Abstract
Background
Acute myeloid leukemia (AML) is a highly heterogeneous hematologic malignancy and the most frequently acute leukemia of stem cell precursors and the myeloid derivatives in adult. Longitudinal studies have indicated the therapeutic landscape and drug resistance for patients with AML are still intractable, which largely attribute to the deficiency of detailed information upon the pathogenesis.
Methods
In this study, we compared the cellular phenotype of resident NK cells (rAML-NKs, rHD-NKs) and expanded NK cells (eAML-NKs, eHD-NKs) from bone marrow of AML patients (AML) and healthy donors (HD). Then, we took advantage of the co-culture strategy for the evaluation of the in vitro cytotoxicity of NK cells upon diverse tumor cell lines (e.g., K562, Nalm6, U937). With the aid of RNA-sequencing (RNA-SEQ) and bioinformatics analyses (e.g., GOBP analysis, KEGG analysis, GSEA, volcano plot), we verified the similarities and differences of the omics features between eAML-NKs and eHD-NKs.
Results
Herein, we verified the sharp decline in the content of total resident NK cells (CD3−CD56+) in rAML-NKs compared to rHD-NKs. Differ from the expanded eHD-NKs, eAML-NKs revealed decline in diverse NK cell subsets (NKG2D+, CD25+, NKp44+, NKp46+) and alterations in cellular vitality but conservations in cytotoxicity. According to transcriptomic analysis, AML-NKs and HD-NKs showed multifaceted distinctions in gene expression profiling and genetic variations.
Conclusions
Collectively, our data revealed the variations in the cytobiological and transcriptomic features between AML-NKs and HD-NKs in bone marrow environment. Our findings would benefit the further development of novel biomarkers for AML diagnosis and NK cell-based cytotherapy in future.
Similar content being viewed by others
Background
Acute myeloid leukemia (AML) has been recognized as a heterogeneous hematological malignancy and the most common acute leukemia in adults, which is mainly caused by multifaceted somatic mutations in myeloid differentiation and monoclonal proliferation of immature progenitors [1, 2]. Despite the approval and application of novel drugs for patients, AML still remains as a major field of unmet medical need among diverse hematologic malignancies attributes to the deficiency of details information upon the cytopathologic and pathogenic features [3]. For decades, considerable progresses have been achieved in elucidating the pathogenesis of AML both at the cellular and molecular levels [4]. For instance, diverse inherited genetic loci and novel biomarkers (e.g., epigenetic, genetic, and protein) involved in myelodysplastic syndrome (MDS) and AML development in patients have been identified for the risk stratification and treatment assessment of AML patients on the basis of epigenetic and omics profiles [3, 5]. According to the World Health Organization (WHO) Classification of leukemia and myeloid neoplasms, AML can be divided into eight categories according to the recurrent genetic abnormalities and into three categories according to the indicated gene mutations. However, the genes with sufficient accuracy for unraveling the risk-stratification schemes and clinically targetable treatment decision-making of AML are still largely obscure [6].
Natural killer (NK) cells are critical innate lymphoid cells (ILCs) for their effect in tumor immunosurveillance and antiviral immunity dispense with presensitization, which have been involved in both innate and adoptive immune responses via the cytotoxic and cytokine-secreting approaches [7,8,9]. NK cells have been considered as heterogeneous populations generated from hematopoietic stem cells (HSCs) in the bone marrow environment (BME) with limited functional and phenotypic diversity, which are composed of two distinct subsets in human, including the cytotoxic CD3−CD56dimCD16high and the IFN-γ-producing CD3−CD56brightCD16+ counterparts [10, 11]. To date, adoptive NK cells and the concomitant chimeric antigen receptor-transduced NK cells (CAR-NKs) have been explored in various cancer settings including AML, and aiming to attain better therapeutic outcomes [12, 13]. For example, Terrén et al. and Albinger et al. reported the application of cytokine-induced memory-like (CIML) NK cells and primary CD33-targeting CAR-NK cells for the treatment of patients with AML, respectively [14, 15]. Furthermore, Soldierer et al. and Xu et al. highlighted the genetic engineering of human cells for the CAR-enhanced immunotherapy of hematological malignancies including AML [16, 17]. Very recently, Gauthier and the colleagues reported the successful control of AML by a trifunctional NKp46−CD16a−NK cell engager (NKCE) with prolonged pharmacodynamic effects and very low inflammatory cytokine induction against CD123 antibody-dependent cell cytotoxicity (ADCC) [18]. Of note, Crinier et al. showed the trajectories of NK cell differentiation from the resident CD56bright NK cells to the CD56dim NK1-like NK cells and the CD56bright NK2-like NK cells, yet the major cytophenotypic and omics features of resident and expanded NK cells in the bone marrow of AML patients (AML-NKs) are largely obscure.
