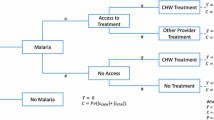
Abstract
Background
Malaria is a public health burden and a major cause for morbidity and mortality in Ethiopia. Malaria also places a substantial financial burden on families and Ethiopia’s national economy. Economic evaluations, with evidence on equity and financial risk protection (FRP), are therefore essential to support decision-making for policymakers to identify best buys amongst possible malaria interventions. The aim of this study is to estimate the expected health and FRP benefits of universal public financing of key malaria interventions in Ethiopia.
Methods
Using extended cost-effectiveness analysis (ECEA), the potential health and FRP benefits were estimated, and their distributions across socio-economic groups, of publicly financing a 10% coverage increase in artemisinin-based combination therapy (ACT), long-lasting insecticide-treated bed nets (LLIN), indoor residual spraying (IRS), and malaria vaccine (hypothetical).
Results
ACT, LLIN, IRS, and vaccine would avert 358, 188, 107 and 38 deaths, respectively, each year at a net government cost of $5.7, 16.5, 32.6, and 5.1 million, respectively. The annual cost of implementing IRS would be two times higher than that of the LLIN interventions, and would be the main driver of the total costs. The averted deaths would be mainly concentrated in the poorest two income quintiles. The four interventions would eliminate about $4,627,800 of private health expenditures, and the poorest income quintiles would see the greatest FRP benefits. ACT and LLINs would have the largest impact on malaria-related deaths averted and FRP benefits.
Conclusions
ACT, LLIN, IRS, and vaccine interventions would bring large health and financial benefits to the poorest households in Ethiopia.
Similar content being viewed by others
Background
Malaria prevention and control has been prioritized over the past decade in many national health sector plans. As a result, remarkable progress was made worldwide in reducing incidence and mortality from malaria [1, 2]. Due to the expansion of effective strategies, between 2001 and 2013, malaria incidence has dropped by 30% [1, 2]. Despite such progress, malaria remains a major public health burden with a huge impact on the socio-economic development of many countries [1, 2]. Nearly one-half of the world population lives in malaria-endemic countries [3]. In 2016 alone, there were an estimated 216 million cases and 445,000 deaths attributable to malaria worldwide [4]. Sub-Saharan Africa accounts for 90% of both cases and deaths due to malaria [4]. Malaria control is unequally distributed across socioeconomic groups and the rates of insecticide- and drug-resistance are increasing. Further scale-up of cost-effective malaria interventions with sustainable financing mechanisms is therefore urgently needed [5].
Ethiopia has made notable progress towards malaria control [6, 7]. Nationally, the prevalence of malaria has declined from 5 to 3% over 2010–2015 [5, 8, 9]. During the same period, malaria-related deaths were reduced by 40% [5]. Scale-up of effective anti-malaria interventions at the primary health care level and improved community engagement were major contributing factors to this progress [10]. There is little evidence from Ethiopia about other factors that might have contributed to malaria decline (e.g. climate change, housing structures and urbanization). However, despite significant progress, much remains to be done in the fight against malaria in Ethiopia, where about 2.6 million cases and 5000 deaths were estimated for the year 2016 [4]. Additionally, the 2015 malaria indicator survey shows that only 40% of the population at risk correctly use insecticide-treated bed nets [9].
Malaria prevention and control are major priorities for Ethiopia’s health sector transformation plan (HSTP) [11]. The primary strategies include rolling out long-lasting insecticide-treated bed nets (LLIN) and insecticide residual spray (IRS) for at-risk population [10, 12]. Similarly, artemisinin-based combination therapy (ACT) is recommended as first-line treatment of uncomplicated malaria [10, 12]. Ethiopia has committed to end malaria by 2030 and adopted global malaria control and elimination strategies [12]. As the country moves towards elimination by 2030, tests that are more sensitive will be required to detect subclinical malaria infection to prevent disease transmission [13]. A malaria vaccine (i.e. RTS,S/AS01) could help curb the malaria burden. However, the efficacy of the vaccine is partial and presents rapid waning immunity [14, 15].
