseases by mosquitoes has turned mosquitoes into the most important group of arthropods in medicine and health [1]. In Iran, mosquitoes are vectors of two protozoan, two bacterial, four filarial, and seven arboviral diseases [2, 3]. There are 70 species and eight (or 12) genera of Iranian mosquitoes depending on the classification of the tribe Aedini [4]. Anopheles species are responsible for the transmission of malaria, but the majority of mosquito species from the genera of Culex and Aedes are responsible for the transmission of arboviruses to humans [5, 6].
Globally, in 2019, there were an estimated 229 million malaria cases in 87 malaria-endemic countries. The disease is a major endemic infectious disease in Iran, especially in the south and southeastern provinces, including the Sistan-Baluchistan, Hormozgan and Kerman Provinces [7,8,9,10,11,12].
Anopheles species are responsible for the transmission of malaria. So far, seven malaria vectors have been recognized and reported in Iran, including Anopheles stephensi, Anopheles culicifacies, Anopheles dthali, Anopheles fluviatilis, Anopheles superpictus, Anopheles maculipennis, and Anopheles sacharovi [13]. The first five of these vectors can be found in the southeast of the country, together with the majority of malaria cases. Also, Anopheles pulcherrimus has been considered as a potential malaria vector in this area based on immunological parasite detection [two-site immunoradiometric assay (IRMA)] [14].
Anopheles maculipennis sensu lato (s.l.) is distributed in Eurasia and North America and comprises nine Palearctic members [15, 16]. Some research indicated the occurrence of this malaria vector in Central Iran, the Caspian coast in the north, and North-West Iran [17,18,19].
Anopheles superpictus s.l. is distributed in Europe, Asia and North Africa [20,21,22,23,24]. This species is one of the seven species of malaria vectors and reported in the Iranian Plateau, the slopes of the Alborz Mountains and southern Zagros, as well as the coastal plains of the Caspian Sea and the Persian Gulf in both malaria-endemic and non-endemic areas [8, 21]. Oshaghi et al. in 2008 reported three genotypes X, Y and Z in Iran. Interestingly, while the sympatric Y and Z genotypes appear to be exclusive to the populations from the southeastern part of the country, genotype X is geographically separated, and present in the North, the West, the South and the Central territories [22].
One of the goals of control methods is to reduce the size of vector populations. There is a risk of insecticide resistance and off-target effects on other arthropod species in chemical control [25]. Biological control is biodegradable and ecologically friendly [26]. Entomopathogenic fungi were first used on Anopheles gambiae with a fungus from the genus of Coelomomyces [27]. Weiser et al. reported Coelomomyces irani from An. maculipennis in Iran [28]. Azari-Hamidian and Abaei reported Coelomomyces sp. from the larvae of An. culicifacies s.l. in Sistan and Baluchistan Province, southeast Iran, where 5.8% of larvae were infected with the fungus [29].
The use of pathogenic insect fungi against mosquito larvae has been reported in many studies, and fungi are proven an effective way of killing mosquito larvae [30,31,32,33,34]. The use of Beauveria bassiana for control of Aedes aegypti [31] and Lagenidium giganteum in California targeted to control Culex tarsalis [32] reduced the survival rate, blood-feeding, fecundity, and disease transmission power of targeted mosquitoes. Some insect pathogenic fungi have been used effectively in the laboratory, small-, and large-scale field studies to control vector mosquitoes especially in Culex [35, 36], Mansonia [37], and Anopheles species [32] and have a wide range of species diversity. This group of pathogens is found among all phyla of fungi. The Ascomycota is the largest group of fungi. This group is extremely ecologically diverse, just like the pathogenesis pathogen of plants, animals and humans. Pathogenic insect ascomycetes include a large group of fungi that attack a wide range of insects and are the most common insect pathogens [38]. The entomopathogenic ascomycete fungi, including Metarhizium anisopliae and Beauveria bassiana have been reported as insecticides [39]. In many studies, spores and secondary metabolites of insect pathogenic fungi have been reported as biocontrol agents against mosquitoes [34, 40,41,42]. The fungal hyphae produce endotoxins and penetrate through the larval body. These toxins cause larval damage and toxicity in the haemocoel and larval mosquito guts [43]. Metabolites of Beauveria bassiana caused changes in the body and tissues of treated Culex pipiens larvae, especially in the cuticle and midgut [44].
