Abstract
Background
Transfusion-transmitted malaria (TTM) is a well-recognized risk of receiving blood transfusions, and has occurred with Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae. The simian parasite Plasmodium knowlesi is also known to be transmissible through inoculation of infected blood, and this species is now the most common cause of malaria in Malaysia with a high rate of severity and fatal cases reported. No confirmed case of accidental transfusion-transmitted P. knowlesi has yet been reported.
Case presentation
A 23-year old splenectomized patient with beta thalassaemia major presented with fever 11 days after receiving a blood transfusion from a pre-symptomatic donor who presented with knowlesi malaria 12 days following blood donation. The infection resulted in severe disease in the recipient, with a parasite count of 84,000/µL and associated metabolic acidosis and multi-organ failure. She was treated with intravenous artesunate and made a good recovery. Sequencing of a highly diverse 649-base pair fragment of the P. knowlesi bifunctional dihydrofolate reductase-thymidylate synthase gene (pkdhfr) revealed that the recipient and donor shared the same haplotype.
Conclusions
This case demonstrates that acquisition of P. knowlesi from blood transfusion can occur, and that clinical consequences can be severe. Furthermore, this case raises the possibility that thalassaemic patients, particularly those who are splenectomized, may represent a high-risk group for TTM and severe malaria. With rising P. knowlesi incidence, further studies in Sabah are required to determine the risk of TTM in order to guide screening strategies for blood transfusion services.
Similar content being viewed by others
Background
Transfusion-transmitted malaria (TTM) was first described in 1911 [1] and remains an important public health problem. In non-endemic countries, stringent screening processes have led to a very low incidence, with less than one case occurring per one million donations [2], however, clinical consequences can be severe due to lack of background immunity in the population [2, 3]. In endemic countries, the incidence of TTM is substantially higher due to a high prevalence of parasitaemia among blood donors; a systematic review in sub-Saharan Africa reported a median prevalence of parasitaemia among blood donors of 10.2 % (range 2–55 %) [4]. In endemic countries outside sub-Saharan Africa, malaria prevalence among blood donors is generally lower [5, 6], however risk of acquiring malaria from infected blood may be higher due to the lower immunity of recipients.
TTM has been reported to occur as a result of infection with Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale [7, 8], and one probable case of transfusion-transmitted Plasmodium knowlesi has been reported [9]. In Sabah, Malaysian Borneo, the overall incidence of clinical malaria is low, and population immunity likely minimal, however human cases of P. knowlesi are increasing, with the species now the most common cause of malaria in Sabah [10]. Plasmodium knowlesi has a 24-hour erythrocytic life cycle, and high parasitaemias can develop rapidly. In adults, the risk of severe disease is at least as high as that of P. falciparum [11], and fatal cases have been reported [10, 12–18]. Transfusion-transmitted P. knowlesi may therefore constitute a significant risk in knowlesi-endemic areas such as Sabah.
Current screening practices in Sabah include a pre-donation questionnaire to exclude donors with history of fever in the previous 7 days, and microscopic examination of a thick and thin blood film for malaria parasites. Microscopy, however, fails to detect parasitaemias <50/µL [19], and for P. falciparum it has been established that as few as ten parasites per unit of red blood cells are sufficient to transmit infection [20]. Screening with microscopy does not therefore eliminate the risk of TTM. Recently, the first reported Malaysian case of TTM was described, involving a child in West Malaysia infected with P. vivax from a donor recently returned from Myanmar [21]. Although P. knowlesi is well known from early experimental and neurosyphilis studies to be transmissible through inoculation of infected blood [22, 23], no confirmed case of accidental transfusion-transmitted P. knowlesi has yet been reported.
This report describes a case of severe transfusion-transmitted P. knowlesi infection in a splenectomized thalassaemic patient, acquired from a pre-symptomatic donor who presented with knowlesi malaria soon after donation.