Malaria is endemic in many regions of Ethiopia with marked seasonal and geographic variation. Nearly 60% of the total population reside in high-risk areas [10, 12]. In addition to its public health impact, malaria imposes a large financial burden on households, consuming on average 7% of household income [16, 17]. Marginalized and economically vulnerable populations are also at a higher risk of acquiring malaria and of experiencing fatal consequences because of limited health care access and the inability to pay for it [1, 18, 19]. Malaria spending is estimated to cost Ethiopia about $200 million annually or 10% of its total health expenditure [20]. Hence, reducing malaria disease burden has the potential to improve socioeconomic development [21].
The recent attention to universal health coverage (UHC) has provided context to explore mechanisms that would expand access to malaria prevention and treatment services in Ethiopia [22]. This would also help address the high rate (33%) of out-of-pocket (OOP) payments [20]. Given that a quarter of the Ethiopian population lives below the national poverty line [23], OOP malaria treatment costs can be an important barrier to access effective treatment and in pushing households into impoverishment in Ethiopia. Accounting for non-health benefits is essential to reduce health inequalities and contribute to the objectives of UHC [22]. Financial risk protection (FRP) is an important policy objective and can improve access to all needed quality health services without financial hardship [24, 25].
In this paper, the aim is to estimate the potential health, FRP, and equity benefits of universal public finance of scaling up selected malaria prevention and treatment interventions in Ethiopia [26]. This will support policymakers in jointly considering health gains, FRP and equity benefits in resource allocation related decisions.
Methods
Using extended cost-effectiveness analysis (ECEA), we consider the costs and health impact of malaria interventions across population subgroups and estimate the FRP impact on households in Ethiopia [26]. Building on a recent ECEA of malaria vaccine [28], and using a static disease model, are quantified, across socioeconomic groups (i.e. income quintiles), for each of four malaria interventions (ACT, LLIN, IRS, and malaria vaccine): the number of malaria-related deaths and OOP expenditures averted; the corresponding household FRP provided; and the implementation costs. Furthermore, ECEA is also applied across malaria transmission intensities to account for geographic variation of malaria (see Additional file 1: Appendix Table S2).
Malaria interventions
Large scale use of LLINs is a key strategy to reduce malaria burden [29]. A meta-analysis showed that LLIN was effective in both reducing malaria cases (by 50%) and malaria deaths (by 18%) [27]. IRS can eliminate malaria vectors by applying a residual insecticide to the internal walls and ceilings of homes [2, 30], and its use has been shown to decrease plasmodium falciparum malaria by 29% [31]. A complete cure can be expected in 95% of falciparum malaria cases treated with ACT [32]. The proportion of Plasmodium falciparum malaria in Ethiopia totals about 80–90% of all malaria cases [9]. Lastly, a recent clinical trial showed a 26% reduction in the number of episodes and hospital admissions, in children under 2 years of age, following three doses of malaria vaccine (currently under development) [14].
Health benefits
Population at risk of malaria (accounting for 60% of total population—defined as areas with annual incidence > 0 per 1000 population) is the target population for LLIN and IRS (Table 1) [12]. Similarly, the estimated number of annual malaria cases and birth cohorts born in at-risk areas were the target populations for ACT and vaccine, respectively [12, 14]. Target populations were split into income quintiles for LLIN, IRS, and ACT interventions. As for the vaccine, quintile-specific total fertility rates were applied in order to differentiate between the number of susceptible individuals per income quintile (see Additional file 1: Appendix). For each intervention, in order to calculate malaria prevalence by at-risk population per income quintile, first the relative risk of malaria prevalence by income quintile is estimated for the general population [9, 10]. These stratified relative risks were multiplied by average malaria prevalence, in order to split prevalence rates across income quintiles for populations at risk (see Additional file 1: Appendix) [9, 10].
The baseline coverage (before introduction of universal public financing) was 40% for LLIN and 29% for IRS and their respective coverage by income quintile was sourced from the 2016 malaria indicator survey (MIS) (Table 1) [9]. LLIN use, rather than its possession, was selected as a proxy parameter because the actual use of LLIN reflects behavioural change [33]. The percentage for whom care was sought among children who had fever in the past 2 weeks was used as a proxy for probability of seeking malaria care and baseline ACT coverage (35%) [34, 35]. A 10% incremental coverage across quintiles was assumed for each intervention. For the vaccine, in addition to the 10% incremental increase in coverage, a scenario with coverage scale-up from 0 to 33% was also considered (since this is the national coverage level of the basic child immunization programme) [34].
