Abstract
Background
Artemether–lumefantrine (AL) is the recommended first-line artemisinin-based combination therapy (ACT) for the treatment of uncomplicated falciparum malaria in most of the malaria-endemic countries, including Tanzania. Recently, dihydroartemisinin–piperaquine (DP) has been recommended as the alternative anti-malarial to ensure effective case management in Tanzania. This study assessed the parasite clearance rate and efficacy of AL and DP among patients aged 6 months to 10 years with uncomplicated falciparum malaria in two sites with different malaria transmission intensity.
Methods
This was an open-label, randomized trial that was conducted at two sites of Muheza Designated District Hospital and Ujiji Health Centre in Tanga and Kigoma regions, respectively. Patients meeting inclusion criteria were enrolled, treated with either AL or DP and followed up for 28 (extended to 42) and 42 (63) days for AL and DP, respectively. Parasite clearance time was monitored in the first 72 h post treatment and the clearance rate constant and half-life were calculated using an established parasite clearance estimator. The primary outcome was parasitological cure on days 28 and 42 for AL and DP, respectively, while secondary outcome was extended parasitological cure on days 42 and 63 for AL and DP, respectively.
Results
Of the 509 children enrolled (192 at Muheza and 317 at Ujiji), there was no early treatment failure and PCR uncorrected cure rates on day 28 in the AL group were 77.2 and 71.2% at Muheza and Ujiji, respectively. In the DP arm, the PCR uncorrected cure rate on day 42 was 73.6% at Muheza and 72.5% at Ujiji. With extended follow-up (to day 42 for AL and 63 for DP) cure rates were lower at Ujiji compared to Muheza (AL: 60.2 and 46.1%, p = 0.063; DP: 57.6 and 40.3% in Muheza and Ujiji, respectively, p = 0.021). The PCR corrected cure rate ranged from 94.6 to 100% for all the treatment groups at both sites. Parasite clearance rate constant was similar in the two groups and at both sites (< 0.28/h); the slope half-life was < 3.0 h and all but only one patient cleared parasites by 72 h.
Conclusion
These findings confirm high efficacy of the first- and the newly recommended alternative ACT for treatments for uncomplicated falciparum malaria in Tanzania. The high parasite clearance rate suggests absence of suspected artemisinin resistance, defined as delayed parasite clearance.
Trial registration This trial is registered at ClinicalTrials.gov under registration number NCT02590627
Similar content being viewed by others
Background
Effective case management based on early diagnosis and prompt treatment with effective drugs has been one of the core malaria interventions [1]. Despite reports of declining burden of malaria attributed to intensified scale-up of interventions, the disease is still a leading cause of morbidity and mortality with estimated 216 million cases and 446,000 deaths in 2016, mostly among under-fives and pregnant women from sub-Saharan Africa [1]. Recent studies have shown that artemisinin-based combination therapy (ACT) is still efficacious and safe for the treatment of uncomplicated falciparum malaria especially in Africa [1, 2], but emergence of resistant Plasmodium falciparum has been reported in Southeast Asian (SEA) countries [3,4,5] and might reverse the recent gains in malaria control, and jeopardize malaria elimination efforts. The World Health Organization (WHO) recommends that malaria-endemic countries should monitor the efficacy of nationally recommended ACT in order to guide national treatment guidelines [2].
Artemisinin-based combinations recommended by WHO includes artemether–lumefantrine (AL), artesunate–amodiaquine (AS + AQ), artesunate–mefloquine (AS + MQ), artesunate–sulphadoxine/pyrimethamine (AS + SP), and dihydroartemisinin–piperaquine (DP) [6]. Of these, AL is the most widely used combination and it is currently the first-line anti-malarial drug in most malaria-endemic countries in the WHO African region [7]. Due to high level of resistance to SP, Tanzania adopted AL in 2006 and the implementation of the new policy started in January 2007 [8]. DP was recommended in 2014 as an alternative drug for the treatment of uncomplicated falciparum malaria in Tanzania in order to improve case management [9]. Baseline data on the efficacy of DP is urgently needed to support future surveillance of its performance once it has been widely used in the country.
Following the WHO recommendation to monitor the efficacy of ACT every 2 years, Tanzania has been conducting series of studies under the national malaria control programme (NMCP) framework to test for efficacy and safety of ACT, particularly after policy changes in 2006 [10]. Studies conducted in Tanzania [10, 11] and elsewhere have showed that AL is still efficacious (91–100%) and highly tolerated when used for the treatment of uncomplicated falciparum malaria [12,13,14,15,16,17,18]. For DP, studies conducted in Uganda [19], Kenya [16] and Mozambique [12] have reported high cure rates and safety profile of the drug with longer post-treatment prophylactic effect due to longer half-life of piperaquine compared to lumefantrine. However, DP was recently introduced in Tanzania as an alternative anti-malarial for treatment of uncomplicated falciparum malaria when indicated and thus, its efficacy and safety need to also be assessed regularly as recommended by WHO. Intensified surveillance of anti-malarial efficacy and safety of AL and DP is urgently needed to monitor their performance.
