Abstract
Background
Plasmodium ovale has two different subspecies: P. ovale curtisi and P. ovale wallikeri, which may be distinguished by the gene potra encoding P. ovale tryptophan-rich antigen. The sequence and size of potra gene was variable between the two P. ovale spp., and more fragment sizes were found compared to previous studies. Further information about the diversity of potra genes in these two P. ovale spp. will be needed.
Methods
A total of 110 dried blood samples were collected from the clinical patients infected with P. ovale, who all returned from Africa in Henan Province in 2011–2016. The fragments of potra were amplified by nested PCR. The sizes and species of potra gene were analysed after sequencing, and the difference between the isolates were analysed with the alignment of the amino acid sequences. The phylogenetic tree was constructed by neighbour-joining to determine the genetic relationship among all the isolates. The distribution of the isolates was analysed based on the origin country.
Results
Totally 67 samples infected with P. o. wallikeri, which included 8 genotypes of potra, while 43 samples infected with P. o. curtisi including 3 genotypes of potra. Combination with the previous studies, P. o. wallikeri had six sizes, 227, 245, 263, 281, 299 and 335 bp, and P. o. curtisi had four sizes, 299, 317, 335 and 353 bp, the fragment sizes of 299 and 335 bp were the overlaps between the two species. Six amino acid as one unit was firstly used to analyse the amino acid sequence of potra. Amino acid sequence alignment revealed that potra of P. o. wallikeri differed in two amino acid units, MANPIN and AITPIN, while potra of P. o. curtisi differed in amino acid units TINPIN and TITPIS. Combination with the previous studies, there were ten subtypes of potra exiting for P. o. wallikeri and four subtypes for P. o. curtisi. The phylogenetic tree showed that 11 isolates were divided into two clusters, P. o. wallikeri which was then divided into five sub-clusters, and P. o. curtisi which also formed two sub-clusters with their respective reference sequences. The genetic relationship of the P. ovale spp. mainly based on the number of the dominant amino acid repeats, the number of MANPIN, AITPIN, TINPIN or TITPIS. The genotype of the 245 bp size for P. o. wallikeri and that of the 299 and 317 bp size for P. o. curtisi were commonly exiting in Africa.
Conclusion
This study further proved that more fragment sizes were found, P. o. wallikeri had six sizes, P. o. curtisi had four sizes. There were ten subtypes of potra exiting for P. o. wallikeri and four subtypes for P. o. curtisi. The genetic polymorphisms of potra provided complementary information for the gene tracing of P. ovale spp. in the malaria elimination era.
Similar content being viewed by others
Background
Plasmodium ovale was first described in 1922 [1], as the fourth malaria parasite of humans [2, 3]. Generally, a P. ovale infection is of low parasitaemia, and the morphology of the parasite is similar to Plasmodium vivax. Also, it frequently presents as a mixed infection with the other Plasmodium species [4,5,6,7,8,9]. As a result, P. ovale attracted less attention compared to other species, and its prevalence has apparently been underestimated. It has long been considered predominantly found in Africa and some islands of Western Pacific [10, 11], with confirmed cases occasionally found in other endemic regions [12, 13].
Currently, P. ovale spp. may be classified into two different subspecies by molecular genotyping: P. o. curtisi (classic type) and P. o. wallikeri (variant type) [14]. The nuclear genome sequences further confirmed that the two species were genetically different, but morphologically indistinct [15], and their duration of latency were seemly different [16]. Both species were considered to exist sympatrically in Africa and Asia, and even both parasites were infected simultaneously [17,18,19,20,21].
Because of the generally low parasitaemia of P. ovale infections, sensitive molecular methods to detect and identify the two subspecies must be used in future investigations, with polymorphic markers as a method to discriminate the different strains. Many protocols showed that the SS rRNA genes [17, 22, 23] were suitable for identification but not for genotyping. The recent study showed that the gene encoding P. ovale tryptophan-rich antigen (potra) could be used to distinguish the two P. ovale subspecies [14]. The sequence and size of the tryptophan-rich antigen gene was variable among the P. ovale subspecies (poctra and powtra) [14]. A nested PCR detection assay was exploited to discriminate the species by the size of the amplified fragments (299 or 317 bp for poctra; 245 bp for powtra), where the conserved sequences were chosen as primers for these two genes [19]. Additionally, a semi-nested PCR protocol was developed by Tanomsing et al. [24] with which the two P. ovale subspecies could be discriminated efficiently, and more fragment sizes were found comparing with previous studies, the 299 bp fragment was overlapping between the two subspecies. This would invalidate amplified fragment size difference, as a means of distinguishing between P. o. curtisi and P. o. wallikeri. The amplified fragment size variations resulted from differences in the number of repeated units, which suggested that a broader range of size variants might occur. In this study, more variations of potra gene were observed.