Case presentation
A 23-years old female presented to Pitas District Hospital in northeast Sabah, Malaysia, with a 5-day history of fever, rigours, headache, and dizziness. She lived with her family in a malaria-endemic village surrounded by palm oil plantations, where long-tailed macaques were regularly seen. She had a history of beta thalassaemia major, diagnosed at 9 months of age and requiring splenectomy at age 13 years. She received iron chelation and second monthly blood transfusions at her local hospital. Her last blood transfusion had been 16 days prior to presentation, and her post-transfusion haemoglobin was 9.8 g/dL. She had recently commenced oral hypoglycaemic agents for type 2 diabetes mellitus.
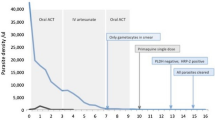
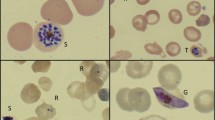
On presentation at the district hospital her vital signs were within normal range, including an oxygen saturation of 98 %. Her haemoglobin was 8.7 g/dL, white cell count 48 × 103/µL and platelet count 129 × 103/µL. She was admitted with a provisional diagnosis of symptomatic anaemia. Later that day she developed respiratory distress with an oxygen saturation of 50 % on room air, improving to 72 % on high-flow oxygen, and she was transferred to a tertiary referral hospital in the state capital, Kota Kinabalu. On arrival her temperature was 38.6 °C, heart rate 139 beats/min, blood pressure 116/69 mm Hg, and oxygen saturation 93 % on high-flow mask with a respiratory rate of 36 breaths per minute. A blood film taken on arrival was positive for malaria parasites resembling P. knowlesi, with a parasite count of 84,000 parasites/µL. Further investigation revealed worsening anaemia (haemoglobin 6.0 g/dL) and further elevation of the white blood cell count (61 × 103/µL), in addition to metabolic acidosis (lactate 16 mmol/L, bicarbonate 8.4 mmol/L, pH 7.14), hyperbilirubinemia (80 µmol/L), acute kidney injury (creatinine 184 µmol/L), increased liver transaminases (alanine transaminase 181 U/L and aspartate transaminase 308 U/L), and hypoglycaemia (blood glucose 2.5 mmol/L) (Table 1). Chest radiograph was initially unremarkable, however, on day 1 showed mild bilateral interstitial infiltrates. She was diagnosed with severe knowlesi malaria, admitted to the intensive care unit, and commenced on intravenous artesunate, intravenous antibiotics (initially piperacillin/tazobactam, then ceftriaxone) and inotropic support; she was transfused two units of whole blood. She made a good recovery, and on day 2 after completing three doses of intravenous artesunate, she was changed to oral artemether-lumefantrine (Riamet®) for 3 days and discharged from the intensive care unit. Her blood film was negative for malaria parasites on day 3, and she was discharged from hospital on day 9. Dengue NS1 antigen and blood cultures taken prior to antibiotics were negative. PCR of her initial blood sample using previously described methods [24] confirmed P. knowlesi.
Donor
In view of the patient’s blood transfusion 16 days prior to admission, the possibility of transfusion-acquired P. knowlesi was investigated. The blood bank was contacted for information regarding the donor, the donor was informed, and donation records were obtained from the blood bank. Medical records of the donor were subsequently retrieved from Pitas District Hospital where he had been admitted, and reviewed for clinical and laboratory details. An EDTA blood sample was retrieved from the State Public Health Laboratory, where it had been sent for Plasmodium PCR.
The blood donor was a 51-years old male farmer who lived in a malaria-endemic village 3 h drive from the patient’s village, and who had donated blood 4 days prior to the patient’s blood transfusion. He was well on the day of the blood donation, and a screening blood film was negative for malaria parasites. Twelve days following the donation however, he was admitted to the same district hospital with a 5-day history of fever, chills, headache, nausea, and malaise. His blood film was reported as P. falciparum ‘2+’ (indicating 40-400 parasites/µL). He was treated with oral artesunate and mefloquine (Artequin®) for uncomplicated malaria, and was discharged after 4 days. PCR performed on an admission EDTA blood sample confirmed P. knowlesi.