In addition to proportion of treatment failure, therapeutic efficacy studies also generate information on parasite clearance in terms of proportion of day 3 parasite positivity or [20] half-life of the parasite clearance slope, estimated through multiple daily parasitaemia in the first 72 h post-treatment (e.g., at 6, 8 or 12 hourly interval) [21, 22]. Most of the studies conducted in Tanzania [10] and elsewhere in Africa [13, 23] have showed that day 3 positivity rate is very low (< 2.0%) which is below the WHO cut-off point of 10%, above which artemisinin resistance is suspected [20]. The few studies incorporating regular parasite sampling conducted in Mali [24], Kenya [16] and Uganda [23] have not reported any signs of delayed parasite clearance, but further surveillance is required to longitudinally monitor and report any occurrence of suspected tolerance/resistance to artemisinins, particularly with continued use of these drugs in Africa. The present study was conducted at two NMCP sentinel sites of Muheza and Kigoma districts in Tanga and Kigoma regions, respectively, and incorporated frequent parasite sampling to assess parasite clearance time and in vivo efficacy of AL and DP for the treatment of uncomplicated falciparum malaria. The data generated provide critical information on efficacy, including parasite clearance time of both DP and AL in Tanzania and will inform the national treatment policy.
Methods
Study area
The study was conducted between May 2014 and January 2015, at Muheza Designated District Hospital in Muheza, Tanga region, and Ujiji Health Centre (Kigoma region) in Northeastern and Western Tanzania, respectively. These sites are among the eight NMCP sentinel sites for monitoring the efficacy of anti-malarial drugs in Tanzania, and were classified as holoendemic (Muheza) and mesoendemic (Ujiji) for malaria in 1990s [25, 26]. However, malaria transmission has changed in recent years with Muheza becoming a low transmission while Ujiji has persistently remained a moderate transmission area [27,28,29]. A detailed description of the study sites has been provided elsewhere [30].
Study design and target population
This was an open-label, randomized trial that assessed the efficacy of AL and DP for the treatment of uncomplicated falciparum malaria. Patients aged 6 months to 10 years were recruited at the outpatient departments (OPDs) and assessed for inclusion in the study based on the WHO protocol of 2009 [31].
Sample size estimation
The sample size was calculated to test the hypothesis that the risk of treatment failure (adjusted by PCR genotyping to distinguish recrudescent from new infections) after day 28, 42 or 63 would not differ between the AL and DP treated children. Based on previous data from areas of high malaria transmission intensity which showed that the efficacy (adjusted by PCR genotyping) after 42 days was estimated to be 98% after treatment with DP and 92% for patients treated with AL [13], 125 patients were targeted in each treatment arm at each site. The estimated sample size was adjusted to allow for 20% loss to follow-up and withdrawals giving a total sample size of 150 patients per treatment arm per site.
Screening and randomization of study participants
Children aged 6 months to 10 years from OPDs of Muheza hospital and Ujiji Health Centre were screened initially with rapid diagnostic tests for malaria (RDTs) and confirmed by microscopy as previously described [30]. Patients meeting inclusion criteria as per WHO protocol of 2009 [31] with minor modifications (to assess cardiotoxicity effects associated with DP [32]) were recruited and randomized to receive therapeutic doses of either AL or DP. The inclusion criteria among others were a microscopically confirmed mono-infection of P. falciparum, and parasitaemia between 250 and 200,000 asexual parasites/µl of blood, axillary temperature ≥ 37.5 °C or a history of fever within the past 24 h and ability to swallow oral medications. Others included the ability and willingness to attend scheduled follow-up visits, providing an informed consent by parent or guardian and stable residence within the catchment areas of the study health facilities throughout the study period.
Exclusion criteria were severe malnutrition, febrile conditions due to diseases other than malaria, presence of danger signs due to severe falciparum malaria, severe anaemia (Hb < 5 g/dl), mixed or mono-infections with other Plasmodium species, severe diarrhoea with dehydration and use of regular medications which could interfere with anti-malarial pharmacokinetics. Patients with a history of hypersensitivity reactions or contra-indications to ACT were also excluded. Assessment was also done to exclude patients taking medicinal products known to prolong QTc interval and treatment with DP in the previous 4 weeks, but not with AL. Patients meeting any of the exclusion criteria were excluded from the study but were treated according to the national guidelines [8].
A randomization list was computer generated for different age-strata (< 2 years; 2 to < 5 years and ≥ 5 to 10 years) using Microsoft Excel. Sequentially numbered, sealed envelopes containing the treatment group assignments were prepared from the randomization list for each age category. These numbers were used to assign patients to the treatment arms based on their age groups, with a target of getting equal numbers of children aged < 2 years, 2 to < 5 years and ≥ 5 to 10 years.