The present study was to isolate and identify entomopathogenic fungi associated with mosquito larvae in Kashan County, Central Iran, and their infection and effects on mosquito larvae.
Methods
Study area
Kashan County is located in central Iran, north of Isfahan Province. This county has four districts, including Central (51°24′43.2″ E, 34°00′16.0″ N), Qamsar (51°27′45.8″ E, 33°45′30.5″ N), Niasar (51°08′47.6″ E, 33°58′39.3″ N), and Barzok (51°13′44″ E, 33°47′32″ N) Districts. The climate of the county varies depending on ups and downs. The uplands are cold, foothills are temperate, and the plains, especially on the margins of the desert, are tropical [45].
A total of 23 larval habitats were selected in Central, Qamsar, Niasar, and Barzok Districts. These larval habitats are natural or artificial, permanent or temporary, with or without vegetation, sunlight or shaded, and clear or stagnant water (Fig. 1).
Larval sampling
Using a standard 350-ml capacity mosquito dipper, larvae and pupae of the mosquitoes were collected from April to late December 2019. Twenty dips were taken in each larval habitat in the morning (08:00–12:00 h) or afternoon (15:00–18:00 h). For sampling larvae from small water bodies, an eyedropper was used. The collected larvae and pupae were transferred into clean plastic jars along with larval habitat water, stored in a cool box with temperature ranging from 8 to 15 °C, the date, collection site and habitat type of larvae recorded with special code on the containers and relevant forms, and then transferred to the medical entomology laboratory of Tehran University of Medical Sciences (TUMS) in less than 5 h.
Immediately after transferring larvae to the laboratory, larvae were observed under a stereomicroscope. Dead larvae, live larvae showing signs of infection, and larvae and pupae with a white coating of fungal mycelium on the outer surface of their bodies were isolated from the rest of the larvae and maintained at 4 °C for isolation and diagnosis of fungi associated with mosquito larvae. Other mosquito larvae were transparent in lactophenol, individually mounted in Berlese's fluid on a microscope slide and identified based on a Culicidae identification key of Iran at the species level [46].
Isolation and diagnosis of fungi associated with mosquito larvae
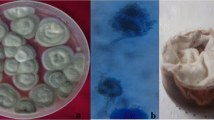
A white coating of fungal mycelium was observed on the surface of some of the larvae and pupae (Fig. 2). These larvae and pupae were removed from the water of the larval habitat at the laboratory and sterilized with 10% sodium hypochlorite for 2 min (to remove surface contaminants that conflict with the main pathogen), then washed twice with distilled water; the remaining water was removed and passed through the filter paper sterilizer [47]. They were then transferred to potato-dextrose-agar (PDA) and water-agar (WA) media. Parts of the body of some other larvae were degraded or broken, or the outer epithelial layer of the larvae were cut and collapsed. Consequently, the outer surface of the larvae was wrinkled (Fig. 3). To determine the possibility of fungal infection, they were transferred to PDA and WA media after surface disinfection, then incubated the Petri dishes in the incubator at 25 ± 2 °C for 3–4 days. Fungi were identified based on phenotypic characteristics and characteristics of the culture medium, such as shape and colour of the fungus colony, filament growth pattern, as well as microscopic properties such as shape, size and colour of spores, mycelium and conidiophore structure [48].
Results
Larval sampling results
A total of 9789 larvae were collected from urban and rural areas of Central, Qamsar, Niasar, and Barzok Districts in Kashan County. Three genera: Anopheles (7.89%), Culiseta (17.42%), and Culex (74.69%), consisting of 13 species were identified (Table 1). Some mosquito specimens were deposited in the Museum of Medical Entomology, TUMS.
Fungi associated with mosquito larvae
Five species of fungi were isolated from mosquito larvae (Table 2). These fungi were isolated from larvae and pupae obtained from natural larval habitats in Qamsar and Barzok Districts. Five out of 13 mosquito species were found to be infected by fungi. A total of 105 larvae revealed morphological or behavioural manifestations of infection and fungal mycelia were detected in all of these larvae.
