Abstract
Background
Prompt diagnosis and effective malaria treatment is a key strategy in malaria control. However, the recommended diagnostic methods, microscopy and rapid diagnostic tests (RDTs), are not supported by robust quality assurance systems in endemic areas. This study compared the performance of routine RDTs and smear microscopy with a simple molecular-based colorimetric loop-mediated isothermal amplification (LAMP) at two different levels of the health care system in a malaria-endemic area of western Kenya.
Methods
Patients presenting with clinical symptoms of malaria at Rota Dispensary (level 2) and Siaya County Referral Hospital (level 4) were enrolled into the study after obtaining written informed consent. Capillary blood was collected to test for malaria by RDT and microscopy at the dispensary and county hospital, and for preparation of blood smears and dried blood spots (DBS) for expert microscopy and real-time polymerase chain reaction (RT-PCR). Results of the routine diagnostic tests were compared with those of malachite green loop-mediated isothermal amplification (MG-LAMP) performed at the two facilities.
Results
A total of 264 participants were enrolled into the study. At the dispensary level, the positivity rate by RDT, expert microscopy, MG-LAMP and RT-PCR was 37%, 30%, 44% and 42%, respectively, and 42%, 43%, 57% and 43% at the county hospital. Using RT-PCR as the reference test, the sensitivity of RDT and MG-LAMP was 78.1% (CI 67.5–86.4) and 82.9% (CI 73.0–90.3) at Rota dispensary. At Siaya hospital the sensitivity of routine microscopy and MG-LAMP was 83.3% (CI 65.3–94.4) and 93.3% (CI 77.9–99.2), respectively. Compared to MG-LAMP, there were 14 false positives and 29 false negatives by RDT at Rota dispensary and 3 false positives and 13 false negatives by routine microscopy at Siaya Hospital.
Conclusion
MG-LAMP is more sensitive than RDTs and microscopy in the detection of malaria parasites at public health facilities and might be a useful quality control tool in resource-limited settings.
Similar content being viewed by others
Background
Malaria remains a major public health problem and an impediment to social and economic development, particularly in sub-Saharan Africa. In 2017, of the estimated 219 million cases and 445,000 deaths attributed to malaria worldwide, approximately 90% of cases and deaths were in sub-Saharan Africa [1]. Between 2000–2015, there has been a significant reduction in the global malaria burden, with decline in incidence by 37% and mortality by 60% [1]. However, over the last two years the rate of decline has stalled and even reversed in some regions. This has been attributed to several interconnected challenges including the fact that many people who are infected are not properly diagnosed and therefore do not receive appropriate treatment [2].
Accurate parasitological diagnosis of a malaria case using either quality-assured microscopy or Rapid Diagnostic Tests (RDTs) and prompt treatment with effective artemisinin-based combination therapy (ACT) remains a key strategy in malaria case management and has played a key role in the reduction of the global malaria burden over the last two decades [3]. In addition, pillar 1 of the Global Technical Strategy for Malaria recommends universal access to malaria prevention, diagnosis and treatment for effective disease management and for surveillance [2]. Concerted efforts by national malaria programmes to improve malaria parasitological diagnosis and strategies such as test, treat and track (T3) launched by the World Health Organization (WHO) in 2012 have led to a significant increase in the number of health facilities in sub-Saharan Africa with capacity for microscopy or RDT [4, 5].
However, this is not supported by robust in-country quality assurance/quality control (QA/QC) programmes. This is evident from the findings of several studies that have evaluated the quality of diagnostic capacities in endemic areas, which have reported gaps in malaria microscopy ranging from shortage of trained personnel [6], lack of well-maintained microscopes and quality reagents [7], high workloads [8] and poor performance in species identification and reporting [9]. Similarly, although RDTs are recommended for malaria diagnosis in health facilities where microscopy is not available and at the community level, their performance depends on several factors including; parasite density, patient anti-malarial treatment history, pfhrp2/3 deletions [10], storage conditions and operator proficiency [11]. Without robust QA/QC systems, these factors could affect the diagnostic performance of RDTs or smear microscopy resulting in erroneous results, poor management of patients, irrational use of anti-malarial drugs and inaccurate surveillance data. Whereas, nucleic acid amplification tests (NAATs) are several orders of magnitude more sensitive than RDTs and microscopy, WHO recommends that NAATs be considered only for epidemiological research and survey mapping of sub-microscopic infections [12]. Nucleic acid amplification tests such as RT-PCR, quantitative nucleic acid sequence-based amplification (QT-NASBA) could be used as reference tests for QA/QC programmes, however, they are prohibitively expensive due to the high cost of equipment required, expensive reagents and the need for highly skilled laboratory personnel [13]. Availability of other NAATs such as the loop-mediated isothermal amplification (LAMP) that do not require thermal cyclers or highly skilled laboratory personnel [14] could be an inexpensive reference test that can be used in a QA/QC programme in resource-limited settings.