Treatment and follow-up
All eligible patients were randomized to receive either AL, a fixed combination of 20 mg artemether and 120 mg lumefantrine in a tablet (Coartem®, IPCa Laboratories Ltd, Kandivil, Mumbai, India) or DP, a fixed combination of 20 mg dihydroartemisinin and 160 mg piperaquine (Duo-cotexin®, Holley Pharm, PR China). The drugs were administered orally under supervision of the study nurse based on patients’ weight. For AL: 1 tablet was given to children weighing 5–14 kg, 2 tablets to those weighing 15–24 kg and 3 tablets to children weighing 25–34 kg. A full course of AL consisted of 6 doses given twice daily (8 hourly apart on day 0 and 12 hourly apart on days 1 and 2). DP was given according to body weight; half a tablet was administered to children weighing 5 to < 7 kg, 1 tablet to those with 7 to < 13 kg, 2 tablets to those weighing 13 to < 24 kg, and 3 tablets for those with 24 to < 36 kg. A full course of DP consisted of three equally divided doses given once daily at an interval of 24 h apart. Patients were observed for 30 min post-treatment; if vomiting occurred within 30 min, a second full dose was repeated. Persistent vomiting of the second dose led to withdrawal from the study and, administration of rescue medicine, with parenteral quinine or injectable artesunate according to the national guidelines for management of complicated and severe malaria [8]. Paracetamol was also given to all patients with body temperature ≥37.5 °C.
Patients were admitted at the health facility for the first 3 days to ensure strict follow-up and adherence to dosing intervals. It also allowed 8 hourly blood slide collection for assessment of parasite clearance until two successive blood smears turned out to be negative. Even after complete clearance of the parasites, patients were retained at the clinic to complete the treatment and collection of the day 3 blood smears before they could be discharged. Patients were seen by study clinicians on days 0, 1, 2, and 3 and then discharged home to attend the weekly follow-up visits from day 7 to day 42 for AL, and day 63 for DP. Parents/care-takers were advised and encouraged to bring back their children to the study clinic at any other time (unscheduled visits) if they felt unwell. During these visits, study clinicians collected medical history, vital signs, malaria blood smears, and adverse events using standard questionnaire guides. Patients who could not attend the scheduled visits by mid-day were traced at their home by a member of the study team and brought to the facility. Those who travelled to other areas and could not be traced for their scheduled follow-up were classified as loss to follow-up and withdrawn from the study.
Sample collection and processing
On the enrolment day, finger-prick blood was initially collected after parents’/guardians’ consent to confirm parasitaemia by RDT and microscopy as earlier described [30]. For patients who were enrolled in the study: venous blood samples (5–7 ml) were collected on the day of enrolment for malaria parasite identification, genomic studies of parasites and human, measurement of haemoglobin levels and preparation of blood spots on filter papers (Whatman No. 3, GE Healthcare Bio-Sciences, PA, USA). During follow-up, blood smears and filter-paper blood spots were prepared from a finger prick. All filter-paper blood samples were air-dried and stored in zip lock envelopes with desiccators for further analysis. Further processing of parasites and genomic analysis targeting parasites and human genes are underway and the findings will be reported elsewhere.
Microscopic diagnosis of malaria parasites
For patients with positive RDT results, two blood smears (thick and thin) were prepared through finger prick at screening and 1 slide was stained with 10% Giemsa for 10–15 min for initial assessment of eligibility to participate in the study, while the second smear was retained. For enrolled patients, the second slide was stained carefully (with 2.5–3% Giemsa stain for 45–60 min) for accurate counting of malaria parasites and detection of parasite species and gametocytes. Similarly, slower staining was used for all slides that were collected at 8-h intervals for assessment of parasite clearance in the first 72 h and during weekly follow-up visits. The thick blood smear for initial screening was used to count the numbers of asexual parasites and white blood cells in a limited number of microscopic fields to determine if the patient was eligible for enrolment. Each of the second blood smear and smears collected during follow-up were examined by two independent microscopists and parasites were counted as asexual parasites per 200 white blood cells (WBCs) while sexual parasites were accounted per 500 WBCs. Parasite density was calculated by multiplying the number of asexual parasites by 40 and 16 for asexual and sexual parasites, respectively; assuming that 1 µl of blood contained 8000 WBCs [33]. A blood slide was declared negative when examination of 100 high power fields did not reveal the presence of any malaria parasite. Blood smears with discordant results (differences between the two microscopists in species diagnosis, presence of parasites or parasite density of > 50%) were re-examined by a third, independent microscopist, and parasite density was calculated by averaging the two closest counts.
Genotyping of malaria parasites
In order to distinguish recrudescence from newly acquired infections, venous blood (3–7 ml) was collected from all study patients on day 0 (before administration of study drugs) and filter paper (Whatman No. 3) blood samples were collected on day 7 and onwards. Parasite DNA was extracted from venous blood or dried blood spots (DBS) using QIAamp DNA blood midi kits (QiAgen GmbH, Hilden, Germany) according to the manufacturer’s instructions. Paired DNA samples (day 0 and day of parasites recurrence) were genotyped by analysing the polymorphic loci of merozoite surface proteins 1 and 2 (msp1 and msp2), and glutamate rich protein (glurp) genes according to the standard protocol [31, 34].
Haematological assessment
A portion of venous blood samples collected on day 0 was used to measure haemoglobin (Hb) levels (g/dl) using a Haemocue® machine (HemoCue, Ångelholm, Sweden). The Hb was also measured using finger prick blood during weekly follow-up visits from day 14 to 42 for AL or day 14 to 63 for DP.