Nattrassia mangiferae was isolated only from An. superpictus s. l. larvae or pupae in Qamsar District in August. The white hyphae of Aspergillus niger, Aspergillus fumigatus and Trichoderma spp. had grown on the surface of the larvae, and penetrated the body. Penicillium spp. was identified from larvae whose parts of their bodies were wrinkled, degenerated or broken (Figs. 4, 5). In this study, Penicillium spp. was isolated from 57 mosquito larvae (54.29% of infected larvae) and it was the most abundant fungus isolated, and identified from larval mosquito habitats in Kashan County (Fig. 6). This fungus was identified from larvae collected from a natural larval habitat with vegetation in Barzok. Anopheles superpictus s. l. had the highest number of larvae infected with the fungi in all larval habitats, and from 105 infected larvae collected, 59 larvae were related to this species (Table 2).
Discussion
Insect pathogenic fungi can grow in liquid and solid environments, and their spores can attack and kill mosquito larvae [49]. In the present study, five fungi species were identified from mosquito pupae or larvae. All of these fungi were isolated from larvae and pupae collected from natural larval habitats.
Effects of fungi mycelia and secondary metabolites on mosquito larvae
Nattrassia mangiferae is common in the tropics and is best known as a plant pathogen (the cause of die‐back and trunk cankers in trees). It can also cause fungal infections in human nails [50]. This fungus had not previously been isolated from insects and this is the first report from mosquito larvae and pupae.
Aspergillus has more than 180 species, some of them are pathogenic or allergenic to humans and animals. In different studies, several species of this fungus have been reported in mosquito larvae. Turky et al. [47] isolated Aspergillus candids, Aspergillus niger, and Aspergillus terreus from Culex quinquefasciatus larvae. Balumahendhiran et al. [51] examined secondary metabolites of Aspergillus flavus and Aspergillus fumigatus in the control of Aedes aegypti, An. stephensi and Cx. quinquefasciatus larvae. Species of Aspergillus produce a variety of secondary metabolites, including aflatoxins. Secondary metabolites of Aspergillus fumigatus had the highest toxicity to Cx. quinquefasciatus and An. stephensi larvae. Secondary metabolites of Aspergillus niger were also effective against the larvae of these three mosquito species [30]. In this study, it was also found that two species of Aspergillus, including Aspergillus niger and Aspergillus fumigatus, can grow in an aquatic environment on mosquito larvae and infect them.
In the present study, Trichoderma spp. were also isolated and identified from mosquito larvae. This fungus is capable of attacking other organisms and microorganisms by producing antibiotics and other extracellular enzymes. Because of this ability, Trichoderma spp. has been known as biocontrol agent of plant pathogens for about 70 years [52]. This fungus is widely used in agriculture to control plant diseases as well as to increase crop yield. Podder and Ghosh investigated the effect of Trichoderma asperellum against anophelinae larvae and their study was the first report the use of Trichoderma asperellum as mosquito larvicides. They observed that the internal tissues of the larvae were destroyed after larval death [53]. It has been confirmed that some fungal toxins can cause tissue damage and dehydration of the host tissues [54].
In a study in 2016, researchers reported Metarhizium brunneum blastospores kill Aedes larvae much faster than conidia of this fungus in natural habitat, in freshwater. Blastopores easily penetrated the larval cuticle and resulted in rapid larval death. Conidia cause stress-induced mortality, which takes a slightly longer time [49].
Effects of fungi on morphological or behavioural manifestations of mosquito larvae
In the present study, Penicillium spp. was isolated from wrinkled and degenerated larvae and the larvae whose parts of their cuticle were destroyed. Ragavendran et al. [55] studied the effect of larvicidal of seven fungal isolates and their metabolites on Ae. aegypti and Cx. quinquefasciatus in vitro and reported that Penicillium spp. had the best larvicidal effect compared to other fungi. The mycelia extract of this fungus had toxic effects on many parts of the larval body, including thorax, abdomen, anal gills, such as the loss of external hair, crumbled epithelial layer of the outer cuticle, and shrinkage of the larvae. After 30 min of exposure of the larvae to the fungal metabolites, the behavioural symptoms of the treated larvae were observed, including upward, downward, horizontal, and vertical movements of the larvae and damage at the bottom of the larval body. Damage to the cuticle layers was also one of the morphological changes in the treated larvae. In this study also, Penicillium spp. was isolated from larvae that had morphological manifestations in the cuticle layers. Lethargy and inactivity were among behavioural manifestations observed in these larvae.