In Kenya, parasitological diagnosis of malaria using microscopy or RDTs is recommended for all patients with suspected malaria [15]. Since microscopy is only available at level 3 (health centre) to level 6 facilities (referral hospital), RDTs are used at level 1 (Community Health Workers) and level 2 facilities (dispensaries) or when microscopy is not available at other levels such as when there is power outage or stock out of reagents for microcopy. As in other endemic countries, the Kenya National Malaria Control Programme has developed malaria diagnosis and QA/QC guidelines and manuals [15]. However, implementation has remained a challenge and this is likely to have an impact on patient management and tracking the malaria burden at different levels of the health care system. Additionally, there is limited information on how many patients are missed by routinely performed RDTs and smear microscopy at different levels of the healthcare system.
The main objective of the current study was to compare the performance of routine RDTs and microscopy against an easy-to-use and highly sensitive molecular diagnostic assay, malachite green loop-mediated isothermal amplification (MG-LAMP) at two government health facilities representing different levels of healthcare delivery in Kenya. Although the use of LAMP has been extensively evaluated for malaria diagnosis in areas of low malaria transmission and elimination settings, there is limited information on its use to support a QA/QC system in resource-limited settings.
Methods
Study area
The study was conducted at two health facilities (Siaya County Referral Hospital and Rota Dispensary), which are located in a malaria endemic area of western Kenya and serve mostly rural populations. Most of the residents in this area belong to the Luo ethnic group and live in scattered family compounds consisting of one or more houses surrounded by agricultural fields. The main occupation of the residents in this area include subsistence farming, fishing and small-scale trading. The community prevalence of Plasmodium falciparum by slide microscopy is 28%, in children aged < 5 years, 42% in the 5 to 15-year old and 18% in those aged > 15 years (KEMRI-CDC unpublished data). Malaria is one of the leading causes of hospital visits and admissions in this area [16]. The primary malaria vectors are Anopheles gambiae sensu lato and Anopheles funestus and entomologic inoculation rates are < 20 infective bites per person per year [17]. Malaria transmission occurs year-round with two peak seasons from May–July and November–December coinciding with the end of the long and short rains.
Both Rota Dispensary and Siaya County Referral Hospital (Fig. 1) are government-owned and are aligned with the country’s health service delivery system; level-1 (community), level-2 (dispensaries/clinics), level-3 (health centres/maternities/nursing homes), level-4 (sub-county hospitals), level-5 (county referral hospitals) and level-6 (regional and national hospitals). Dispensaries are headed by a nurse and provide promotive and preventive care. Malaria diagnosis at dispensary is primarily by RDT. Country hospitals are headed by a medical officer and undertake mainly curative and rehabilitative services. Malaria diagnosis at this level is performed by laboratory technologists mainly by microscopy, but RDTs can be used if microscopy is unavailable, for example when there is a prolonged power outage or stock outs of reagents for microscopy. Rota Dispensary records 20–30 patients per day mainly for outpatient consultations while complicated cases are referred to levels 3, 4 or 5 facilities. Siaya Hospital outpatient department (OPD) records 40–60 patients per day with both minor and complicated ailments and has inpatient facilities. Any complicated cases are referred to level-6 facilities.
Study participants
Study participants were recruited at the outpatient departments of the two health facilities if they presented with symptoms suggestive of malaria and were referred to the laboratory for malaria parasitological diagnosis. At Rota Dispensary, RDT (SD Bioline Malaria Ag P.f/Pan 05FK60, Standard Diagnostics, Kyonggi, Republic of Korea) was used for diagnosis. Routine microscopy was used for diagnosis at Siaya Hospital as per the national malaria diagnosis and treatment guidelines. Participants were enrolled into the study after obtaining written informed consent for participants aged over 18 years, parental/caregiver consent for those aged less than 18 years or written assent for emancipated minors. Participants were excluded if they presented with severe disease or reported use of anti-malarial drugs during the past four weeks. Participants found to be malaria positive were treated with artemether-lumefantrine (AL) as per the Kenya Ministry of Health national guidelines.