Outcome classification
The primary endpoint was adequate clinical and parasitological response (ACPR) on day 28 for AL, and day 42 for DP as per WHO protocol [31]. Secondary endpoints included extended parasitological cure on day 42 and day 63 for AL and DP, respectively, parasite clearance by 72 h, improvement in haemoglobin levels comparing baseline and follow-up visits and reduction in gametocyte carriage during follow-up compared to day 0 baseline.
Treatment outcomes were classified as per WHO protocol of 2009 either as early treatment failure (ETF), late clinical failure (LCF), late parasitological failure (LPF), or adequate clinical and parasitological response (ACPR) [31].
Ethical considerations
The study protocol was reviewed and approved by the Tanzanian Medical Research coordinating Committee (MRCC) of the National Institute for Medical Research (NIMR). Permission to conduct the study in Muheza–Tanga and Ujiji–Kigoma was sought in writing and obtained from the district and regional medical officers. Written informed consent was obtained from children’s parents or guardians before screening. Appropriate information (about the study and the protocol/methods) in a language that was understood by the parents/guardians of the study patients was compiled and provided before consent was obtained.
Data management and analysis
The data was double-entered into a Microsoft Access database followed by validation, cleaning and analysis using STATA version 11 (STATA Corporation, TX-USA). Descriptive statistics (mean, standard deviation and proportions) was used to describe the study population and present treatment outcomes. The data were also transferred to the WHO Excel software programme [35], for automatic analysis of treatment outcomes. Differences in proportions of treatment outcomes within and between the two study sites were compared using Chi squared test. Student’s t test (for continuous variables) or Mann–Whitney U test (a non-parametric test for non-normally distributed data) were applied for analysis of continuous variables such as parasite density, age and Hb levels. Analysis was performed based on per protocol method and Kaplan–Meier survival analysis. Analysis of parasite clearance was performed using the parasite clearance estimator as previously described [21, 22]. The minimum detectable parasitaemia was set at 16 asexual parasite/µl of blood. During the analysis of parasite clearance (0–72 h), samples with too few data points and/or too low parasitaemia were excluded. Different estimates were generated including clearance rate constant (estimated clearance rate constant/h), slope half-life (estimated time in hours for parasitaemia to decline by half), and P50 and P95 (estimated time in hours for the parasitaemia to decline by 50 and 95% of the initial values, respectively). p values < 0.05 were considered statistically significant.
Results
Trial profile and baseline characteristics
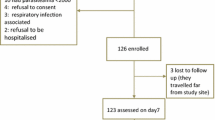
Overall, 1090 patients were screened, 671 patients (61.5%) were positive for P. falciparum and 509 were recruited at both sites of Muheza (n = 192) and Ujiji (n = 317). Of these, 257 (50.5%) were randomized to receive AL and 252 (49.5%) received DP. Twenty-two patients (4.3%) were excluded from the study due to various reasons while 18 (3.5%) were lost to follow-up (Fig. 1). Within each site, most of the baseline characteristics were similar in the 2 groups (Table 1). However, children recruited at Ujiji had significantly higher mean age (p < 0.001), haemoglobin concentration (p < 0.001) and parasitaemia (p < 0.001). The proportion of male patients recruited at Muheza was higher compared to Ujiji (p = 0.029) (Table 1).
Treatment outcome
There was no ETF, however cases with recurrent infections increased at the two sites and within the treatment groups as the follow up was extended (Table 2). Among patients treated with AL, uncorrected ACPR on day 28 was slightly higher at Muheza compared to Ujiji (77.2 and 71.2%, respectively). However, when the follow up was extended to 42 days, uncorrected ACPR was lower at Ujiji compared to Muheza (46.1 and 60.2%, respectively), although the difference was not statistically significant (p = 0.063). Similarly, the uncorrected ACPR among patients treated with DP was not significantly different at the two sites (73.6% at Muheza and 72.5% at Ujiji) during the 42 days of follow-up, but it was significantly lower at Ujiji compared to Muheza when the follow-up was extended to 63 days (40.3 and 57.6%, respectively; p = 0.021) (Table 2). However, when the treatment outcomes were adjusted by PCR, the cure rate of the two drugs was similar, and ranged from 95.1 to 100% at different follow-up points in both sites (Table 2).
Parasite clearance
A total of 35 individuals were excluded in the analysis of parasite clearance leaving 474 patients. The rates of parasite clearance based on different estimates were similar for both drugs and at both sites (Table 3). However, the slope half-life was slightly higher at Muheza compared to Ujiji in both treatment groups and all patients had cleared parasites by day 3 except one patient in the AL arm (1.1%) at Muheza (Table 4).
Gametocyte clearance
Gametocyte carriage by microscopy was higher at Muheza where individuals with gametocytes increased between days 1 and 2 and then declined on day 3, with complete clearance by day 7. Low gametocyte carriage was observed at Ujiji where only one patient treated with AL had gametocytes on days 1 and 2 and total clearance was attained by day 3 (Table 4).