Infection with all fungi identified in this study was present in An. superpictus s.l. larvae. Omrani et al. reported the first case of a microsporidium infection (a microsporidium species from the genus Parathelohania) in An. superpictus s.l. from Iran [56]. Parathelohania legeri was reported in An. maculipennis s.l. about 110 years ago [57].
In addition to parasitic effects and their potential for mosquito control, mosquito-associated fungi also have non-pathogenic interactions with mosquitoes, such as the impact on breeding site selection and impact on larval and adult feeding behaviour. It has been demonstrated that secondary metabolites produced by Trichoderma viride have effects on attracting gravid Cx. quinquefasciatus females and find oviposition sites [58, 59].
There are several reports of insecticide resistance in malaria and West Nile vectors in Iran [13, 60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75]. Studies on non-pathogenic fungi of mosquitoes are very scarce and have not been done in Iran. A study of the impact of pathogenic and non-pathogenic fungi on the behaviour of mosquitoes can help to develop new vector control strategies. In addition, some fungi are recommended for combinations of insecticides for indoor residual spraying as adult control. Using these fungi in combination with other vector control measures is appropriate for decision-makers [76,77,78].
Conclusion
This study did not examine the lethal effects of these fungi on larvae in the laboratory, and reports only natural fungi infection in mosquito larvae in their natural habitats. Therefore, it is suggested that their effects on mosquito larvae be investigated in the laboratory. The most important point, however, is the proper way of exploiting these biocontrol agents to maximize their effect on reducing the population of vector mosquito larvae without any negative effect on non-target organisms.
Availability of data and materials
Not applicable.
Abbreviations
- PDA:
-
Potato-dextrose-agar
- WA:
-
Water-agar
References
Service MW. Management of vectors. In: Youdeowei A, Service MW, editors. Pest and vectors management in the tropics. London: Longman; 1983. p. 265–80.
Azari-Hamidian S, Norouzi B, Harbach RE. A detailed review of the mosquitoes (Diptera: Culicidae) of Iran and their medical and veterinary importance. Acta Trop. 2019;194:106–22.
Pouriayevali MH, Rezaei F, Jalali T, Baniasadi V, Fazlalipour M, Mostafavi E, et al. Imported cases of Chikungunya virus in Iran. BMC Infect Dis. 2019;19:1004.
Azari-Hamidian S, Abai MR, Norouzi B. Mansonia uniformis (Diptera: Culicidae), a genus and species new to southwestern Asia, with a review of its medical and veterinary importance. Zootaxa. 2020;4772:385–95.
Tabachnick WJ. Evolutionary genetics and arthropod-borne disease: the yellow fever mosquito. Am Entomol. 1991;37:14–26.
Solomon T, Vaughn D. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol. 2002;267:171–94.
Nejati J, AnsariMoghadam S, Tabatabai A, Keyhani A. Effects of immigration on malaria incidence and its foci classification. Hormozgan Med J. 2012;16:283–91.
Nejati J, Vatandoost H, Oshghi MA, Salehi M, Mozafari E, Moosa-Kazemi SH. Some ecological attributes of malarial vector Anopheles superpictus Grassi in endemic foci in southeastern Iran. Asian Pac J Trop Biomed. 2013;3:1003–8.
Nejati J, Moosa-Kazemi SH, Saghafipour A, Soofi K. Knowledge, attitude and practice (KAP) on malaria, from high malaria burden rural communities, southeastern Iran. J Parasit Dis. 2018;42:62–7.
Nejati J, Saghafipour A, Vatandoost H, Moosa-Kazemi SH, Motevalli Haghi A, Sanei-Dehkordi A. Bionomics of Anopheles subpictus (Diptera: Culicidae) in a malaria endemic area, southeastern Iran. J Med Entomol. 2018;55:1182–7.