Molecular analysis
Molecular finger-printing of the patient and donor infections was undertaken using sequence data generated on a 649-base pair fragment of the P. knowlesi bifunctional dihydrofolate reductase-thymidylate synthase gene (pkdhfr, PKNH_0509600), which has been demonstrated to be highly diverse in Sabah [25]. DNA extraction and pkdhfr sequencing was conducted using the methods described by Grigg et al. [25]. Additional pkdhfr sequence data on 21 P. knowlesi infections sourced from the same district were retrieved from Grigg et al. to assess the local pkdhfr diversity. Analysis using DnaSP (version 5.10.01) confirmed high diversity amongst the 22 baseline samples. A total of 18 distinct haplotypes were observed, with haplotype diversity estimated at 0.978 (i.e., approximately 2 % probability that two independent infections randomly selected from the population would have identical haplotypes by chance alone). The patient and donor infections shared the same haplotype along with one other infection (QEM131) (Fig. 1).
Sequence polymorphisms in the pkdhfr gene fragment in Plasmodium knowlesi infections in patients from the transfusion recipient’s district, including the transfusion recipient and donor (T1 and T2). Nineteen polymorphic sites are presented: from left to right representing nucleotide positions 36, 90, 101, 135, 192, 240, 252, 271, 288, 386, 418, 429, 446, 459, 495, 528, 570, 600 and 636 with numbering beginning from the first nucleotide of the start codon of pkdhfr in the P. knowlesi H strain (PkH1) reference sequence (GeneDB version 2015-06-18). Dots represent identical nucleotide residues
Discussion
This report describes a case of severe transfusion-transmitted knowlesi malaria, the occurrence of which may have implications for blood transfusion practices in knowlesi-endemic areas. Plasmodium knowlesi is the most common cause of malaria in Malaysia [10, 26, 27], and the species may cause severe and fatal disease [10, 12]. Cases of transfusion-transmitted falciparum malaria can rapidly be fatal in susceptible individuals [28]. Transfusion-transmitted P. knowlesi may represent a significant risk in endemic areas, particularly in those at high risk, such as the asplenic thalassaemic patient described in this report.
The risk of being exposed to P. knowlesi through blood transfusion in Sabah is difficult to estimate. Although no cross-sectional prevalence survey has yet been reported in Sabah, a recent molecular-based survey of individuals residing in households or villages of symptomatic malaria patients in northeast Sabah found that in this population the prevalence of asymptomatic P. knowlesi infection was 6.9 % [29]. In a recent study in Ghana, where 55 % of blood donors were parasitaemic when tested by PCR, the incidence of TTM in non-parasitaemic recipients receiving parasitaemic blood was around 14–28 % [30]. The risk of acquiring malaria from infected blood may be higher in Sabah where immunity of the population is likely to be low.
Beta thalassaemia has been shown to be a risk factor for other severe infections, with increasing risk associated with duration of thalassaemia, number of transfusions, previous splenectomy, and receiving the iron chelator deferoxamine [31]. Additional factors thought to contribute to infection risk include anaemia, reticulo-endothelial dysfunction resulting from iron overload and haemolysed erythroblasts, and altered immune responses [32]. The incidence of TTM among thalassaemic patients in endemic countries is difficult to quantify, as recipients frequently have pre-existing parasitaemia [20]. In a study in Sri Lanka, a higher frequency of P. vivax antibodies was found in thalassaemia patients compared to age-matched controls, with frequency particularly high in splenectomized patients [33]. An Indian study demonstrated post-transfusion malaria occurring in 6.4 % of beta thalassaemia patients who received repeated blood transfusion [34]. Malaysia has a high prevalence of thalassaemia; in 2009 there were 4541 registered thalassaemia patients, of whom 3310 were transfusion-dependent with either beta thalassaemia major or HbE beta thalassaemia. The state of Sabah has the highest number of registered thalassaemia cases, accounting for 28 % of the total cases in Malaysia [35]. TTM among the beta thalassaemia population in malaria-endemic regions of Sabah may be under-reported and requires further investigation.