Before intervention, 2.6 million cases and 5000 deaths attributed to malaria were assumed to occur annually in Ethiopia [4]. On average, 1% of all malaria cases would be hospitalized, according to the integrated disease surveillance database [36, 37]. Severe and mild cases were treated as inpatient and outpatient cases, respectively. Deaths averted by each intervention were calculated as a product of disease incidence, case fatality ratio, intervention efficacy and incremental coverage (see Additional file 1: Appendix).
Financial consequences for households
Both inpatient and outpatient care of malaria can impose an economic burden to individual households. Direct medical, non-medical, and indirect costs were extracted from two previously published studies [18, 42]. Before universal public finance (UPF) of each intervention, individuals seeking malaria care would pay about $6 and $66 out-of-pocket (OOP) costs for outpatient and inpatient treatment, respectively [18, 42]. Even if there were no OOP payments for preventive interventions, the three malaria preventive interventions (i.e. LLIN, IRS, vaccine) would lower the risk of malaria and thus household OOP expenditures related to malaria treatment. The amount of OOP expenditures averted per income quintile was quantified, before and after UPF. OOP expenditures averted depended on: target population, incremental coverage, health care use, OOP payments, and preventive intervention effectiveness (see Additional file 1: Appendix).
Financial risk protection benefits
The financial risk faced by households depends on the malaria burden, intervention coverage, and probability of seeking treatment. Annual consumption expenditures were extracted from the Ethiopian Household Income Consumption and Expenditure and Welfare Monitoring Survey as a proxy for income [48]. In this study, a case of catastrophic health expenditures (CHE) was counted when total OOP spending for malaria treatment exceeded 10% of total household consumption expenditures or 40% of capacity to pay (i.e. non-food total household consumption) [49, 50]. UPF introduction would avert a number of CHE cases following the reduction in incidence of OOP expenditures.
Intervention costs
The cost of each intervention was estimated from the health system perspective. Average unit cost estimates for preventive (LLIN, IRS, and vaccine) and curative (ACT) interventions were obtained from published studies (Table 1) [44,45,46,47]. The unit cost for LLIN included net price and delivery cost. Similarly, for IRS, insecticide cost accounted for 50%, spray campaign operations and labour for 26%, capital cost for 23% and other commodities accounted for 1% [44, 46, 47]. The average unit cost per fully vaccinated child included vaccine price, and supplies accounted for 84%, and the remaining costs (16%) included training, transportation, waste management [45]. Unit cost of ACT comprised of human resources at 58%, drug and pharmaceutical supplies at 25% and rest was indirect costs [43]. Patient and health system costs were extracted from the literature and converted for the year 2016 using Ethiopia’s gross domestic product (GDP) deflator [38]. The total costs considered: target population, intervention coverage and intervention unit cost.
Sensitivity analyses
The robustness of the findings were tested by using one-way sensitivity analyses. Specifically, the value of malaria prevalence, case fatality ratio, intervention effectiveness, health services utilization, and intervention unit cost were varied by ± 20%, one at a time, to evaluate the interventions impact on the deaths and CHE averted, across income quintiles.
Results
Deaths and cases of CHE averted by malaria interventions
Increasing coverage (by 10%) of ACT, LLIN, IRS and vaccine among the population at risk would avert 358, 188, 107 and 38 deaths per year in Ethiopia, respectively. The four interventions would also avert 440 (i.e. 10% of the baseline CHE), 220 (5%), 125 (3%) and 18 (2%) CHE cases annually, respectively. Among the interventions, LLIN and ACT would have the largest number of deaths averted and CHE cases averted. In addition, ACT and LLIN would avert $4,277,000 and $214,000 of OOP expenditure, respectively (Table 2).
Distribution of deaths and CHE cases averted by malaria intervention
All four interventions would save larger numbers of lives among the poor, due to the fact that the poor would face a higher malaria prevalence and associated risk factors. For example, ACT would avert twice as many deaths in the poorest income quintile as compared to the richest quintile (Fig. 1). 50% of the deaths averted would be concentrated in the poorest two quintiles. The distribution of deaths averted (by LLIN, IRS and ACT), from poorest to richest quintiles, would be 30, 20, 23, 14 and 13%, respectively. Similarly, the distribution of deaths averted by the malaria vaccine would be 30, 22, 21, 16, and 11%, respectively (Fig. 1).