Haemoglobin recovery
At both sites, a highly significant increase in mean Hb (g/dl) was observed between day 0 and day 28 in the AL arm (p < 0.001) (Fig. 2). With extended follow-up, the mean Hb increased further at Muheza but significant changes were reported at Ujiji (p < 0.001). For the patients treated with DP (at both sites), the mean Hb increased between day 0 and day 28 while no significant changes were seen at Muheza and a slight decrease was reported at Ujiji during extended follow-up to day 63 (Fig. 2).
Discussion
The findings of this study showed that both AL and DP are highly efficacious for the treatment of uncomplicated falciparum malaria in the study areas; with PCR corrected ACPR of 97.4–100% on day 28 for AL and 97.0–99.1% on day 42 for DP. The observed high efficacy of AL was similar to that of other studies from different parts of Tanzania [10, 11] and elsewhere in Africa [12,13,14,15,16,17,18], where similar PCR corrected cure rates were reported supporting the maintained high efficacy of AL despite its use in Africa for more than a decade. However, studies done in Angola in 2013 and 2015 reported low efficacy of AL (PCR corrected cure rate of 88%) at one of the sites [36, 37] and that is lower compared to cure rates reported in the majority of studies that were conducted in the sub-Saharan-African region [12,13,14,15,16,17,18]. However, patients enrolled in both Angolan studies took the evening doses of AL at home unsupervised, and this might explain the high treatment failure rate.
This study also showed high PCR corrected ACPR of DP, which is consistent with the findings from recent studies conducted in Tanzania (Kakolwa et al. manuscript in preparation) and other African countries [16, 18, 19, 36, 38, 39]. DP has been deployed as an alternative ACT for the treatment of uncomplicated falciparum malaria in mainland Tanzania since 2014. The finding of PCR corrected cure rate > 97% with DP treatment (during the 42 days of follow-up) supports the decision made by NMCP and is also in line with WHO recommendation that an ACT should have cure rate of > 95% before including it in the treatment policy [6]. The rapid development and spread of piperaquine resistance in SEA, leading to high treatment failure with DP [4, 40], underscores the need to closely monitor the efficacy of DP and molecular marker associated with piperaquine resistance.
There were high and similar parasite recurrences in both sites, which were due to re-infection as confirmed by PCR analysis. However, re-infection rate during extended follow-up was higher at Ujiji site for both drugs, possibly related to relatively higher malaria transmission in this site compared to Muheza [27,28,29]. From operational perspective, WHO recommends to consider parasite recurrence before 28 days of treatment with ACT as recrudescence (true treatment failure) and they should be treated with the second line anti-malarial; those which occur after 28 days as new infections and should be treated with the same drug [6]. However, efforts should be directed at intensifying malaria control so that the risk of malaria infection in the community can be further reduced or eliminated.
According to WHO [20], delayed parasite clearance (slope half-life > 5 h or day 3 positivity rate > 10%) indicate suspected artemisinin resistance. This study showed that both drugs had fast parasite clearance in terms of parasite clearance rate constant and slope half-life as well as day 3 positivity rates, indicating absence of suspected artemisinin resistance. Similar findings have also been reported from other African countries [16, 23, 24] suggesting that artemisinin resistance has not emerged in Africa. In addition, molecular studies in Africa have showed absence of the known mutations in the kelcher 13 (k-13) gene associated with artemisinin resistance in SEA [41,42,43], further suggesting that artemisinins are still effective and their parasite clearance capacity has not been altered. However, due to the spread of artemisinin resistance in SEA [3,4,5, 20], African countries have to be vigilant about its potential emergence through continuous monitoring of the efficacy and parasite clearance of ACT, and surveillance of polymorphism in the k-13 gene.
It was reported that only one patient from Ujiji had gametocytes up to day 2, possibly suggesting that patients were captured at early stage of the infection before developing falciparum gametocytes. On the other hand, microscopy could have failed to detect sub-microscopic gametocytes as previously shown [44]. Similar findings of gametocytes carriage among patients enrolled in clinical trials were reported in previous studies from Africa and elsewhere [45, 46]. However, the findings that only one patient from Ujiji had gametocytes in the first 3 days of the study was not expected and suggest that further assessment using more sensitive methods such as PCR may be needed, to confirm if most of febrile patients from this site do not carry gametocytes, suggesting that the transmission could possibly be driven and maintained by asymptomatic patients. A study conducted in Bagamoyo showed that asymptomatic individuals were more likely to carry gametocytes compared to symptomatic patients reporting to health facilities [44]. As also shown by other studies [47, 48], carriage of gametocytes by asymptomatic individuals could be critical in maintaining transmission in both malaria hyper and hypo-endemic areas suggesting that they should be targeted with transmission reducing interventions.
In this study, a progressive haematological improvement was observed from baseline to day 28 between the two sites and within the two treatment groups. However, patients from Ujiji had relatively higher Hb at enrolment which was maintained throughout the follow-up. Higher mean Hb at Ujiji than Muheza might be attributed to differences in nutritional status and other conditions associated with anaemia such as concurrent infections and helminth infestations [49,50,51,52,53]. It could also be due to differences in age, since patients enrolled at Ujiji had significantly higher mean age compared to Muheza (3.9 vs 3.2 years for Ujiji and Muheza, respectively). Improvements in Hb during follow-up could suggest that malaria might be a major contributing factor to the low haemoglobin levels and anaemia at recruitment as reported in other studies done in Tanzania [10] and elsewhere in sub-Saharan Africa [14, 15].