In addition to being a possible risk factor for TTM, the lack of a spleen has been associated with severe malaria following infection with P. falciparum [36], and severe knowlesi malaria in splenectomized patients has also been reported [11, 37]. High parasite counts and severe disease, as occurred in this case, usually occur in older patients, being relatively uncommon among young patients with knowlesi malaria [11]. Over 100 cases of severe knowlesi malaria have now been reported [10–17, 37–44]; to our knowledge, only 6 cases have been reported in patients <30 years old [11, 13, 37], with only 2 of these having more than one severity criteria [13, 37]. Notably, the only previous report of multi-organ failure in a patient <30 years old occurred in a splenectomized patient [37]. It is therefore possible that in this 23-years old patient the lack of spleen contributed to her severe disease. Furthermore, direct inoculation of P. knowlesi-infected blood may have contributed to the development of severe disease; early studies from the neurosyphilis era demonstrated increased virulence of infection with serial blood passage through humans [45, 46], and more recently in a murine model parasite, virulence was found to be modified by vector transmission [47].
To reduce the risk of TTM in endemic countries the World Health Organization recommends several strategies, including donor selection and deferral, and/or screening of all donated blood for malaria parasites [48]. However, current screening strategies are associated with significant limitations. In Sabah, microscopy is used as the current screening policy, however it has poor sensitivity for low parasitaemias [29], and hence will fail to detect a significant proportion of sub-clinical infections, as occurred in the current case. Rapid diagnostic tests are also insufficiently sensitive, particularly for the diagnosis of knowlesi malaria [49, 50], and PCR, although sensitive, is limited by expense. Loop-mediated isothermal amplification (LAMP) assays have been developed recently and offer potential as a low–cost sensitive screening tool, but have been evaluated in only small numbers of patients [51–53]. Larger studies are required to assess the potential of this method for screening donated blood for malaria parasites.
An additional strategy proposed to prevent TTM in endemic countries is default malaria treatment of transfusion recipients [48], although the feasibility of this option is limited by the cost of artemisinin-based combination therapy. Moreover, in a country of low prevalence such as Sabah, this strategy would result in significant over-treatment of recipients. Recently, a whole blood pathogen reduction treatment was shown to reduce the incidence of TTM in addition to other transfusion-transmitted pathogens, demonstrating the potential of this technology to enhance safety of blood transfusions in malaria-endemic regions [54].
Conclusion
This case demonstrates that acquisition of P. knowlesi through blood transfusion may occur, and that clinical consequences can be severe. It is possible that thalassaemic and/or splenectomized individuals constitute a particular risk group for TTM, and may be at high risk of severe disease. With a rising incidence of knowlesi malaria, further studies are required to determine the prevalence of malaria among blood donors in Sabah, and the risk of TTM among recipients, in order to guide screening strategies for blood transfusion services in the region. Finally, clinicians should be aware of the risk of TTM in any patient having received a blood transfusion, with post-transfusion febrile illnesses appropriately investigated.
Abbreviations
- pkdhfr :
-
P. knowlesi bifunctional dihydrofolate reductase-thymidylate synthase gene
- LAMP:
-
loop-mediated isothermal amplification
- TTM:
-
transfusion transmitted malaria
- PCR:
-
polymerase chain reaction
References
Woolsey G. Transfusion for pernicious anaemia: two cases. Ann Surg. 1911;53:132.
O’Brien SF, Delage G, Seed CR, Pillonel J, Fabra CC, Davison K, et al. The epidemiology of imported malaria and transfusion policy in 5 nonendemic countries. Transfus Med Rev. 2015;29:162–71.
Centers for Disease Control and Prevention (CDC). Transfusion-transmitted malaria–Missouri and Pennsylvania, 1996–1998. MMWR Morb Mortal Wkly Rep. 1999;48:253–6.
Owusu-Ofori AK, Parry C, Bates I. Transfusion-transmitted malaria in countries where malaria is endemic: a review of the literature from sub-Saharan Africa. Clin Infect Dis. 2010;51:1192–8.
Batista-dos-Santos S, Raiol M, Santos S, Cunha MG, Ribeiro-dos-Santos A. Real-time PCR diagnosis of Plasmodium vivax among blood donors. Malar J. 2012;11:345.
Fernandes H, D’souza PF, D’souza PM. Prevalence of transfusion transmitted infections in voluntary and replacement donors. Indian J Hematol Blood Transfus. 2010;26:89–91.