Methods
Sample collection and DNA extraction
Dried blood spots on filter paper (Whatman 3M) were collected from patients returned from Africa with P. ovale infection before treatment. All the patients were diagnosed by nested PCR and microscopy. All the dried blood spots were labelled with a unique identification number, air-dried and individually placed in plastic bags with desiccant and stored at − 20 °C until laboratory analysis. DNA was extracted from the dried blood spots using a QIAamp DNA mini kit (Qiagen, Germany).
Nested PCR amplification and DNA sequencing
The fragments of potra was amplified with nested PCR using the primers as described previously [14, 19]. The amplified products were identified by agarose gel electrophoresis. Bidirectional sequencing was performed for the secondary potra PCR products using the secondary primers by Sangon Biotech Co Ltd (Shanghai, China).
Sequencing alignments and analysis
All the genes sequences were analysed with multiple sequence alignment using the Clustal X software. HM594180–HM594183 [19], KF018430–KF018433 [24] and KX417700–KX417704 [25] from the GenBank would be as the reference sequences of P. ovale spp. Phylogenetic trees were constructed using the Molecular Evolutionary Genetics Analysis (MEGA) 6.06.
Results
Amplification of the potra gene of Plasmodium ovale spp.
A total of 110 dried blood samples, from patients returned from Africa to Henan Province with P. ovale infection, were collected. The amplified nested PCR products of the potra gene of 110 samples were blasted in the GenBank. The blast data showed that 67 samples infected with P. o. wallikeri and 43 samples infected with P. o. curtisi. More fragment sizes of the potra gene from this study were found comparing with the previous reports. P. o. wallikeri had five different sizes including 227, 245, 263, 281 and 299 bp, while P. o. curtisi had three sizes including 299, 317 and 335 bp. Also, the amplified fragment size differed as a result of differences in 18 bases of units, with the overlap of 299 bp between the two species (Fig. 1). The size of 245 bp was the main type for the P. o. wallikeri, accounting for 82.1% (55/67), and the 299 bp was the main size for P. o. curtisi, accounting for 67.4% (29/43). The number of isolates for each size was shown in the Table 1.
Oguike reported that the sizes of the potra gene was 245 bp for P. o. wallikeri and 299 bp and 317 bp for P. o. curtisi [19] in Congo, Uganda and Equatorial Guinea. While there were three sizes (245, 299 and 335 bp) for P. o. wallikeri and three sizes (299, 317 and 353 bp) for P. o. curtisi in the study of Tanomsing et al. [24]. Combination with the previous studies, P. o. wallikeri had six sizes, 227, 245, 263, 281, 299 and 335 bp, and P. o. curtisi had four sizes, 299, 317, 335 and 353 bp, the fragment sizes of 299 and 335 bp were the overlaps between the two species (shown in Table 1).
Genotypes of potra of Plasmodium ovale spp.
There were 8 genotypes of the potra gene for the 67 isolates infected with P. o. wallikeri and three genotypes of potra for the 43 isolates infected with P. o. curtisi. The sizes of 277, 245 and 263 bp for P. o. wallikeri all had two different subtypes. The sequences of the 11 genotypes of potra gene were deposited in GenBank under accession number MG588144-MG588154. For P. o. wallikeri, the genotype of MG588146 was the same with that of the reference sequences HM594180 and HM594181, but the reference sequences KF018430 and KF018431 were different with any of MG588144-MG588151. For P. o. curtisi, the genotype of MG588152 was the same with that of the reference sequences HM594182 and KF018433, and the genotype of MG588153 was the same with that of the reference sequence HM594183. Combination with the previous studies, there were ten genotypes of potra exited for P. o. wallikeri, and four genotypes for P. o. curtisi. The number of isolates for each genotype was shown in the Table 2.