Conclusion
The study showed that AL still remains highly efficacious for the treatment of uncomplicated falciparum malaria at the two sites and possibly in mainland Tanzania after its use for a decade. The study also revealed high efficacy of DP supporting the recommendations by NMCP to deploy it as an alternative ACT for improved case management in the country.
Abbreviations
- ACPR:
-
adequate clinical and parasitological response
- ACT:
-
artemisinin-based combination therapy
- AL:
-
artemether–lumefantrine
- AS + AQ:
-
artesunate + amodiaquine
- AS + MQ:
-
artesunate + mefloquine
- AS + SP:
-
artesunate + sulfadoxine/pyrimethamine
- DBS:
-
dried blood spots on filter papers
- DNA:
-
deoxyribonucleic acid
- DP:
-
dihydroartemisinin–piperaquine
- ETF:
-
early treatment failure
- glurp :
-
glutamate-rich protein gene
- Hb:
-
haemoglobin
- LCF:
-
late clinical failure
- LPF:
-
late parasitological failure
- MRCC:
-
Medical Research Coordinating Committee
- RDTs:
-
malaria rapid diagnostic tests
- msp1 :
-
merozoite surface protein-1 gene
- msp2 :
-
merozoite surface protein-2 gene
- NIMR:
-
National Institute for Medical Research
- NMCP:
-
National Malaria Control Programme
- OPD:
-
outpatient department
- PCR:
-
polymerase chain reaction
- PCT:
-
parasite clearance time
- SEA:
-
Southeast Asia
- WBCs:
-
white blood cells
- WHO:
-
World Health Organization
References
WHO. World malaria report. Geneva: World Health Organization; 2017.
WHO. Global report on antimalarial drug efficacy and drug resistance: 2000–2010. Geneva: World Health Organization; 2010.
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–23.
Leang R, Taylor WR, Bouth DM, Song L, Tarning J, Char MC, et al. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in Western Cambodia: dihydroartemisinin–piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59:4719–26.
Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, Hlaing T, et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis. 2015;15:415–21.
WHO. Guidelines for treatment of malaria. 3rd ed. Geneva: World Health Organization; 2015.
WHO. World malaria report 2011. Geneva: World Health Organization; 2011.
Ministry of Health. National guidelines for malaria diagnosis and treatment. Edited by A. Mwita and F. Molten. Dar es Salam, United Republic of Tanzania; 2006.
Ministry of Health. National Guidelines for diagnosis and treatment of malaria. Dar es Salam: Ministry of Health and Social Welfare, United Republic of Tanzania; 2014.
Shayo A, Buza J, Ishengoma DS. Monitoring of efficacy and safety of artemisinin-based anti-malarials for treatment of uncomplicated malaria: a review of evidence of implementation of anti-malarial therapeutic efficacy trials in Tanzania. Malar J. 2015;14:135.
Mwaiswelo R, Ngasala BE, Jovel I, Gosling R, Premji Z, Poirot E, et al. Safety of a single low-dose of primaquine in addition to standard artemether–lumefantrine regimen for treatment of acute uncomplicated Plasmodium falciparum malaria in Tanzania. Malar J. 2016;15:316.
Bassat Q, Mulenga M, Tinto H, Piola P, Borrmann S, Menendez C, et al. Dihydroartemisinin–piperaquine and artemether–lumefantrine for treating uncomplicated malaria in African children: a randomised, non-inferiority trial. PLoS ONE. 2009;4:e7871.
Kamya MR, Yeka A, Bukirwa H, Lugemwa M, Rwakimari JB, Staedke SG, et al. Artemether–lumefantrine versus dihydroartemisinin–piperaquine for treatment of malaria: a randomized trial. PLoS Clin Trials. 2007;2:e20.
Ndiaye JL, Faye B, Gueye A, Tine R, Ndiaye D, Tchania C, et al. Repeated treatment of recurrent uncomplicated Plasmodium falciparum malaria in Senegal with fixed-dose artesunate plus amodiaquine versus fixed-dose artemether plus lumefantrine: a randomized, open-label trial. Malar J. 2011;10:237.
Ogouyemi-Hounto A, Azandossessi C, Lawani S, Damien G, de Tove YS, Remoue F, et al. Therapeutic efficacy of artemether–lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Benin. Malar J. 2016;15:37.
Ogutu BR, Onyango KO, Koskei N, Omondi EK, Ongecha JM, Otieno GA, et al. Efficacy and safety of artemether–lumefantrine and dihydroartemisinin–piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children aged less than five years: results of an open-label, randomized, single-centre study. Malar J. 2014;13:33.
Sow D, Ndiaye JL, Sylla K, Ba MS, Tine RC, Faye B, et al. Evaluation of the efficacy and safety of three 2-drug combinations for the treatment of uncomplicated Plasmodium falciparum malaria in Senegal: artesunate–amodiaquine, dihydroartemisinin–piperaquine, and artemether–lumefantrine. Med Sante Trop. 2016;26:45–50 (in French).