Bruce-Chwatt LJ. Transfusion malaria revisited. Trop Dis Bull. 1982;79:827–40.
Mungai M, Tegtmeier G, Chamberland M, Parise M. Transfusion-transmitted malaria in the United States from 1963 through 1999. N Engl J Med. 2001;344:1973–8.
Traipattanakul J, Changpradub D, Trakulhun K, Phiboonbanakit D, Mungthin M. A first case of Plasmodium knowlesi malaria in Phramongkutklao Hospital. Journal of Infectious Diseases and Antimicrobial Agents. 2014;31:91–100.
Rajahram G, Barber B, William T, Grigg MJ, Menon J, Yeo TW, et al. Falling Plasmodium knowlesi malaria death rate among adults despite rising incidence, Sabah, Malaysia, 2010–2014. Emerg Infect Dis. 2016;22:41–8.
Barber BE, William T, Grigg MJ, Menon J, Auburn S, Marfurt J, et al. A prospective comparative study of knowlesi, falciparum and vivax malaria in Sabah, Malaysia: high proportion with severe disease from Plasmodium knowlesi and P. vivax but no mortality with early referral and artesunate therapy. Clin Infect Dis. 2013;56:383–97.
Rajahram G, Barber BE, William T, Menon J, Anstey NM, Yeo TW. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: association with reporting as P. malariae and delayed parenteral artesunate. Malar J. 2012;11:284.
William T, Menon J, Rajahram G, Chan L, Ma G, Donaldson S, et al. Severe Plasmodium knowlesi malaria in a tertiary hospital, Sabah, Malaysia. Emerg Infect Dis. 2011;17:1248–55.
Cox-Singh J, Davis TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, et al. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–71.
Cox-Singh J, Hiu J, Lucas SB, Divis PC, Zulkarnaen M, Chandran P, et al. Severe malaria-a case of fatal Plasmodium knowlesi infection with post-mortem findings. Malar J. 2010;9:10.
Daneshvar C, Davis TM, Cox-Singh J, Rafa’ee M, Zakaria S, Divis P, et al. Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis. 2009;49:852–60.
Willmann M, Ahmed A, Siner A, Wong I, Woon L, Singh B, et al. Laboratory markers of disease severity in Plasmodium knowlesi infection: a case control study. Malar J. 2012;11:363.
Rajahram GS, Barber BE, Tan WM, Yeo TW, William T. Case report: fatal Plasmodium knowlesi malaria following an atypical clinical presentation and delayed diagnosis. Med J Malaysia. 2013;68:71–2.
Milne LM, Kyi MS, Chiodini PL, Warhurst DC. Accuracy of routine laboratory diagnosis of malaria in the United Kingdom. J Clin Pathol. 1994;47:740–2.
Seed CR, Kitchen A, Davis TME. The current status and potential role of laboratory testing to prevent transfusion-transmitted malaria. Transfus Med Rev. 2005;19:229–40.
Anthony CN, Lau YL, Sum JS, Fong MY, Ariffin H, Zaw WL, et al. Malaysian child infected with Plasmodium vivax via blood transfusion: a case report. Malar J. 2013;12:308.
Knowles RM, Das Gupta B, Knowles R. A study of monkey-malaria and its experimental transmission to man. Indian Medical Gazette. 1932;67:246–9.
van Rooyen CE, Pile G. Observations on infection by Plasmodium knowlesi (ape malaria) in the treatment of general paralysis of the insane. BMJ. 1935;2:662–6.
Imwong M, Tanomsing N, Pukrittayakamee S, Day NPJ, White NJ, Snounou G. Spurious amplification of a Plasmodium vivax small-subunit RNA gene by use of primers currently used to detect P. knowlesi. J Clin Microbiol. 2009;47:4173–5.
Grigg MJ, Barber BE, Marfurt J, Imwong M, William T, Bird E, et al. Dihydrofolate-reductase mutations in Plasmodium knowlesi appear unrelated to selective drug pressure from putative human-to-human transmission in Sabah, Malaysia. PLoS One. 2016;11:e0149519.