Collection of blood samples
Approximately 300 µL of capillary blood was collected into ethylenediaminetetraacetic acid (EDTA) microtainers from enrolled participants. The whole blood was used for preparation of blood smears for expert microscopy, performing malaria RDT, performing MG-LAMP assay and preparation of dried blood spots (DBS) on Whatman® 903 protein saver filter paper (GE Healthcare, USA) for RT-PCR. All samples were assigned a unique study identification number. Blood smears and DBSs were transported to Kenya Medical Research Institute/Centre for Global Health Research (KEMRI/CGHR) malaria laboratories, located about 6.1 km from Rota Dispensary and 56 km from Siaya County Referral Hospital, for storage and analysis.
Rapid diagnostic tests and malaria microscopy at the health facilities
Rapid diagnostic tests were performed at Rota dispensary using 5 µL of blood according to manufacturer’s instructions. At the Siaya County Referral Hospital, malaria microscopy was performed by a laboratory technologist using the hospital standard operating procedure (SOP) and involved collection of finger prick blood, preparation of thick and thin blood smears using 6 and 2 µL of blood, respectively, staining with 10% Giemsa for 15 min and examination of slides under a microscope. A smear was considered negative if no parasites were detected in 100 high power microscopic fields.
Expert microscopy at the KEMRI/CGHR malaria laboratories
For endpoint analysis, malaria microscopy was carried out according to WHO basic malaria microscopy guidelines, 2010 [18]. Thick (for parasite density determination) and thin (for Plasmodium species identification) smears were prepared from 6 µL and 2 µL of whole blood sample, respectively. The smears were allowed to air dry and then stained with 3% Giemsa for 1 h. The slides were read at 100× objective lens under oil immersion using a compound microscope for determination of both asexual and sexual stage of parasites. A blood smear was considered negative if 100 microscopic high-powered fields showed no parasites. If a blood smear was positive, malaria parasites were counted in 40 microscopic high-powered fields and parasite densities expressed per microlitre (µL). All blood smears were examined independently by two expert microscopists blinded to each other’s results. Where the two readings differed in results (one reader positive and the other negative), parasite species, or if the higher count divided by the lower count was ≥ 2 (for high and medium parasitaemia) and ≥ 10 (for low parasitaemia), smears were re-examined by a tie-breaker microscopist who was blinded to the results of the first two readers. All the microscopists were enrolled and had passed a quarterly external quality assurance programme administered by the National Institute of Communicable Diseases (NICD), South Africa.
MG-LAMP assay
The MG-LAMP was performed at the health facilities by boil-and-spin method [19] using 50 µL of the collected blood. DNA was released from whole blood by boiling in a heat-block at 95 °C for 10 min. The samples were centrifuged for 3 min at 15,000 × g and the supernatant (containing the DNA) was collected and used for the MG-LAMP assay. Five µL of the supernatant was used in the MG-LAMP assay and the rest stored in the -80ºC freezer. The MG-LAMP assay was performed in a 20 μL total reaction volume containing 2X in-house buffer (40 mM Tris–HCL pH 8.8, 20 mM KCl, 16 mM MgSO4, 20 mM (NH4)SO4, 0.2% Tween-20, 0.8 M Betaine, 2,8 mM of dNTPs each), 0.004% MG, 8 units of Bst polymerase (New England Biolabs, Ipswich, MA) and 5 μL of template DNA [20]. Mitochondria Plasmodium genus-specific primers were used to amplify the DNA at 63 °C for 60 min using a simple heat block. The samples were allowed to cool for 15 min before being scored by three independent readers by visual inspection of color change. Positive (known P. falciparum) and negative (no template/DNA) control samples were included in each run. Positive samples retained a light green/blue malachite green colour while negative samples were colourless. For the purpose of quality control, 20% of the samples were randomly selected and retested at KEMRI/CGHR Malaria Laboratories.
Quantitative real-time PCR
The QIAamp DNA Mini Blood Kit (Quigen, Valencia, CA) was used to extract DNA from DBS prepared from 50 µL of blood. Commercially available TaqMan Universal Master Mix (Applied Biosystems) was used. Species-specific probes corresponding to P. falciparum was used to detect the presence of P. falciparum. The Rougemont real-time PCR was performed using standard equipment and methods as previously described [21]. Positive (known P. falciparum positive sample) and negative (no template/DNA) control sample were included in each run. All samples were run in duplicates. A threshold cycle number (Ct) of 40 was used as the cut-off in order to consider a sample positive or negative: all samples which did not amplify and those that amplified after a Ct value of 40 were considered negative and all samples that amplified before Ct value of 40 were considered positive.