Soleimani Ahmadi M, Vatandoost H, Shaeghi M, Raeisi A, Abedi F, Eshraghian M, et al. Vector ecology and susceptibility in a malaria-endemic focus in southern Islamic Republic of Iran. East Mediterr Health J. 2012;18:1034–41.
Vatandoost H, Emami S, Oshaghi M, Abai M, Raeisi A, Piazzak N, et al. Ecology of malaria vector Anopheles culicifacies in a malarious area of Sistan va Baluchestan Province, south-east Islamic Republic of Iran. East Mediterr Health J. 2011;17:439–45.
Vatandoost H, Raeisi A, Saghafipour A, Nikpour F, Nejati J. Malaria situation in Iran: 2002–2017. Malar J. 2019;18:200.
Zaim M, Subbarao S, Manouchehri A, Cochrane A. Role of Anopheles culicifacies s.l. and An. pulcherrimus in malaria transmission in Ghassreghand (Baluchistan), Iran. J Am Mosq Control Assoc. 1993;9:23–6.
Djadid ND, Gholizadeh S, Tafsiri E, Romi R, Gordeev M, Zakeri S. Molecular identification of Palearctic members of Anopheles maculipennis in northern Iran. Malar J. 2007;6:6.
Harbach R. The classification of genus Anopheles (Diptera: Culicidae) a working hypothesis of phylogenetic relationships. Bull Entomol Res. 2004;94:537–53.
Manouchehri A, Zaim M, Emadi A. A review of malaria in Iran, 1975–90. J Am Mosq Control Assoc. 1992;8:381–5.
Oshaghi M, Sedaghat M, Vatandoost H. Molecular characterization of the Anopheles maculipennis complex in the Islamic Republic of Iran. Eastern Mediterr Health J. 2003;9:659–66.
Sedaghat M, Linton Y-M, Oshaghi M, Vatandoost H, Harbach R. The Anopheles maculipennis complex (Diptera: Culicidae) in Iran: molecular characterization and recognition of a new species. Bull Entomol Res. 2003;93:527–35.
Hanafi-Bojd AA, Azari-Hamidian S, Vatandoost H, Zabihollah C. Spatio—temporal distribution of malaria vectors (Diptera: Culicidae) across different climatic zones of Iran. Asian Pac J Trop Med. 2011;4:498–504.
Hanafi-Bojd AA, Sedaghat MM, Vatandoost H, Azari-Hamidian S, Pakdad K. Predicting environmentally suitable areas for Anopheles superpictus Grassi (s.l.), Anopheles maculipennis Meigen (s.l.) and Anopheles sacharovi Favre (Diptera: Culicidae) in Iran. Parasit Vectors. 2018;11:382.
Oshaghi M, Yaghobi-Ershadi M, Shemshad K, Pedram M, Amani H. The Anopheles superpictus complex: introduction of a new malaria vector complex in Iran. Bull Soc Pathol Exot. 2008;101:429–34.
Sinka M, Bangs M, Manguin S, Coetzee M, Mbogo C, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117.
Rowland M, Mohammed N, Rehman H, Hewitt S, Mendis C, Ahmad M, et al. Anopheline vectors and malaria transmission in eastern Afghanistan. Trans R Soc Trop Med Hyg. 2002;96:620–6.
WHO. Pesticides and their application: for the control of vectors and pests of public health importance. 6th ed. 2006. Geneva, World Health Organization. https://apps.who.int/iris/handle/10665/69223. Accessed 17 June 2012.
Huang Y-JS, Higgs S, Vanlandingham DL. Biological control strategies for mosquito vectors of arboviruses. Insects. 2017;8:21.
Muspratt J. Destruction of the larvae of Anopheles gambiae Giles by a Coelomomyces fungus. Bull World Health Organ. 1963;29:81–6.
Weiser J, Zaim M, Saebi E. Coelomomyces irani sp.n. infecting Anopheles maculipennis in Iran. J Invertebr Pathol. 1991;57:290–1.
Azari-Hamidian S, Abaei MR, Nourouzi. The first record of the fungal pathogen Coelomomyces (Blastocladiales: Coelomomycetaceae) in the malaria vector Anopheles culicifacies s.l. (Diptera: Culicidae) in Iran. In 4th international and 11th national congress on parasitology and parasitic diseases of Iran (NICOPA11) Urmia University of Medical Sciences. 2019.