Alignment of the translated amino acid sequence of potra fragments
Interestingly, the translated amino acid sequence of potra fragments were composed with multiple amino acid units, and six amino acids was considered as a unit. For P. o. wallikeri, it was mainly composed with nine kinds of amino acid units, TANPIN, MANPIN, MAKPIN, TITPIN, AITPIN, AITPIK, TITSIN, TITPMN and TITPIS. While P. o. curtisi had six kinds of amino acid units, TANPIN, AANPIN, TINPIN, KINPIN, TITPIN and TITPIS. The two subspecies of P. ovale had three common amino acid units, TANPIN, TITPIN and TITPIS. Amino acid sequence alignment revealed that the types of P. o. wallikeri differed in two amino acid units, MANPIN and AITPIN, while the types of P. o. curtisi differed in amino acid units TINPIN and TITPIS. The amplified fragment size differed as a result of differences in amino acid of repeat units. The dominant amino acid repeats of potra were showed in Table 2. As the same with the genotypes of potra, there were 10 subtypes exiting for P. o. wallikeri and 4 subtypes for P. o. curtisi. The detail information is shown in Fig. 2 and Table 2.
Amino acid sequence alignment of the distinct potra fragments amplified from 11 genotypes of Plasmodium ovale and the reference sequences. a P. ovale wallikeri; b P. ovale curtisi. Six amino acids were one amino acid unit which were underlined. P. o. wallikeri was mainly composed with nine kinds of amino acid units, TANPIN, MANPIN, MAKPIN, TITPIN, AITPIN, AITPIK, TITSIN, TITPMN and TITPIS. While P. o. curtisi had six kinds of amino acid units, TANPIN, AANPIN, TINPIN, KINPIN, TITPIN and TITPIS. Boxed sequences represented the dominant amino acid repeats that differed in the number of amino acid units. The sequences of P. o. wallikeri were characterized by two amino acid units, MANPIN and AITPIN. The sequences of P. o. curtisi were characterized by amino acid units TINPIN and TITPIS
Phylogenetic relationship among potra subtype families
Neighbour-joining was used to cluster the potra gene sequences. The 11 genotypes were classified into two clusters, eight genotypes infected with P. o. wallikeri were as one cluster and other three genotypes infected with P. o. curtisi formed the other one. Meanwhile the cluster of P. o. wallikeri was classified into five sub-clusters, distinctive phylogenetic relationship presented between the eight genotypes of P. o. wallikeri. The sequence 1-227, formed a sub-cluster with reference sequences HM594180, HM594181 and KX417700, having the same 2 repeats of AITPIN. The sequences 2-227 and 3-245 existed in the same sub-cluster with a closer genetic relationship, having the same repeat of MANPIN. The sequences 4-245 and 5-263 formed a sub-cluster with reference sequence KX417704, having the same two repeats of MANPIN. The sequences 6-263 and 7-281 formed another sub-cluster with reference sequences KF018430 and KF018431, having the same three repeats of AITPIN. Having significantly difference sequences, the three genotypes of P. o. curtisi also formed two sub-clusters. The sequence 9-299 formed a sub-cluster with reference sequences, having the same repeat of TITPIS. The sequences 10-317 and 11-335 had a closer genetic relationship, which formed another sub-cluster with reference sequences, having the same two repeats of TITPIS (Fig. 3).
Distribution of the Plasmodium ovale spp.
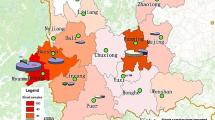
All the 110 P. ovale patients returned from twenty counties of Africa, and 70.0% (77/110) were from Angola, Congo, Equatorial Guinea, Liberia and Nigeria. Only one patient originated from Algeria, Tanzania, Sudan, Libya and Ivory Coast. 82.1% (55/67) P. o. wallikeri infection were the same subtype with 245 bp size of the potra gene, and this type was identified in 16 countries except Gabon, Algeria, Tanzania and Libya; three isolates (4.5%, 3/67) with 227 bp size originated from Congo and Liberia,; four isolates (6.0%, 4/67) of 263 bp size were from Angola, Liberia and Gabon; 4 isolates (6.0%, 4/67) of 281 bp size were from Congo, Liberia, Nigeria and Sierra Leone; only one isolate of 299 bp size was from Congo. In contrast, the predominant type of P. o. curtisi (67.4%, 29/43) was the potra gene with 299 bp size, which originated from 12 countries; 27.9% (12/43) with 317 bp size and 4.7% (2/43) with 335 bp size were also identified from nine countries and two countries, respectively (Table 3).
Discussion
Malaria elimination is a long-term goal to be achieved worldwide. As one species of human Plasmodium, the identification of P. ovale is more widespread than formerly known. Plasmodium ovale, like P. vivax, has hypnozoites that cause relapses [26, 27], and it consists of two different subspecies: P. ovale curtisi and P. ovale wallikeri [14]. Therefore, the differentiation of the two P. ovale species, especially with respect of molecular phylogeny will need to be better understood.