Statistical analysis
All data was collected using standardized forms and entered into Microsoft Excel (Microsoft Corp, Redmond, Washington, USA). Data analysis was carried out using Stata version 14.2 (StataCorp, College Station, TX, USA). The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were determined for rapid diagnostic test (RDT), routine microscopy, expert microscopy and MG-LAMP, using RT-PCR as the reference standard. Sensitivity was calculated as (number of true positives)/(number of true positives + number of false negatives) × 100, and specificity was calculated as (number of true negatives)/(number of true negatives + number of false positives) × 100. The PPV was calculated as (number of true positives)/(number of true positives + number of false positives) × 100, and the NPV was calculated as (number of true negatives)/(number of true negatives + number of false negatives) × 100. Kappa coefficient was calculated to assess the agreement among the different diagnostic methods. P value below 0.05 was considered statistically significant.
Results
Characteristics of study participants
A total of 416 patients presenting to the two health facilities with suspected malaria were screened for enrollment into the study. Two hundred and sixty-four were enrolled, 197 at Rota Dispensary and 67 at Siaya Hospital and one hundred and fifty-two participants were excluded for various reasons (Fig. 2). The characteristics of the study participants are shown on Table 1. There was no significant difference in gender and mean parasite densities by expert microscopy for participants enrolled at the two health facilities. However, participants enrolled at Rota Dispensary were older, 16.8 years (range 6 months–62 years) compared to those who were enrolled at Siaya Hospital, 7.2 years (range 8 months-–51 years).
Malaria positivity by RDT, routine microscopy, expert microscopy, MG-LAMP and RT-PCR
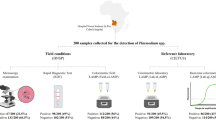
At Rota Dispensary, where RDTs are used for malaria diagnosis, the malaria positivity by RDT, MG-LAMP, RT-PCR and expert microscopy were comparable, 37%, 44%, 42% and 30% respectively. Similarly, at Siaya Hospital where microscopy is used for routine diagnosis, there was no significant difference in positivity rate by routine microscopy, MG-LAMP, RT-PCR and expert microscopy (Fig. 3).
The malaria positivity by RDT, routine microscopy, MG-LAMP, RT-PCR and expert microscopy at Rota Dispensary and Siaya Hospital. Routine microscopy carried out at Siaya Hospital by technicians hired by the hospital, while expert microscopy was carried at KEMRI/CGHR Malaria Laboratories by study staff
Discordant results between RDT, routine microscopy, expert microscopy, MG-LAMP and RT-PCR
Table 2 shows the number of patients identified as positive or negative by RDT, routine microscopy, expert microscopy, MG-LAMP and RT-PCR at the two health facilities. At Rota dispensary, there were a total of 15 participants who were positive by RDT but negative by MG-LAMP (14), RT-PCR (8) and expert microscopy (15). At the same health facility, a total of 29 participants were positive by MG-LAMP (29), RT-PCR (18) and expert microscopy (2) but negative by RDT. At Siaya Hospital, there was agreement between routine and expert microscopy on the number of positive participants but the results of 3 were discordant and scored as positive by routine microscopy but negative by MG-LAMP (3) and RT-PCR (3). At the same health facility, a total of 13 participants were positive by MG-LAMP (13), RT-PCR (5) and expert microscopy (1) but negative by routine microscopy.
The performance characteristics of RDT, routine microscopy, expert microscopy and MG-LAMP using RT-PCR as a reference
The sensitivity, specificity, positive and negative predictive values of the different diagnostic tests are shown in Table 3. At Rota dispensary, the sensitivity of RDT, MG-LAMP and expert microscopy was 78.1% (CI 67.5–86.4), 82.9% (CI 73.0–90.3) and 72.0% (CI 61–81.3) respectively. At the same facility, the specificity for the three diagnostic tests was 93.0% (CI 86.8–97), 83.5% (CI 75.4–89.8) and 100% (CI 96.8–100) respectively. At Siaya County Referral Hospital, the sensitivity of routine microscopy, MG-LAMP and expert microscopy was 83.3% (CI 65.3- 94.4), 93.3% (CI 77.9–99.2) and 86.7% (CI 69.3–96.2) respectively. At this facility, the specificity for the three diagnostic tests was 91.9% (CI 78.1–98.3), 73.0% (CI 55.9–86.2) and 91.9% (CI 78.1–98.3), respectively.
Discussion
The WHO recommends universal diagnosis of all suspected malaria cases using quality-assured microscopy and RDTs [22]. Due to weak or non-existence of QA/QC systems in endemic areas to support this recommendation, it is important to periodically check the performance of routine diagnostic methods and whether the results compare with those of more sensitive methods such as expert microscopy and molecular methods. In this study, diagnostic results obtained by routine RDTs and smear microscopy at two different levels of health care facilities in an area of high and perennial malaria transmission of western Kenya were compared with results obtained by expert microscopy and more sensitive molecular diagnostic methods; a simple calorimetric-based LAMP and RT-PCR.