Soni N, Prakash S. Aspergillus niger metabolites efficacies against the mosquito larval (Culex quinquefasciatus, Anopheles stephensi and Aedes aegypti) population after column chromatography. Am J Microbiol Res. 2011;2:15–20.
Darbro JM, Johnson PH, Thomas MB, Ritchie SA, Kay BH, Ryan PA. Effects of Beauveria bassiana on survival, blood-feeding success, and fecundity of Aedes aegypti in laboratory and semi-field conditions. Am J Trop Med Hyg. 2012;86:656–64.
Kerwin JL, Washino RK. Ground and aerial application of the asexual stage of Lagenidium giganteum for control of mosquitoes associated with rice culture in the Central Valley of California. J Am Mosq Control Assoc. 1987;3:59–64.
Agudelo-Silva F, Wassink H. Infectivity of a Venezuelan strain of Metarhizium anisopliae to Aedes aegypti larvae. J Invertebr Pathol. 1984;43:435–6.
Pinnock D, Garcia R, Cubbin C. Beauveria tenella as a control agent for mosquito larvae. J Invertebr Pathol. 1973;22:143–7.
Merriam TL, Axtell RC. Evaluation of the entomogenous fungi Culicinomyces clavosporus and Lagenidium giganteum for control of the salt marsh mosquito, Aedes taeniorhynchus. Mosq News. 1982;42:594–602.
Jaronski S, Axtell R. Persistence of the mosquito fungal pathogen Lagenidium giganteum (Oomycetes; Lagenidiales) after introduction into natural habitats. Mosq News. 1983;43:332–7.
Cuda J, Hornby J, Cotterill B, Cattell M. Evaluation of Lagenidium giganteum for biocontrol of Mansonia mosquitoes in Florida (Diptera: Culicidae). Biol Control. 1997;8:124–30.
Araújo JP, Hughes DP. Diversity of entomopathogenic fungi: which groups conquered the insect body? Adv Genet. 2016;94:1–39.
Goettel M, Inglis G. Fungi: hyphomycetes. In: Lacey LA, editor. Manual of techniques in insect pathology. London: Academic Press; 1997. p. 213–49.
Scholte E, Takken W, Knols B. Pathogenicity of five east African entomopathogenic fungi against adult Anopheles gambiae s.s. mosquitoes (Diptera, Culicidae). Proc Exper Appl Entomol. 2003;14:25–9.
Prasad A, Veerwal B. Toxicological effect of entomopathogenic fungus Beauveria bassiana (balsamo) vuillemin against malaria vector Anopheles stephensi (s.l.). Int J Pharma Bio Sci. 2012;3:625–37.
Bezalwar P, Gomashe A, Gulhane P. Laboratory-based evaluation of the potential of Beauveria bassiana crude metabolites for mosquito larvae. Annihilation. 2014;9:15–20.
Crisan EV. Mechanism responsible for release of toxin by Metarrhizium spores in mosquito larvae. J Invertebr Pathol. 1971;17:260–4.
Farida B, Sonia H, Hakima M, Fatma B, Fatma H. Histological changes in the larvae of the domestic mosquito Culex pipiens treated with the entomopathogenic fungus Beauveria bassiana. Sci Res Essays. 2018;13:1–10.
I.R. of Iran meteorological organization. Climate profile of Kashan County. 2014. http://www.kashanmet.ir. Accessed 15 Sept 2015.
Azari-Hamidian S, Harbach RE. Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae). Zootaxa. 2009;2078:1–33.
Turky W, Hassoon A, Al-Taee M. Isolation and diagnosis of fungi associated with the larvae of the mosquitoes Culex quinquefasciatus and its biological control. JMEST. 2018;5:9028–35.
Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. 4th ed. St Paul: APS Press; 1998.
Alkhaibari AM, Carolino AT, Yavasoglu SI, Maffeis T, Mattoso TC, Bull JC, et al. Metarhizium brunneum blastospore pathogenesis in Aedes aegypti larvae: attack on several fronts accelerates mortality. PLoS Pathog. 2016;12: e1005715.