For each intervention, the gradient in private OOP expenditures averted would be flat across quintiles as malaria prevalence would decrease with increasing income, but the probability of seeking malaria care would increase as income goes up (Table 3). Therefore, the gains in private expenditures would be evenly distributed across income quintiles. Across the first three income quintiles, a greater number of CHE cases would be averted and the largest benefits would be among the poorest income quintile (Fig. 2).
The annual policy costs of UPF for 10% incremental coverage of ACT, LLIN, IRS and vaccine would be $5.7, 16.5, 32.6, and 5.1 million, respectively. Similarly, due to declines in malaria cases through preventive interventions, $241,000, $137,000 and $16,000 of government expenditures on malaria treatment would be averted annually by LLIN, IRS and malaria vaccine, respectively.
Most of these government savings would be observed within quintile one to three and LLINs would contribute to more than half of these savings. The rollout of malaria vaccines at 10% incremental coverage, under the routine immunization program in the country, would cost around $5 million and avert 38 deaths and reach $17 million and avert about 120 deaths with 33% coverage.
Deaths and cases of CHE averted per million spent
The health benefits per $1 million invested on ACT, LLIN, IRS, and vaccine interventions would be 63, 11, 3, and 7 lives, respectively. Similarly, they would reduce OOP expenditures by $1,560,000, 13,000, 3700 and 2800, respectively; with varying numbers of CHE cases averted by income quintile (see Additional file 1: Appendix, Figs. S1–S3).
Sensitivity analyses
The results of our univariate sensitivity analyses are described in Table 4 (and Additional file 1: Tables S3–S6). Generally, the distribution of health gains is highly prone to variations in malaria prevalence, case fatality ratio and intervention efficacy. The distributions in OOP expenditures averted and CHE cases averted would be more sensitive to malaria prevalence, health care utilization, probability of seeking inpatient care, intervention efficacy and OOP expenditures.
Discussion
In this paper, the health and financial benefits of UPF for malaria interventions were estimated across Ethiopian households at all income levels. Overall, all four interventions showed substantial benefits, with ACT and LLIN accounting for the larger shares of malaria-related deaths and CHE cases averted.
All the interventions showed a greater number of deaths averted among the poorest 40% of the population, averted similar OOP expenditures across all income groups, and relatively higher FRP benefits for the poorest 40%. Even if the poor had lower access for care and higher baseline malaria risk, for each of the intervention greater benefits would go toward the poor. This suggests that the malaria interventions analysed in this paper benefit the worse-off and poor populations in remote areas of Ethiopia, who suffer the disease risk at most. Given the relatively lower malaria burden, the four malaria interventions would avert fewer deaths annually, as compared to, other interventions addressing childhood diarrhoea and pneumonia for example [51, 52]. Rapid decline of malaria deaths in Ethiopia over the last two decades and a relatively lower prevalence were the main reasons [6]. Among the four interventions, LLIN and ACT were the two strategies with the highest impact on malaria mortality. In contrast, the malaria vaccine would prevent the smallest number of deaths averted (i.e. 38 per year) as compared to the other interventions. This is largely because the vaccine would be relatively less efficacious [14, 41]: only 2% of malaria-related child deaths would be prevented from the vaccine in this study.
Even though the rich had more access to health services and less malaria burden, the private OOP savings would be similar across all income quintiles. This might be due to the fact that the poor and rich are spending similar OOP expenditures for malaria care. In absolute terms, the gains in private OOP expenditures could be lower as compared to findings from other Ethiopian ECEAs [51,52,53]. This might be due to less OOP payments for malaria care as compared to the other diseases. As for the FRP benefits, LLIN and ACT prevented a higher number of CHE cases, and for all interventions, the greatest number of CHE cases averted would occur in the poorest income quintile. In addition, the annual cost of implementing IRS at a 10% incremental coverage for the at-risk population was about $33 million, 2 times higher than that of the LLIN intervention. This corresponds to more than 16% of malaria-related health care spending in Ethiopia [20]. Lastly, though ACT, LLIN, IRS, and malaria vaccine are critical for malaria control and elimination, these interventions would need to be combined with other interventions, such as behavioural change, correct use and implementation, to yield full impact.