There was no significant difference in malaria positivity rate by routine RDT and microscopy at the two health facilities and the comparative diagnostic methods-expert microscopy, RT-PCR and MG-LAMP at the two health facilities. The malaria positivity rate by RDT at Rota Dispensary was slightly higher than the other diagnostics tests used in this study. This is similar to what has been reported in previous studies. In an analysis of 85,000 children enrolled in Demographic and Health Surveys and Malaria Indicator Surveys across 15 countries in sub-Saharan Africa, the mean malaria prevalence was 24.5% by microscopy and 30.3% by RDTs [23]. Similar discrepancies have been shown in São Tomé, 37% by microscopy and 53% by RDT [24]. A study in Tanzania 57.9% malaria positivity by RDT and 52% by microscopy [25], and in Cameroon, 31% by microscopy and 45% by RDT [26]. The higher positivity by RDTs compared to microscopy can be attributed to the fact that HRP-2 based RDTs can remain positive remain positive for longer periods due to persistence of HRP-2 antigens in the blood even after treatment or past infection [27]. This is a challenge for health managers who use results based on RDTs only to estimate malaria case burdens and thus the need for robust secondary diagnostic methods or QA/QC systems in settings where RDTs are the main diagnostic methods. Similar to what has been reported in previous studies that have compared the diagnostic performance of RDTs, routine microscopy and molecular tests, the positivity rates by molecular tests was higher [26,27,28,29]. This is not surprising since the threshold of parasite detection for the molecular tests is significantly lower, 1–2 parasites/µL for PCR assays compared to RDTs (100–200 parasites/µL) and microscopy [30].
In this study, 29 and 13 patients at Rota Dispensary and Siaya Hospital, respectively, were negative by the routine diagnostic tests used at these health facilities but they were positive by MG-LAMP, RT-PCR and expert microscopy. This implies that these patients had malaria but were not treated. In the absence of differential diagnosis such as access to blood cultures or PCR to rule out causes of clinical symptoms at many public health facilities in endemic areas, especially in young children, untreated P. falciparum malaria can progress rapidly to severe and life-threatening forms of the disease [31]. This can lead to deaths and undermine both the clinical confidence and credibility of health services if patients who might have malaria but are not treated and the cause of symptoms for the hospital visit is not identified [32]. Additionally, untreated cases can contribute to the transmission of malaria in an area [33]. The study also found 15 positive cases by RDT but negative by MG-LAMP, RT-PCR and expert microscopy at the dispensary and 3 cases which were positive by routine microscopy but negative by MG-LAMP and RT-PCR at Siaya Hospital. According to the WHO, both microscopy and RDTs must be supported by a quality assurance programme [34]. This reduces the chance of misdiagnosis and improves patient management. Previous studies have also reported discrepancy between different diagnostic methods [35,36,37]. Since NAATs diagnostic methods are more sensitive and have a lower limit of parasite detection than RDTs and microscopy, discrepancies are expected in the results obtained by NAATs and other diagnostic methods. Previous studies have reported a limit of detection of < 6 parasites/µL for NAATs compared to 100–200 parasites/µL for RDTs and 50 parasites/µL for microscopy [12, 38]. The sensitivity of MG-LAMP was higher than RDT and microscopy, but a lower specificity at the two health facilities. These results are consistent with previous studies showing the higher sensitivity but lower specificity of MG-LAMP compared to microscopy and RDT [37, 39, 40]. There are many factors which could affect the sensitivity of different diagnostic methods including sample collection method and preparation, efficiency of nucleic acid extraction procedure, amount of blood, amount of template used in the reaction, copy number of target sequence and the buffers, enzymes and other materials used [12]. However, these limitations can be overcome by standardization of the methods and use of quality-assured reagents.
There are several reports highlighting the challenges of malaria diagnostic tests in endemic areas [7,8,9, 41,42,43]. Despite these shortcomings, RDTs and microscopy procedures that are not quality assured continue to be used in many malaria endemic areas. This could result in misdiagnosis leading to inappropriate patient management, irrational use of anti-malarial drugs and generation of inaccurate data on the malaria burden. Therefore, there is a need to strengthen the quality assurance processes for malaria diagnostics using inexpensive and novel strategies such as placing simple, inexpensive and more sensitive molecular diagnostic assays at regional centres to strengthen the national QA/QC programmes.