In 2011, Oguike et al. [19] published the discrimination of the two P. ovale subspecies by the size of the amplified fragments of the potra gene (299 or 317 bp for P. o. curtisi; 245 bp for P. o. wallikeri), using nested PCR. Although this technique was specific for P. ovale spp., the sizes of the amplified fragment varied with the number of repeat units, which reduced the discrimination between species: for P. o. curtisi, 299, 317 and 353 bp, and for P. o. wallikeri, 245, 299 and 335 bp [24]. Tanomsing et al. [24] suggested the number of potra size variations might be more than those evaluated and this speculation was confirmed in this study. Using the same primes and method, more fragment sizes were identified in this study, while some sizes overlapped between the two subspecies: for P. o. curtisi having sizes 299, 317 and 335 bp, and for P. o. wallikeri having sizes 227, 245, 263, 281 and 299 bp. The results of the three studies [19, 24] were also combined, as shown in Table 1. Four different sizes for P. o. curtisi and six sizes for P. o. wallikeri have been reported, and that the fragment sizes of 299 and 335 bp were overlaps between the two species. As more samples were analysed, it was likely that the number of potra size variants would be more than expected. Conceivably, more size variants may be identified in future studies.
Potra gene was used to discriminate the two P. ovale species, because the tryptophan-rich antigen was encoded by a repeat pattern of variable length 3-amino acid [14]. Sutherland et al. [14] and Oguike et al. [19] had also proposed that the potra gene of P. o. curtisi (poctra) could be identified by the pattern of the six amino acids of the repeat region, TITPIS, while the potra gene of P. o. wallikeri (powtra) were different in two non-synonymous positions. By alignment of the amino acid sequence of potra fragments, 11 genotypes of potra were found from the 110 isolates in this study. Combination with the previous studies of Oguike et al. [19] and Tanomsing et al. [24], showed ten subtypes of potra gene for P. o. wallikeri and four subtypes for P. o. curtisi, and 14 genotypes of potra gene were under analysis. This study showed that the amino acid sequence of potra fragments were composed with multiple amino acid units, six amino acids were as one unit. There was different dominant amino acid repeat of potra for the two P. ovale species, which could be used to discriminate the subtype of P. ovale spp. The repeat of six amino acids as one unit to analyse the difference of the genotypes between the two P. ovale species was first reported, which could make the results more clear and simple. The sizes of 277, 245 and 263 bp for P. o. wallikeri all had two different subtypes, and this phenomenon did not find in the P. o. curtisi.
The sizes of the reference sequences KX417700–KX417704 [25] from Genbank were short for discriminating the differences of dominant amino acid repeats. In our study, the genetic relationship of the P. ovale spp. was analysed by the neighbour-joining tree mainly based on the number of the dominant amino acid repeats including MANPIN, AITPIN, TINPIN or TITPIS.
The distribution of the two P. ovale spp. was different. The isolates of P. o. wallikeri was more than that of P. o. curtisi, and the genotype of the 245 bp size was the predominant type for P. o. wallikeri in most Africa countries, but the other genotypes were less. For P. o. curtisi, the genotypes of the 299 and 317 bp size were commonly in Africa and the genotype of 335 bp size was less.
Molecular epidemiological studies on genetic diversity of Plasmodium vivax have been based mainly on single copy polymorphic genes which code for parasite surface antigens such as circumsporozoite protein (csp), merozoite surface protein-1 (msp-1) and merozoite surface protein 3 alpha (msp 3α) [28]. Pvcsp comprises of central domain of tandem repeated sequences flanked by two non-repeated conserved sequences [29,30,31,32]. Two types of repeat elements, either VK210 or VK247 types were detected in clinical isolates of P. vivax and thus pvcsp serves as a useful tool for genotyping [33, 34]. Potra has the similar characteristics with pvcsp, which also could be used for parasite genotyping.
Conclusions
Considering the change of malaria epidemiology and the approaching of malaria elimination, P. ovale spp. deserves more attention. Molecular techniques are a good tool for detecting and identifying the two P. ovale subspecies and their relative distribution.