Official Portal: Sarawak State Health Department [http://jknsarawak.moh.gov.my/bm/] Accessed 25th May 2016.
Yusof R, Lau Y, Mahmud R, Fong M, Jelip J, Ngian H, et al. High proportion of knowlesi malaria in recent malaria cases in Malaysia. Malar J. 2014;13:168.
Mungai M, Tegtmeier G, Chamberland M, Parise M. Transfusion-transmitted malaria in the United States from 1963 through 1999. N Engl J Med. 2001;344:1973–8.
Fornace KM, Nuin NA, Betson M, Grigg MJ, William T, Anstey NM, et al. Asymptomatic and submicroscopic carriage of Plasmodium knowlesi malaria in household and community members of clinical cases in Sabah, Malaysia. J Infect Dis. 2015;213:784–5.
Freimanis G, Sedegah M, Owusu-Ofori S, Kumar S, Allain JP. Investigating the prevalence of transfusion transmission of Plasmodium within a hyperendemic blood donation system. Transfusion (Paris). 2013;53:1429–41.
Rahav G, Volach V, Shapiro M, Rund D, Rachmilewitz EA, Goldfarb A. Severe infections in thalassaemic patients: prevalence and predisposing factors. Br J Haematol. 2006;133:667–74.
Modi C, Padalia U, Patil RC. A case study of possible relationship between post transfusion malaria and thalassaemia. Asian J Exp Sci. 2008;22:109–12.
O’Donnell A, Premawardhena A, Arambepola M, Samaranayake R, Allen SJ, Peto TE, et al. Interaction of malaria with a common form of severe thalassemia in an Asian population. Proc Natl Acad Sci USA. 2009;106:18716–21.
Choudhury NJ, Dubey ML, Jolly JG, Kalra A, Mahajan RC, Ganguly NK. Post-transfusion malaria in thalassaemia patients. Blut. 1990;61:314–6.
Ministry of Health. Management of transfusion dependent thalassemia. Malaysia: Putrajaya; 2009.
Demar M, Legrand E, Hommel D, Esterre P, Carme B. Plasmodium falciparum malaria in splenectomized patients: two case reports in French Guiana and a literature review. Am J Trop Med Hyg. 2004;71:290–3.
Boo YL, Lim HT, Chin PW, Lim SY, Hoo FK. A case of severe Plasmodium knowlesi in a splenectomized patient. Parasitol Int. 2016;65:55–7.
Lee CE, Adeeba K, Freigang G. Human Plasmodium knowlesi infections in Klang Valley, Peninsular Malaysia: a case series. Med J Malaysia. 2010;65:63–5.
Ninan T, Nalees K, Newin M, Sultan Q, Than MM, Shinde S, et al. Plasmodium knowlesi malaria infection in human. Brunei Int Med J. 2012;8:358–61.
Nakaviroj S, Kobasa T, Teeranaipong P, Putaporntip C, Jongwutiwes S. An autochthonous case of severe Plasmodium knowlesi malaria in Thailand. Am J Trop Med Hyg. 2015;92:569–72.
Lee W-C, Chin P-W, Lau Y-L, Chin L-C, Fong M-Y, Yap C-J, Supramaniam R, et al. Hyperparasitaemic human Plasmodium knowlesi infection with atypical morphology in peninsular Malaysia. Malar J. 2013;12:1.
van Hellemond J, Rutten M, Koelewijn R, Zeeman A, Verweij J, Wismans P, et al. Human Plasmodium knowlesi infection detected by rapid diagnostic tests for malaria. Emerg Infect Dis. 2009;15:1478.
Seilmaier M, Hartmann W, Beissner M, Fenzl T, Haller C, Guggemos W, et al. Severe Plasmodium knowlesi infection with multi-organ failure imported to Germany from Thailand/Myanmar. Malar J. 2014;13:422.
Orth H, Jensen B, Holtfreter M, Kocheril S, Mallach S, MacKenzie C, et al. Plasmodium knowlesi infection imported to Germany, January 2013. Euro Surveill. 2013;18:1–3.