This study has several limitations. There was a selection bias since only patients who presented to the health facilities with symptoms suggestive of malaria were enrolled. However, since the main objective of the study was to evaluate the performance of routine diagnostic methods at two different levels of heath care system, these results could reflect the performance of the diagnostics tests evaluated at health facilities in malaria endemic areas. Another limitation is comparison of results using diagnostic methods that use different input samples, that is, extraction of DNA from DBS versus whole blood. However, these methods are well standardized and are used widely for malaria diagnosis for different objectives such as clinical management-RDTs and microscopy-research and in elimination settings-NAATs. Another limitation is the small sample size at the Siaya Hospital where only 67 participants were enrolled. Patients attending a referral hospital are typically more sick and likely referred from either a dispensary or health centre. Therefore, the results from the dispensary where a larger number of participants were enrolled compensates for the low numbers enrolled at the referral hospital. A major limitation of the LAMP assay is the inability to quantify parasite density. Since the objective of this study was to evaluate whether LAMP can be used as a reference method for RDTs and microscopy in a QA/QC programme, this might not be a major drawback since it is more sensitive than RDTs and microscopy.
Conclusion
The MG-LAMP evaluated in this study is a simple and sensitive assay for the detection of malaria parasites compared to RDTs and microscopy, which are used for routine malaria diagnosis at health facilities in endemic areas. It could be an ideal and inexpensive reference test in a quality control scheme to monitor the performance of RDTs and smear microscopy in resource-limited settings. This will improve both patient management of suspected malaria cases and the quality of health facility surveillance data.
Availability of data and materials
The data can be obtained from the corresponding author upon request.
Abbreviations
- DNA:
-
Deoxyribonucleic Acid
- EDTA:
-
Ethylene diamine tetra-acetic acid
- HRP-2:
-
Histidine Rich Protein-2
- JICA:
-
Japan International Cooperation Agency
- JKUAT:
-
Jomo Kenyatta University of Agriculture and Technology
- KEMRI/CGHR:
-
Kenya Medical Research Institute/Centre for Global Health Research
- KU-ERC:
-
Kenyatta University Ethical Review Committee
- MG-LAMP:
-
Malachite green-loop-mediated isothermal amplification
- NPV:
-
Negative predictive value
- P/µL:
-
Parasite per microlitre
- PPV:
-
Positive predictive value
- QA:
-
Quality assurance
- QC:
-
Quality control
- RDTs:
-
Rapid diagnostic tests
- RT-PCR:
-
Real-time polymerase chain reaction
- SERU:
-
Scientific and Ethical Review Unit
- WHO:
-
World Health Organization
References
WHO. World malaria report 2018. Geneva, World Health Organization, 2018.
WHO. Global Technical Strategy for Malaria 2016–2030. Geneva, World Health Organization; 2015.
WHO. Malaria. Geneva, World Health Organization, 2017.
Bastiaens GJ, Bousema T, Leslie T. Scale-up of malaria rapid diagnostic tests and artemisinin-based combination therapy: challenges and perspectives in sub-Saharan Africa. PLoS Med. 2014;11:e1001590.
Kachur SP. Beyond “test and treat” – malaria diagnosis for improved pediatric fever management in sub-Saharan Africa by Emily White Johansson. Glob Health Action. 2016;9:34416.
Sori G, Zewdie O, Tadele G, Samuel A. External quality assessment of malaria microscopy diagnosis in selected health facilities in Western Oromia. Ethiopia Malar J. 2018;17:233.
Wanja E, Achilla RA, Obare P, Adeny R, Moseti C, Otieno V, et al. Evaluation of a laboratory quality assurance pilot programme for malaria diagnostics in low-transmission areas of Kenya, 2013. Malar J. 2017;16:221.
Odhiambo F, Buff AM, Moranga C, Moseti CM, Wesongah JO, Lowther SA, et al. Factors associated with malaria microscopy diagnostic performance following a pilot quality-assurance programme in health facilities in malaria low-transmission areas of Kenya, 2014. Malar J. 2017;16:371.
Jemere KA, Melaku MY, Jemeber TH, Abate MA. Performance evaluation of laboratory professionals on malaria microscopy at health facilities in Bahir Dar city administration, Northwest Ethiopia. PLoS One. 2018;13.
Beshir KB, Sepúlveda N, Bharmal J, Robinson A, Mwanguzi J, Busula AO, et al. Plasmodium falciparum parasites with histidine-rich protein 2 (pfhrp2) and pfhrp3 gene deletions in two endemic regions of Kenya. Sci Rep. 2017;7:14718.
WHO. Malaria rapid diagnostic test performance. Results of WHO product testing of malaria RDTs: Round 3 (2010–2011). Geneva, World Health Organization; 2011.