Ursing J, Rombo L, Rodrigues A, Kofoed PE. Artemether–lumefantrine versus dihydroartemisinin–piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in children aged less than 15 years in Guinea-Bissau—an open-label non-inferiority randomised clinical trial. PLoS ONE. 2016;11:e0161495.
Zwang J, Ashley EA, Karema C, D’Alessandro U, Smithuis F, Dorsey G, et al. Safety and efficacy of dihydroartemisinin–piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS ONE. 2009;4:e6358.
WHO. Artemisinin and artemisinin-based combination resistance. Geneva: World Health Organization; 2017.
Flegg JA, Guerin PJ, White NJ, Stepniewska K. Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J. 2011;10:339.
Stepniewska K, Ashley E, Lee SJ, Anstey N, Barnes KI, Binh TQ, et al. In vivo parasitological measures of artemisinin susceptibility. J Infect Dis. 2010;201:570–9.
Muhindo MK, Kakuru A, Jagannathan P, Talisuna A, Osilo E, Orukan F, et al. Early parasite clearance following artemisinin-based combination therapy among Ugandan children with uncomplicated Plasmodium falciparum malaria. Malar J. 2014;13:32.
Maiga AW, Fofana B, Sagara I, Dembele D, Dara A, Traore OB, et al. No evidence of delayed parasite clearance after oral artesunate treatment of uncomplicated falciparum malaria in Mali. Am J Trop Med Hyg. 2012;87:23–8.
East African Network for Monitoring Antimalarial Treatment (EANMAT). Monitoring antimalarial drug resistance within National Malaria Control Programmes: the EANMAT experience. Trop Med Int Health. 2001;6:891–8.
East African Network for Monitoring Antimalarial Treatment (EANMAT). The efficacy of antimalarial monotherapies, sulphadoxine-pyrimethamine and amodiaquine in East Africa: implications for sub-regional policy. Trop Med Int Health. 2003;8:860–7.
Ministry of Health, Community Development, Gender, Elderly and Children, MoH/Zanzibar, National Bureau of Statistics, OCGS/Zanzibar, and ICF. Tanzania demographic and health survey and malaria indicator survey (TDHS-MIS), 2015–16. Dar es Salaam, Tanzania; 2016.
TACAIDS, ZAC, NBS, OCGS, and Macro International Inc. Tanzania HIV/AIDS and malaria indicator survey 2007/2008. Dar es Salaam, United Republic of Tanzania; 2008.
TACAIDS, ZAC, NBS, OCGS, and ICF International. Tanzania HIV/AIDS and malaria indicator survey 2011–2012. Dar es Salaam, United Republic of Tanzania; 2014.
Ishengoma DS, Shayo A, Mandara CI, Baraka V, Madebe RA, Ngatunga D, et al. The role of malaria rapid diagnostic tests in screening of patients to be enrolled in clinical trials in low malaria transmission settings. Health Syst Policy Res. 2016;3:1–10.
WHO. Methods for surveillance of antimalarial drug efficacy, 2009. Geneva: World Health Organization; 2015.
WHO. Evidence review group on the cardiotoxicity of antimalarial medicines. Geneva: World Health Organization; 2017.
WHO. Basic malaria microscopy: part I. Learner’s guide. 2nd ed. Geneva: World Health Organization; 2010.
WHO. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite population. Edited by Informal consultation organized by the Medicines for Malaria Venture and cosponsored by the World Health Organization. Amsterdam: Medicine for Malaria Venture and The World Health Organization; 2007.
WHO. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva: World Health Organization; 2003. http://www.who.int/malaria/publications/atoz/whohtmrbm200350/en/. Accessed 8 Aug 2017.
Plucinski MM, Dimbu PR, Macaia AP, Ferreira CM, Samutondo C, Quivinja J, et al. Efficacy of artemether–lumefantrine, artesunate–amodiaquine, and dihydroartemisinin–piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in Angola, 2015. Malar J. 2017;6:62.
Plucinski MM, Talundzic E, Morton L, Dimbu PR, Macaia AP, Fortes F, et al. Efficacy of artemether–lumefantrine and dihydroartemisinin–piperaquine for treatment of uncomplicated malaria in children in Zaire and Uige Provinces, angola. Antimicrob Agents Chemother. 2015;59:437–43.
Zani B, Gathu M, Donegan S, Olliaro PL, Sinclair D. Dihydroartemisinin–piperaquine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database Syst Rev. 2014;1:CD010927.
Zongo I, Dorsey G, Rouamba N, Dokomajilar C, Sere Y, Rosenthal PJ, et al. Randomized comparison of amodiaquine plus sulfadoxine–pyrimethamine, artemether–lumefantrine, and dihydroartemisinin–piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clin Infect Dis. 2007;45:1453–61.
Amaratunga C, Lim P, Suon S, Sreng S, Mao S, Sopha C, et al. Dihydroartemisinin–piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis. 2016;6:357–65.
Kamau E, Campino S, Amenga-Etego L, Drury E, Ishengoma D, Johnson K, et al. K13-propeller polymorphisms in Plasmodium falciparum parasites from sub-Saharan Africa. J Infect Dis. 2015;211:1352–5.