Roy P, Bhatt P. Nattrassia mangiferae: an uncommon agent of onychomycosis. Med J Armed Forces India. 2015;71:297–9.
Balumahendhiran K, Vivekanandhan P, Shivakumar MS. Mosquito control potential of secondary metabolites isolated from Aspergillus flavus and Aspergillus fumigatus. Biocatal Agric Biotechnol. 2019;21: 101334.
Weindling R, Fawcett H. Experiments in the control of Rhizoctonia damping-off of citrus seedlings. Hilgardia. 1936;10:1–16.
Podder D, Ghosh SK. A new application of Trichoderma asperellum as an anopheline larvicide for eco friendly management in medical science. Sci Rep. 2019;9:1108.
Ferron P. Pest control by the fungi Beauveria and Metarhizium. In: Burge HD, editors. Microbial control of pests and plant diseases 1970–1980. New York: Academic Press; 1981. p. 465–81.
Ragavendran C, Manigandan V, Kamaraj C, Balasubramani G, Prakash JS, Perumal P, et al. Larvicidal, histopathological, antibacterial activity of indigenous fungus Penicillium sp. against Aedes aegypti L and Culex quinquefasciatus (Say) (Diptera: Culicidae) and its acetylcholinesterase inhibition and toxicity assessment of zebrafish (Danio rerio). Front Microbiol. 2019;10:427.
Omrani SM, Moosavi SF, Manouchehri K. Microsporidium infecting Anopheles superpictus (Diptera: Culicidae) larvae. J Arthropod Borne Dis. 2016;10:413–20.
Andreadis TG. Microsporidian parasites of mosquitoes. J Am Mosq Control Assoc. 2007;23:3–29.
Geetha I, Paily K, Padmanaban V, Balaraman K. Oviposition response of the mosquito, Culex quinquefasciatus to the secondary metabolite (s) of the fungus, Trichoderma viride. Mem Inst Oswaldo Cruz. 2003;98:223–6.
Malassigné S, Valiente Moro C, Luis P. Mosquito mycobiota: an overview of non-entomopathogenic fungal interactions. Pathogens. 2020;9:564.
Shemshadian A, Abai MR, Vatandoost H, Dinparast-Djadid N, Oshaghi MA, Mojahedi A. Assessment the changing trend of susceptibility to two insecticides among field-population Culex quinquefasciatus compared with the same population undergoing to multiple colonization. J Arthropod Borne Dis. 2020;14:185–92.
Vatandoost H, Hanafi-Bojd AA, Nikpoor F. Study on insecticide resistance and irritability of malaria vector, Anopheles dthali in Iran. Int J Mosq Res. 2020;7:19–24.
Nikookar SH, Fazeli-Dinan M, Ziapour SP, Ghorbani F, Salim-Abadi Y, Vatandoost H, et al. First report of biochemical mechanisms of insecticide resistance in the field population of Culex pipiens (Diptera: Culicidae) from Sari, Mazandaran, north of Iran. J Arthropod Borne Dis. 2019;13:378–90.
Hazratian T, Paksa A, Sedaghat MM, Vatandoost H, Moosa-Kazemi SH, Sanei-Dehkordi A, et al. Baseline susceptibility of Culiseta longiareolata (Diptera: Culicidae) to different imagicides, in eastern Azerbaijan. Iran J Arthropod Borne Dis. 2019;13:407–15.
Rahimi S, Vatandoost H, Abai MR, Raeisi A, Hanafi-Bojd AA. Status of resistant and knockdown of West Nile vector, Culex pipiens complex to different pesticides in Iran. J Arthropod Borne Dis. 2019;13:284–96.
Zeidabadinezhad R, Vatandoost H, Abai MR, Djadid ND, Abbasali RA, Sedaghat MM, et al. Target site insensitivity detection in deltamethrin resistant Culex pipiens complex in Iran. Iran J Public Health. 2019;48:1091–8.
Gorouhi MA, Oshaghi MA, Vatandoost H, Enayati AA, Raeisi A, Abai MR, et al. Biochemical basis of cyfluthrin and DDT resistance in Anopheles stephensi (Diptera: Culicidae) in malarious area of Iran. J Arthropod Borne Dis. 2018;12:310–20.