Abbreviations
- Potra :
-
Plasmodium ovale tryptophan- rich antigen
- Spp.:
-
plural form of species
- PCR:
-
polymerase chain reaction
- NCBI:
-
national center for biotechnology information
- DNA:
-
desoxyribonucleic acid
- MEGA:
-
molecular evolutionary genetics analysis
References
Stephens JWW. A new malaria parasite of man. Ann Trop Med Parasitol. 1922;16:383–8.
White NJ. Plasmodium knowlesi: the fifth human malaria parasite. Clin Infect Dis. 2008;46:172–3.
Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis. 2004;4:327–36.
Trape JF, Rogier C, Konate L, Diagne N, Bouganali H, Canque B, et al. The Dielmo project: a longitudinal study of natural malaria infection and the mechanisms of protective immunity in a community living in a holoendemic area of Senegal. Am J Trop Med Hyg. 1994;51:123–7.
Koita OA, Sangare L, Sango HA, Dao S, Keita N, Maiga M, et al. Effect of seasonality and ecological factors on the prevalence of the four malaria parasite species in northern Mali. J Trop Med. 2012;2012:367160.
Roucher C, Rogier C, Sokhna C, Tall A, Trape JF. A 20-year longitudinal study of Plasmodium ovale and Plasmodium malariae prevalence and morbidity in a West African population. PLoS ONE. 2014;9:e87169.
Trape JF, Tall A, Sokhna C, Ly AB, Diagne N, Ndiath O, et al. The rise and fall of malaria in a West African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis. 2014;14:476–88.
Doderer-Lang C, Atchade PS, Meckert L, Haar E, Perrotey S, Filisetti D, et al. The ears of the African elephant: unexpected high seroprevalence of Plasmodium ovale and Plasmodium malariae in healthy populations in Western Africa. Malar J. 2014;13:240.
Faye FB, Spiegel A, Tall A, Sokhna C, Fontenille D, Rogier C, et al. Diagnostic criteria and risk factors for Plasmodium ovale malaria. J Infect Dis. 2002;186:690–5.
Lysenko AJ, Beljaev AE. An analysis of the geographical distribution of Plasmodium ovale. Bull World Health Organ. 1969;40:383–94.
Collins WE, Jeffery GM. Plasmodium ovale: parasite and disease. Clin Microb Rev. 2005;18:570–81.
Smith AD, Bradley DJ, Smith V, Blaze M, Behrens RH, Chiodini PL, et al. Imported malaria and high risk groups: observational study using UK surveillance data 1987–2006. BMJ. 2008;337:a120.
Mueller I, Zimmerman PA, Reeder JC. Plasmodium malariae and Plasmodium ovale—the “bashful” malaria parasites. Trends Parasitol. 2007;23:278–83.
Sutherland CJ, Tanomsing N, Nolder D, Oguike M, Jennison C, Pukrittayakamee S, et al. Two non recombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis. 2010;201:1544–50.
Ansari HR, Templeton TJ, Subudhi AK, Ramaprasad A, Tang J, Lu F, et al. Genome-scale comparison of expanded gene families in Plasmodium ovale wallikeri and Plasmodium ovale curtisi with Plasmodium malariae and with other Plasmodium species. Int J Parasitol. 2016;46:685–96.
Nolder D, Oguike MC, Maxwell-Scott H, Niyazi HA, Smith V, Chiodini PL, et al. An observational study of malaria in British travellers: Plasmodium ovale wallikeri and Plasmodium ovale curtisi differ significantly in the duration of latency. BMJ Open. 2013;3:e002711.
Calderaro A, Piccolo G, Perandin F, Gorrini C, Peruzzi S, Zuelli C, et al. Genetic polymorphisms influence Plasmodium ovale PCR detection accuracy. J Clin Microbiol. 2007;45:1624–7.
Win TT, Jalloh A, Tantular IS, Tsuboi T, Ferreira MU, Kimura M, et al. Molecular analysis of Plasmodium ovale variants. Emerg Infect Dis. 2004;10:1235–40.
Oguike MC, Betson M, Burke M, Nolder D, Stothard JR, Kleinschmidt I, et al. Plasmodium ovale curtisi and Plasmodium ovale wallikeri circulate simultaneously in African communities. Int J Parasitol. 2011;41:677–83.
Fuehrer HP, Habler VE, Fally MA, Harl J, Starzengruber P, Swoboda P, et al. Plasmodium ovale in Bangladesh: genetic diversity and the first known evidence of the sympatric distribution of Plasmodium ovale curtisi and Plasmodium ovale wallikeri in southern Asia. Int J Parasitol. 2012;42:693–9.