Nevertheless, the analysis presented here has several limitations. First, the disease model was static and did not address the dynamics of malaria transmission. Second, because of the unavailability of key input parameters by socioeconomic group, proxy input parameters were used. For example, the percentage who sought treatment for fever in the past 2 weeks was used as a proxy indicator for seeking malaria care. This might have overestimated malaria cases as there are other causes of fever among individuals (besides malaria). The Ethiopian 2016 DHS, the Malaria indicator survey and the ACT malaria consortium guidance on health equity analysis use health care utilisation due to fever in the past 2 weeks as a proxy for seeking care for malaria [9, 34, 35]. Third, due to the lack of disaggregated data, constant rates for case fatality ratio, intervention effectiveness, and inpatient cost inputs were assumed across quintiles. Fourth, unit costs for the vaccine were not specific to Ethiopia. However, despite the limitations, the analysis is crucial as the findings could assist policymakers decide on which health interventions to rollout to reduce malaria disease burden affecting 60% of the Ethiopian population [9].
The ECEA can also answer some of the equity concerns by providing valuable information on how malaria prevention or treatment strategies would decrease both malaria burden and financial risk incurred by households across various socioeconomic groups in Ethiopia. This study shows that malaria interventions could improve FRP across all income groups, especially among the bottom income groups in Ethiopia. Furthermore, this analysis can help reorienting malaria interventions to target elimination across selected segments of the population, especially among the poor.
Conclusions
All four malaria interventions would save more lives among the poor than among the rich. Preventing and treating malaria provides substantial health benefits and FRP, especially among poor Ethiopians. ACT and LLINs would generate the largest impact on malaria-related deaths averted and FRP benefits. Improving health equity and reducing poverty are major objectives of the Sustainable Development Goals, and the findings of the study presented here would provide insight for policymakers on how to prioritize malaria interventions for targeted population groups including the poorest.
Availability of data and materials
Not applicable.
Abbreviations
- ACT:
-
artemisinin-based combination therapy
- CFR:
-
case fatality ratio
- CHE:
-
catastrophic health expenditure
- CTP:
-
capacity to pay
- ECEA:
-
extended cost-effectiveness analysis
- EDHS:
-
Ethiopia Demographic and Health Survey
- FRP:
-
financial risk protection
- GDP:
-
gross domestic product
- IRS:
-
indoor residual spraying
- LLIN:
-
long-lasting insecticidal nets
- MIS:
-
malaria indicator survey
- OOP:
-
out-of-pocket payment
- UHC:
-
universal health coverage
- UPF:
-
universal public financing
- USD:
-
United States dollar
- WHO:
-
World Health Organization
References
WHO. Global technical strategy for malaria 2016–2030. Geneva: World Health Organization; 2015.
WHO on behalf of the Roll Back Malaria Partnership Secretariat. Action and investment to defeat Malaria 2016–2030 (AIM)—for a malaria-free world. 2015. https://www.mmv.org/sites/default/files/uploads/docs/publications/RBM_AIM_Report.pdf. Accessed 23 Sept 2018.
WHO. World malaria report 2015. Geneva: World Health Organization; 2015.
WHO. World malaria report 2017. Geneva: World Health Organization; 2017.
WHO. World malaria report 2016. Geneva: World Health Organization; 2016.
Deribew A, Dejene T, Kebede B, Tessema GA, Melaku YA, Misganaw A, et al. Incidence, prevalence and mortality rates of malaria in Ethiopia from 1990 to 2015: analysis of the global burden of diseases 2015. Malar J. 2017;16:271.
Accorsi S, Bilal NK, Farese P, Racalbuto V. Countdown to 2015: comparing progress towards the achievement of the health Millennium Development Goals in Ethiopia and other sub-Saharan African countries. Trans R Soc Trop Med Hyg. 2010;104:336–42.
Ethiopian Public Health Institute. The 2011 Ethiopia National Malaria Indicator Survey (EMIS). Addis Ababa: Ethiopian Public Health Institute; 2012.
Ethiopian Public Health Institute. The 2016 Ethiopia National Malaria Indicator Survey (EMIS). Addis Ababa: Ethiopian Public Health Institute; 2016.
Ethiopia Federal Ministry of Health. Ethiopian External Malaria Programme midterm review. Addis Ababa: Ethiopia Federal Ministry of Health; 2017.
Federal Ministry of Health Ethiopia. Health sector transformation plan 2015/16–2019/20 (2008–2012 EFY). Addis Ababa: Federal Ministry of Health Ethiopia; 2015.
Federal Ministry of Health. National malaria strategic plan 2017–2020. Addis Ababa: Federal Ministry of Health Ethiopia; April 2017.