WHO. Global Malaria Programme: Policy brief on malaria diagnostics in low-transmission settings. Geneva: World Health Organization; 2014.
Berzosa P, de Lucio A, Romay‑Barja M, Herrador Z, González V, García. L, et al. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from Equatorial Guinea. Malar J. 2018;17:333.
Mahmud Y-LL, Meng-Yee L, Mun-Yik F, Jenarun J, Rohela. Loop-mediated isothermal amplification assay for identification of five human Plasmodium species in Malaysia. Am J Trop Med Hyg. 2016;94:336–9.
Ministry of Health. National guidelines for the diagnosis, treatment and prevention of malaria in Kenya. Nairobi, 2016.
Kapesa A, Kweka EJ, Zhou G, Atieli HE, Kamugisha E, Mazigo HD, et al. Utility of passive malaria surveillance in hospitals as a surrogate to community infection transmission dynamics in western Kenya. Arch Public Health. 2018;76:39.
Bayoh MN, Walker ED, Kosgei J, Ombok M, Olang GB, Githeko AK, et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasit Vectors. 2014;7:380.
WHO. Basic Malaria Microscopy: Part 1. Learner's Guide. 2nd edition. Geneva, World Health Organization; 2010.
Lucchi NW, Ljolje D, Silva-Flannery L, Udhayakumar V. Use of malachite green-loop mediated isothermal amplification for detection of Plasmodium spp. parasites. PLoS One. 2016; 11:e0151437.
McConnico DB, Alessandra EF, Michael CH, John DB, Caitlin C. Development of new malaria diagnostics: matching performance and need. Malar J. 2016;15:406.
Lucchi NW, Narayanan J, Karell MA, Xayavong M, Kariuki S, DaSilva AJ, et al. Molecular diagnosis of malaria by photo-induced electron transfer fluorogenic primers: PET-PCR. PLoS ONE. 2013;8:e56677.
WHO-FIND-CDC. Methods manual for product testing of malaria rapid diagnostic tests. Geneva, World Health Organization; 2018.
Watson OJ, Sumner KM, Janko M, Goel V, Winskill P, Slater HC, et al. False-negative malaria rapid diagnostic test results and their impact on community-based malaria surveys in sub-Saharan Africa. BMJ Glob Health. 2019;4:e001582.
Shaio P-WL, Dar-Der J, Chia-Tai L, Herodes SR, Virgilio EdR, Lin IF, et al. Application of loop-mediated isothermal amplification for malaria diagnosis during a follow-up study in São Tomé. Malar J. 2012;11:408.
Bwire GM, Ngasala B, Kilonzi M, Mikomangwa WP, Fatuma F. Felician FF, et al. Diagnostic performance of CareStart™ malaria HRP2/pLDH test in comparison with standard microscopy for detection of uncomplicated malaria infection among symptomatic patients, Eastern Coast of Tanzania. Malar J. 2019;18:354.
Mfuh KO, Achonduh-Atijegbe OA, Bekindaka ON, Esemu LF, Mbakop CD, Gandhi K, et al. A comparison of thick-film microscopy, rapid diagnostic test, and polymerase chain reaction for accurate diagnosis of Plasmodium falciparum malaria. Malar J. 2019;18:73.
Waitumbi JN, Gerlach J, Afonina I, Anyona SB, Koros JN, Siangla J, et al. Malaria prevalence defined by microscopy, antigen detection, DNA amplification and total nucleic acid amplification in a malaria-endemic region during the peak malaria transmission season. Trop Med Int Health. 2011;16:786–93.
Masanja IM, McMorrow ML, Maganga MB, Sumari D, Udhayakumar V, McElroy PD, et al. Quality assurance of malaria rapid diagnostic tests used for routine patient care in rural Tanzania: microscopy versus real-time polymerase chain reaction. Malar J. 2015;14:85.
Wanja EK, Moranga N, Hickman C, Johnson M, Moseti J, Anova C, et al. Field evaluation of diagnostic performance of malaria rapid diagnostic tests in western Kenya. Malar J. 2016;15:456.
Tambo M, Mwinga M, Mumbengegwi DR. Loop-mediated isothermal amplification (LAMP) and Polymerase Chain Reaction (PCR) as quality assurance tools for Rapid Diagnostic Test (RDT) malaria diagnosis in Northern Namibia. PLoS ONE. 2018;13:e0206848.
ACTwatch Group, Musuva A, Ejersa W, Kiptui R, Memusi D, Abwao E. The malaria testing and treatment landscape in Kenya: results from a nationally representative survey among the public and private sector in 2016. Malar J. 2017;16:494.