Ciuca M, Chelarescu M, Sofletea A, Constantenescu P, Teriteanu E, Cortez P, et al. Contribution expérimentale a l’étude de l’immunité dans le paludisme. Bucarest: L’Academia; 1955.
Milam DF, Kusch E. Observations on Plasmodium knowlesi malaria in general paresis. South Med J. 1938;31:947–9.
Spence PJ, Jarra W, Levy P, Reid AJ, Chappell L, Brugat T, et al. Vector transmission regulates immune control of Plasmodium virulence. Nature. 2013;498:228–31.
WHO. Screening Donated Blood for Transfusion-Transmissible Infections. Geneva: World Health Organization; 2010.
Barber BE, William T, Grigg MJ, Piera K, Yeo TW, Anstey NM. Evaluation of the sensitivity of a pLDH-based and an aldolase-based rapid diagnostic test for the diagnosis of uncomplicated and severe malaria caused by PCR-confirmed Plasmodium knowlesi, Plasmodium falciparum and Plasmodium vivax. J Clin Microbiol. 2013;51:1118.
Grigg MJ, William T, Barber BE, Paramaswaran U, Bird E, Piera K, et al. Combining parasite lactate dehydrogenase-based rapid tests to improve specificity for the diagnosis of malaria due to Plasmodium knowlesi and other Plasmodium species in Sabah, Malaysia. J Clin Microbiol. 2014;52:2053–60.
Lau Y-L, Fong M-Y, Mahmud R, Chang P-Y, Palaeya V, Cheong F-W, et al. Specific, sensitive and rapid detection of human Plasmodium knowlesi infection by loop-mediated isothermal amplification (LAMP) in blood samples. Malar J. 2011;10:197.
Iseki H, Kawai S, Takahashi N, Hirai M, Tanabe K, Yokoyama N, et al. Evaluation of a loop-mediated isothermal amplification method as a tool for diagnosis of infection by the zoonotic simian malaria parasite Plasmodium knowlesi. J Clin Microbiol. 2010;48:2509.
Britton S, Cheng Q, Grigg MJ, William T, Anstey NM, McCarthy JS. A sensitive, colorimetric, high throughput, loop-mediated isothermal amplification (HtLAMP) assay for the detection of Plasmodium knowlesi. Am J Trop Med Hyg 2016; pii:15-0670 (Epub ahead of print).
Allain J-P, Owusu-Ofori AK, Assennato SM, Marschner S, Goodrich RP, Owusu-Ofori S. Effect of Plasmodium inactivation in whole blood on the incidence of blood transfusion-transmitted malaria in endemic regions: the African Investigation of the Mirasol System (AIMS) randomised controlled trial. Lancet. 2016;387:1753–61.
Authors’ contributions
EMB retrieved and reviewed the case notes and contacted the Blood Bank for additional information. EMB and BEB wrote the first draft of the article with assistance from NMA. TW, TMK, UP and EMB managed the patient. AA, JM and SA designed and performed the PCR assays, sequenced the dhfr gene, and analysed the molecular data. All authors reviewed final manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank the donor and the recipient for allowing publication of this case review; staff at Pitas District Hospital for assistance with obtaining medical records; staff at Pitas Blood Bank for provision of information and assistance with contacting the donor; staff at the Sabah State Public Health Laboratory for providing the donor’s EDTA blood sample; research staff Rita Wong, Thecla Tasius, Florena Maidik, and Kelly Nestor for assistance with the case review; the Director General of Health, Ministry of Health Malaysia, for permission to publish this report.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
All relevant data are included in this report.
Consent
Written informed consent was obtained from the patient and the donor for publication of this Case report.
Funding
This study was supported by the National Health Medical Research Council of Australia (Grant Number 10451516; Fellowships to NMA, TWY and BEB; and scholarship to MJG).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bird, E.M., Parameswaran, U., William, T. et al. Transfusion-transmitted severe Plasmodium knowlesi malaria in a splenectomized patient with beta-thalassaemia major in Sabah, Malaysia: a case report. Malar J 15, 357 (2016). https://doi.org/10.1186/s12936-016-1398-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-016-1398-z