Imwong M, Nguyen TN, Tripura R, Peto T, Lee SJ, Lwin KM, Suangkanarat P, et al. The epidemiology of subclinical malaria infections in South-East Asia: findings from cross-sectional surveys in Thailand–Myanmar border areas, Cambodia, and Vietnam. Malar J. 2015;14:381.
WHO. Malaria vaccine: WHO position paper, January 2016—recommendations. Vaccine. 2016;2018(36):3576–7.
Cropper ML, Haile M, Lampietti J, Poulos C, Whittington D. The demand for a malaria vaccine: evidence from Ethiopia. J Dev Econ. 2004;75:303–18.
Fonkwo PN. Pricing infectious disease. The economic and health implications of infectious diseases. EMBO Rep. 2008;9 Suppl 1:S13–7.
Gallup JL, Sachs JD. The economic burden of malaria. Am J Trop Med Hyg. 2001;64(Suppl 1):85–96.
Deressa W, Hailemariam D, Ali A. Economic costs of epidemic malaria to households in rural Ethiopia. Trop Med Int Health. 2007;12:1148–56.
Russell S. The economic burden of illness for households in developing countries: a review of studies focusing on malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome. Am J Trop Med Hyg. 2004;71(2 Suppl):147–55.
Federal Ministry of Health. Ethiopia national health accounts, 2013/2014. Addis Ababa: Federal Ministry of Health; 2017.
Jobin WR. Economic aspects of suppressing malaria in Africa. Malar World J. 2014;5:8.
WHO. Technical Consultation meeting report. Universal access to core malaria interventions in high-burden countries. Malaria Policy Advisory Committee Meeting. Geneva: World Health Organization; 2018.
National Planning Commission Ethiopia. Ethiopia’s progress towards eradicating poverty: an interim report on 2015/16 poverty analysis study. 2017.
O’Donnell O. Financial protection against medical expenses (January 31, 2019). Tinbergen Institute Discussion Papers 2019-010/V. SSRN: https://ssrn.com/abstract=3329189 or http://dx.doi.org/10.2139/ssrn.3329189. Accessed 30 Oct 2019.
Saksena P, Hsu J, Evans DB. Financial risk protection and universal health coverage: evidence and measurement challenges. PLoS Med. 2014;11:e1001701.
Verguet S, Kim JJ, Jamison DT. Extended cost-effectiveness analysis for health policy assessment: a tutorial. Pharmacoeconomics. 2016;34:913–23.
Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;2:CD000363.
Liu L, Portnoy A, True Z, Fink G, Verguet S. The health and financial benefits for households from averting malaria with RTS, S/AS01 vaccine in Zambia: an extended cost-effectiveness analysis. Disease control priorities in developing countries. 3rd edn. Working Paper No. 26. Seattle: University of Washington; 2018.
WHO, Malaria Control Unit, UNICEF. The Africa malaria report 2003. Geneva: World Health Organization; 2003.
Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;4:CD006657.
Kesteman T, Randrianarivelojosia M, Rogier C. The protective effectiveness of control interventions for malaria prevention: a systematic review of the literature. F1000Res. 2017;6:1932.
Global Partnership to Roll Back Malaria. Antimalarial drug combination therapy: report of a WHO technical consultation. Geneva: World Health Organization; 2001.
Birhanu Z, Abebe L, Sudhakar M, Dissanayake G, Yihdego Y, Alemayehu G, et al. Access to and use gaps of insecticide-treated nets among communities in Jimma Zone, southwestern Ethiopia: baseline results from malaria education interventions. BMC Public Health. 2015;15:1304.
Central Statistical Agency, Ethiopia. Ethiopia demographic and health survey. Addis Ababa: Central Statistical Agency; 2016.
Mangham L. ACT consortium guidance on health equity analysis. 2009. http://www.actconsortium.org/healtheconomicsguidance. Accessed 7 Oct 2019.
Jima D, Wondabeku M, Alemu A, Teferra A, Awel N, Deressa W, et al. Analysis of malaria surveillance data in Ethiopia: what can be learned from the Integrated Disease Surveillance and Response System? Malar J. 2012;11:330.
President’s Malaria Initiative (PMI). Ethiopia malaria operational plan. 2017.