Menard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374:2453–64.
Taylor SM, Parobek CM, DeConti DK, Kayentao K, Coulibaly SO, Greenwood BM, et al. Absence of putative artemisinin resistance mutations among Plasmodium falciparum in Sub-Saharan Africa: a molecular epidemiologic study. J Infect Dis. 2015;211:680–8.
Sumari D, Mwingira F, Selemani M, Mugasa J, Mugittu K, Gwakisa P. Malaria prevalence in asymptomatic and symptomatic children in Kiwangwa, Bagamoyo district, Tanzania. Malar J. 2017;16:222.
WWARN Gametocyte Study Group. Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systematic review and meta-analysis of individual patient data. BMC Med. 2016;14:79.
Stepniewska K, Price RN, Sutherland CJ, Drakeley CJ, von Seidlein L, Nosten F, et al. Plasmodium falciparum gametocyte dynamics in areas of different malaria endemicity. Malar J. 2008;7:249.
Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J. 2010;9:136.
Goncalves BP, Kapulu MC, Sawa P, Guelbeogo WM, Tiono AB, Grignard L, et al. Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun. 2017;8:1133.
Ngui R, Lim YA, Chong KL, Sek CC, Jaffar S. Association between anaemia, iron deficiency anaemia, neglected parasitic infections and socioeconomic factors in rural children of West Malaysia. PLoS Negl Trop Dis. 2012;6:e1550.
Koukounari A, Estambale BB, Njagi JK, Cundill B, Ajanga A, Crudder C, et al. Relationships between anaemia and parasitic infections in Kenyan schoolchildren: a Bayesian hierarchical modelling approach. Int J Parasitol. 2008;38:1663–71.
Magalhaes RJ, Clements AC. Mapping the risk of anaemia in preschool-age children: the contribution of malnutrition, malaria, and helminth infections in West Africa. PLoS Med. 2011;8:e1000438.
Sousa-Figueiredo JC, Gamboa D, Pedro JM, Fancony C, Langa AJ, Magalhaes RJ, et al. Epidemiology of malaria, schistosomiasis, geohelminths, anemia and malnutrition in the context of a demographic surveillance system in northern Angola. PLoS ONE. 2012;7:e33189.
Said K, Hella J, Knopp S, Nassoro T, Shija N, Aziz F, et al. Schistosoma, other helminth infections, and associated risk factors in preschool-aged children in urban Tanzania. PLoS Negl Trop Dis. 2017;11:e0006017.
Authors’ contributions
CIM, DSI, RAK, MML, RN, SM, RM, EN, PM designed the study and CIM did the field data collection while DSI, RAK and MML supervised data collection, and overall implementation of the study. CIM, MW and DSI managed, cleaned and analysed the data. CIM and DSI wrote the manuscript with contribution from MW. All authors read and approved the final manuscript.
Acknowledgements
The team is indebted to the children and their parents/guardians for accepting to take part in the study and attending the follow-up visits, despite the long duration of follow-up. The continued support by colleagues at Muheza DDH and Ujiji Health Centres and offices of regional and district medical officers who supported the study teams during data collection at their respective sites is greatly acknowledged. Thanks to the staff of NIMR Tanga Centre (Geofrey Makenga, Filbert Francis, Ezekiel Malecela, Zakaria Savael, Hatibu Athumani, Juma Tupa, Thomas Semdoe, Seth Nguhu, Neema Barua, Fides Mumburi and Mary Lukindo) for taking part in the implementation of the study at different stages. The support provided by the district authorities (particularly the district executive directors and district medical officers) of Muheza and Kigoma is highly appreciated. The authors are grateful to Dr. Michael Alifrangis for his support with PCR reagents and technical support in the laboratory genotyping of malaria parasites. Holy Pharmacy (Dar es Salaam, Tanzania) donated some doses of DP while WHO provided AL and filter papers. Permission to publish this paper has been granted by the Director General of NIMR.
Competing interests
The authors declare that they have no competing interests.
Availability of data
The data set used in this study is available and can be shared upon reasonable request to the corresponding author.
Disclaimer
Marian Warsame and Ritha Njau are staff members of the World Health Organization and they alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization.
Ethics approval and consent to participate
This study was approved by the Medical Research Coordinating Committee (MRCC) of the National Institute for Medical Research (NIMR) and permission to conduct the study in the 2 districts was sought and obtained from the district authorities of Kigoma and Muheza. Parents/guardians of all study participants signed informed consents before enrolment.
Funding
Funding was obtained from the World Bank Project—East African Public Health Laboratory Network Project (EAPHLNP) through the Tanzanian Ministry of Health, Community Development, Gender, Elderly and Children (MoHCDGEC) and the Tanzanian Commission for Science and Technology (COSTECH).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mandara, C.I., Kavishe, R.A., Gesase, S. et al. High efficacy of artemether–lumefantrine and dihydroartemisinin–piperaquine for the treatment of uncomplicated falciparum malaria in Muheza and Kigoma Districts, Tanzania. Malar J 17, 261 (2018). https://doi.org/10.1186/s12936-018-2409-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-018-2409-z