Bauffe F, Desplans J, Fraisier C, Parzy D. Real-time PCR assay for discrimination of Plasmodium ovale curtisi and Plasmodium ovale wallikeri in the Ivory Coast and in the Comoros Islands. Malar J. 2012;11:307.
Calderaro A, Piccolo G, Gorrini C, Montecchini S, Rossi S, Medici MC, et al. A new real-time PCR for the detection of Plasmodium ovale wallikeri. PLoS ONE. 2012;7:e48033.
Fuehrer HP, Stadler MT, Buczolich K, Bloesch I, Noedl H. Two techniques for simultaneous identification of Plasmodium ovale curtisi and Plasmodium ovale wallikeri by use of the small-subunit rRNA gene. J Clin Microbiol. 2012;50:4100–2.
Tanomsing N, Imwong M, Sutherland CJ, Dolecek C, Hien TT, Nosten F, et al. Genetic marker suitable for identification and genotyping of Plasmodium ovale curtisi and Plasmodium ovale wallikeri. J Clin Microbiol. 2013;51:4213–6.
Daniels RF, Deme AB, Gomis JF, Dieye B, Durfee K, Thwing JI, et al. Evidence of non-Plasmodium falciparum malaria infection in Kedougou, Senegal. Malar J. 2017;16:9.
Garnham PCC, Bray RS, Cooper W, Lainson R, Awad FI, Williamson J. The pre-erythrocytic stage of Plasmodium ovale. Trans R Soc Trop Med Hyg. 1955;49:158–67.
Chin W, Coatney GR. Relapse activity in sporozoite-induced infections with a West African strain of Plasmodium ovale. Am J Trop Med Hyg. 1971;20:825–7.
Cui L, Escalante AA, Imwong M, Snounou G. The genetic diversity of Plasmodium vivax populations. Trends Parasitol. 2003;19:220–6.
Rosenberg R, Wirtz RA, Lanar DE, Sattabongkot J, Hall T, Waters AP, et al. Circumsporozoite protein heterogeneity in human malaria parasite Plasmodium vivax. Science. 1989;245:973–6.
Arnot DE, Barnwell JW, Tam JP, Nussenzweig V, Nussenzweig RS, Enea V. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science. 1985;230:815–8.
Qari SH, Shi YP, Goldman IF, Udhayakumar V, Alpers MP, Collins WE, et al. Identification of Plasmodium vivax-like human malaria parasites. Lancet. 1993;341:780–3.
Qari SH, Shi YP, Povoa MM, Alpers MP, Deloron P, Murphy GS, et al. Global occurrence of Plasmodium vivax-like human malaria parasite. J Infect Dis. 1993;168:1485–9.
Kain KC, Brown AE, Webster HK, Wirtz RA, Keystone JS, Rodriguez MH, et al. Circumsporozoite genotyping of global isolates of Plasmodium vivax from dried blood specimens. J Clin Microbiol. 1992;30:1863–6.
González JM, Hurtado S, Arévalo-Herrera M, Herrera S. Variants of the Plasmodium vivax circumsporozoite protein (VK210 and VK247) in Colombian isolates. Mem Inst Oswaldo Cruz. 2001;96:709–12.
Authors’ contributions
RMZ was responsible for the molecular genetic analysis and data interpretation and drafted the manuscript. YL participated in sample detection and data analysis. HWZ and BLX conceived the study and revised the manuscript. FH revised the manuscript. SUL participated in sample collection and sample detection. YLZ, YD and DLL provided the administrative coordination. CYY and DQ participated in the data collection and analysed the data. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank all the patients who participated in these surveys and the staff of the hospitals and centers for disease control and prevention in Henan Province.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the first/corresponding author on reasonable request.
Ethics approval and consent to participate
The purpose of this study was demonstrated to participants before sampling, who agreed to participate in the survey, and the consent was obtained from them. Approval of the study was obtained from the Ethical Committee of Henan Province Center for Disease Control and Prevention, prior to commencement of the study.
Funding
The study was funded by the Project of Medical Science and Technology of Henan Province (No. 201602318) and the “51282” Project of Innovative Talents of Science and Technology of Henan province (No. 1030412). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhou, R., Liu, Y., Li, S. et al. Polymorphisms analysis of the Plasmodium ovale tryptophan-rich antigen gene (potra) from imported malaria cases in Henan Province. Malar J 17, 127 (2018). https://doi.org/10.1186/s12936-018-2261-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-018-2261-1