World Bank. The World Bank in Ethiopia. 2017. https://data.worldbank.org/country/ethiopia. Accessed 21 May 2018.
Institute for Health Metrics and Evaluation. Global burden of disease (GBD) 2016 data. https://vizhub.healthdata.org/gbd-compare/. Accessed 10 May 2018.
Deressa W, Yihdego YY, Kebede Z, Batisso E, Tekalegne A, Dagne GA. Effect of combining mosquito repellent and insecticide treated net on malaria prevalence in Southern Ethiopia: a cluster-randomised trial. Parasit Vectors. 2014;7:132.
Penny M, Pemberton-Ross P, Smith T. The time-course of protection of the RTS,S vaccine against malaria infections and clinical disease. Malar J. 2015;14:437.
Hailu A, Lindtjørn B, Deressa W, et al. Economic burden of malaria and predictors of cost variability to rural households in south-central Ethiopia. PLoS ONE. 2017;12:e0185315.
Berman P, Mann C, Agarwal A, Abdella E. Costs of publicly funded primary care facilities, departments, and exempted services in Ethiopia. Boston: Harvard TH Chan School of Public Health; Breakthrough International Consultancy, PLC; 2016.
Pulkki-Brännström A-M, Wolff C, Brännström N, Skordis-Worrall J. Cost and cost effectiveness of long-lasting insecticide-treated bed nets—a model-based analysis. Cost Eff Resour Allocation. 2012;10:5.
Galactionova K, Bertram M, Lauer J, Tediosi F. Costing RTS,S introduction in Burkina Faso, Ghana, Kenya, Senegal, Tanzania, and Uganda: a generalizable approach drawing on publicly available data. Vaccine. 2015;33:6710–8.
Macdonald M. New ways of approaching indoor residual spraying for malaria. Glob Health Sci Pract. 2016;4:511.
Johns B. PMI IRS Country Programs: 2015 comparative cost analysis PMI Africa Indoor Residual Spraying Project, Abt Associates Inc. 2016.
The Federal Democratic Republic of Ethiopia. Central Statistical Agency. The 2015/16 Ethiopian household consumption—expenditure (HCE) survey. Addis Ababa; January 2018.
Wagstaff A, Doorslaer E. Catastrophe and impoverishment in paying for health care: with applications to Vietnam 1993–1998. Health Econ. 2003;12:921–34.
WHO. Monitoring the building blocks of health systems: a handbook of indicators and their measurement strategies. Geneva: World Health Organization; 2010.
Johansson KA, Memirie ST, Pecenka C, Jamison DT, Verguet S. Health gains and financial protection from pneumococcal vaccination and pneumonia treatment in Ethiopia: results from an extended cost-effectiveness analysis. PLoS ONE. 2015;10:e0142691.
Pecenka CJ, Johansson KA, Memirie ST, Jamison DT, Verguet S. Health gains and financial risk protection: an extended cost-effectiveness analysis of treatment and prevention of diarrhoea in Ethiopia. BMJ Open. 2015;5:e006402.
Verguet S, Olson ZD, Babigumira JB, Desalegn D, Johansson KA, Kruk ME, et al. Health gains and financial risk protection afforded by public financing of selected interventions in Ethiopia: an extended cost-effectiveness analysis. Lancet Glob Health. 2015;3:e288–96.
Acknowledgements
We are indebted to Emily Coles and Dr. Eyersulam Kassaye for language editing. Previous versions of this paper were presented during seminars at Addis Ababa University College of Health Sciences and the Harvard T.H. Chan School of Public Health, where we received valuable inputs from participants.
Funding
We thank the Bill & Melinda Gates Foundation (OPP1162384) for funding this study.
Author information
Authors and Affiliations
Contributions
LFA, SV, KAJ, MTT, OFN conceived and designed the study. LFA performed the analysis with input from XJK, DW, LL, SV, KAJ, AJ and MTT. LFA, KAJ, MTT wrote the first draft of the paper, which SV, KAJ, MTT, and AJ subsequently reviewed. All authors provided constructive feedback. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Additional Appendix, Figures S1–S3 and Tables S1–S6.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Assebe, L.F., Kwete, X.J., Wang, D. et al. Health gains and financial risk protection afforded by public financing of selected malaria interventions in Ethiopia: an extended cost-effectiveness analysis. Malar J 19, 41 (2020). https://doi.org/10.1186/s12936-020-3103-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-020-3103-5