Hailu HA, Shiferaw MB, Demeke L, Derebe MM, Gelaw ZD, Emiru MA, et al. External quality assessment of malaria microscopy diagnosis among public health facilities in West Amhara Region. Ethiopia BMC Res Notes. 2017;10:764.
Beshir KB, Sutherland CJ, Sawa P, Drakeley CJ, Okell L, Mweresa CK, et al. Residual Plasmodium falciparum parasitemia in Kenyan children after artemisinin-combination therapy is associated with increased transmission to mosquitoes and parasite recurrence. J Infect Dis. 2013;208:2017–24.
WHO. Guidelines for the treatment of malaria. 3rd Edn. Geneva, World Health Organization; 2015.
Oriero EC, Okebe J, Jacobs J, Van geertruyden J-P, Nwakanma D, D’Alessandro U. Diagnostic performance of a novel loop-mediated isothermal amplification (LAMP) assay targeting the apicoplast genome for malaria diagnosis in a field setting in sub-Saharan Africa. Malar J. 2015;14:396.
Morris U, Khamis M, Aydin-Schmidt B, Abass AK, Msellem MI, Nassor MH, et al. Field deployment of loop-mediated isothermal amplification for centralized mass-screening of asymptomatic malaria in Zanzibar: A pre-elimination setting. Malar J. 2015;14:205.
Kudyba HM, Louzada J, Ljolje D, Kudyba KA, Muralidharan V, Oliveira-Ferreira J, et al. Field evaluation of malaria malachite green loop-mediated isothermal amplification in health posts in Roraima state. Brazil Malar J. 2019;18:98.
Cheaveau J, Nguyen H, Chow B, Marasinghe D, Mohon AN, Yuan H, et al. Clinical validation of a commercial LAMP test for ruling out malaria in returning travelers: A prospective diagnostic trial. Open Forum Infect Dis. 2018;5:11.
Vincent JP, Komaki-Yasuda K, Iwagami M, Kawai S, Kano S. Combination of PURE-DNA extraction and LAMP-DNA amplification methods for accurate malaria diagnosis on dried blood spots. Malar J. 2018;17:373.
Björkman JC, Berit A-S, Iveth JG, David B, Elin E, Majda HN, et al. Loop-mediated isothermal amplification (LAMP) for point-of-care detection of asymptomatic low-density malaria parasite carriers in Zanzibar. Malar J. 2015;14:43.
Kabaghe AN, Phiri MD, Phiri KS, van Vugt M. Challenges in implementing uncomplicated malaria treatment in children: a health facility survey in rural Malawi. Malar J. 2017;16:419.
Diallo MA, Diongue K, Seck MC, Ndiaye M, Diallo I, Diedhiou Y, et al. Quality control of malaria microscopy reveals misdiagnosed non-falciparum species and other microscopically detectable pathogens in Senegal. Ann Clin Microbiol Antimicrob. 2018;17:8.
Zimmerman PA, Howes RE. Malaria diagnosis for malaria elimination. Curr Opin Infect Dis. 2015;28:446–54.
Acknowledgements
We would like to acknowledge the study participants, the Rota Dispensary and Siaya County Referral Hospital staff, KEMRI-CCHR Malaria Laboratories Staff, Kenya Medical Research Institute and Japan International Corporation Agency, Africa-ai-Japan Project Office, Jomo Kenyatta University of Agriculture and Technology. This paper is published with the permission of the Director General, KEMRI.
Funding
The study was funded by Kenya Medical Research Institute Internal Research Grants (IRG), Nairobi, Kenya. JG received support for his post-graduate studies from Japan International Cooperation Agency (JICA), Africa-ai-Japan Project Office, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya.
Author information
Authors and Affiliations
Contributions
SK, KO, JG and CWN conceived and designed the study. JG, AG, WC, SK and KO participated in training of study staff and carrying out the laboratory assays. JG, WC, SK and KO analysed the data. JG, WC, KO, SK drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol was reviewed and approved by the KEMRI Scientific and Ethics Review Unit (SERU) and the Kenyatta University Ethical Review Committee (KU-ERC). All participants or parents/guardians were informed about the objectives of the study and signed written consent before enrollment into the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gachugia, J., Chebore, W., Otieno, K. et al. Evaluation of the colorimetric malachite green loop-mediated isothermal amplification (MG-LAMP) assay for the detection of malaria species at two different health facilities in a malaria endemic area of western Kenya. Malar J 19, 329 (2020). https://doi.org/10.1186/s12936-020-03397-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-020-03397-0