Vatandoost H, Hanafi-Bojd AA, Raeisi A, Abai MR, Nikpour F. Bioecology of dominant malaria vector, Anopheles superpictus sl (Diptera: Culicidae) in Iran. J Arthropod Borne Dis. 2018;12:196–218.
Ghorbani F, Vatandoost H, Hanafi-Bojd AA, Abai MR, Nikookar H, Enayati AA. High resistance of vector of West Nile virus, Culex pipiens Linnaeus (Diptera: Culicidae) to different insecticides recommended by WHO in northern Iran. J Arthropod Borne Dis. 2018;12:24–30.
Vatandoost H, Hanafi-Bojd AA, Raeisi A, Abai MR, Nikpour F. Ecology, monitoring and mapping of insecticide resistance of malaria vector, Anopheles culicifacies (Diptera: Culicidae) to different imagicides in Iran. Asian Pac J Trop Dis. 2017;7:53–6.
Chavshin AR, Dabiri F, Vatandoost H, Bavani MM. Susceptibility of Anopheles maculipennis to different classes of insecticides in West Azarbaijan Province, northwestern Iran. Asian Pac J Trop Biomed. 2015;5:403–6.
Abai MR, Hanafi-Bojd AA, Vatandoost H. Laboratory evaluation of temephos against Anopheles stephensi and Culex pipiens larvae in Iran. J Arthropod Borne Dis. 2016;10:510–8.
Salim-Abadi Y, Oshaghi MA, Enayati AA, Abai MR, Vatandoost H, Eshraghian MR, et al. High insecticides resistance in Culex pipiens (Diptera: Culicidae) from Tehran, capital of Iran. J Arthropod Borne Dis. 2016;10:483–92.
Gorouhi MA, Vatandoost H, Oshaghi MA, Raeisi A, Enayati AA, Mirhendi H, et al. Current susceptibility status of Anopheles stephensi (Diptera: Culicidae) to different imagicides in a malarious area, southeastern of Iran. J Arthropod Borne Dis. 2016;10:493–500.
Fathian M, Vatandoost H, Moosa-Kazemi SH, Raeisi A, Yaghoobi-Ershadi MR, Oshaghi MA, et al. Susceptibility of Culicidae mosquitoes to some insecticides recommended by WHO in a malaria endemic area of southeastern Iran. J Arthropod Borne Dis. 2015;9:22–34.
Ataie A, Moosa-Kazemi SH, Vatandoost H, Yaghoobi-Ershadi MR, Bakhshi H, Anjomruz M. Assessing the susceptibility status of mosquitoes (Diptera: Culicidae) in a dirofilariasis focus, northwestern Iran. J Arthropod Borne Dis. 2015;9:7–21.
Hancock PA. Combining fungal biopesticides and insecticide-treated bednets to enhance malaria control. PLoS Comput Biol. 2009;5: e1000525.
Paula AR, Carolino AT, Paula CO, Samuels RI. The combination of the entomopathogenic fungus Metarhizium anisopliae with the insecticide imidacloprid increases virulence against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasit Vectors. 2011;4:8.
Samuels RI, Paula AR, Carolino AT, Gomes SA, Paula CO, Cypriano MB, et al. Entomopathogenic organisms: conceptual advances and real-world applications for mosquito biological control. J Insect Physiol. 2016;6:25–31.
Acknowledgements
The authors are grateful to Prof. A. A. HanafiBojd for reviewing the manuscript, and Dr. S. M. Asgarian for helping with field collections.
Funding
This work was supported as No.: IR.TUMS.VCR.REC.1397.1001.
Author information
Authors and Affiliations
Contributions
SHMK and MMS developed the study concept, design, revised and edited the manuscript. TSA collected the data, did the laboratory work, and wrote the manuscript. SJ did the laboratory work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical clearance was earned from the Tehran University of Medical Sciences, Tehran, Iran.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Moosa-Kazemi, S.H., Asgarian, T.S., Sedaghat, M.M. et al. Pathogenic fungi infection attributes of malarial vectors Anopheles maculipennis and Anopheles superpictus in central Iran. Malar J 20, 393 (2021). https://doi.org/10.1186/s12936-021-03927-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-021-03927-4