For the purpose, in this study, we isolated resident NK cells (rNKs) from both AML patients (rAML-AMLs) and healthy donors (rHD-AMLs), and conducted ex vivo NK cell expansion and activation from the corresponding rNKs after a 14-day’s induction (eAML-AMLs, eHD-AMLs). With the aid of multifaceted cellular and molecular analyses, we verified the cytophernotic (e.g., cytomorphology, immunophenotyping, cellular vitality, and cytotoxicity against diverse tumor cell lines) and transcriptomic characteristics (e.g., gene expression profiling, genetic variations) between AML-NKs and HD-NKs in the bone marrow microenvironment, which collectively indicated the pathogenic effect of NK cells in patients with AML and would benefit the further development of novel biomarkers for clinical diagnosis and NK cell-based cytotherapy in future.
Methods
Preparation of mononuclear cells (MNCs) from bone marrow
Human bone marrow was obtained with the consent of AML patients and healthy donors (HD) and the approval of the Ethics Committee of Gansu Provincial Hospital and the guideline of Helsinki Declaration (2023-120). For MNCs isolation from bone marrow of AML patients (AML-MNCs) and HD (HD-MNCs), the Ficoll-based density gradient centrifugation were conducted as we described recently with several modifications [19, 20]. As sample collection, 3–5 ml fresh bone marrow in 10 HD (healthy donors satisfied the selection criteria for bone-marrow transplantation donation) and 9 AML patients before treatment (without receiving clinical treatment course and regimen) was collected by bone marrow aspiration in sterile anti-coagulant tubes with inverted mixing for 10 times. Then, the blood samples were immediately turned to MNC isolation as abovementioned according to the manufacturer’s instructions. The detail data of AML was available in Additional file 1: Table S1.
NK cell expansion and activation from MNCs
For ex vivo NK cell expansion and activation, 2 × 106/ml HD-MNCs or AML-MNCs were seeded in NK MACS basal Medium (Miltenyi Biotech, Germany) with the indicated recombinant human interleukin (rhIL) addition, including 1000U/ml rhIL-2, 10 ng/ml rhIL-15, and 50 ng/ml rhIL-18 [20]. The medium was half replaced every other day for 7 days, and then completely replaced every day for the other 7 days as we previously reported [19, 21]. The numbers of total NK cells (CD3−CD56+) were calculated based on Trypan Blue-based viable cell counting, and the proportions of total NK cells and the subsets (CD16+, NKG2A+, NKG2D+, NKp44+, NKp46+) were detected by flow cytometry (FCM) analysis at day 0 and day 14 of ex vivo NK cell induction. The list of the aforementioned cytokines was shown in Additional file 1: Table S2.
Flow cytometry (FCM) analysis
FCM analysis was performed as we recently reported [20, 22]. In brief, the fresh-enriched MNCs (day 0) and the MNC-derived cells (day 14) were harvested by centrifugation at 300×g for 5 min and resuspended by 1 × PBS (Solarbio, China) for twice. After that, the cells were incubated in 1 × PBS (Solarbio, China) with 2% fetal bovine serum (FBS) (Australia) and the fluorescence-conjugated antibodies such as anti-CD3-PE (BioLegend, USA), anti-CD3-APC (BioLegend, USA), anti-CD4-PE (BioLegend, USA), anti-CD8-PE-Cy7 (BioLegend, USA), anti-CD56-APC (BioLegend, USA), anti-CD16-FITC (BioLegend, USA), anti-CD25-FITC (BioLegend, USA), anti-NKG2D-perCP-Cy5.5 (BioLegend, USA), anti-NKp44-APC-Cy7, anti-NKp46-PE-Cy7 (BD Biosci, USA), anti-NKG2A-PE (BD Biosci, USA), anti-CD107a-PE (BD Biosci, USA), 7-AAD (BD Pharmigen), Propidium iodide (PI) (BD Pharmigen, USA) or Annexin V-FITC (Tianjin Sungene Biotech, China) in dark for 30 min. Finally, the cells were washed and turned to FACS Canto II (BD Biosci, USA) and FlowJo 10.0 software (Tree Star, USA) for analysis. The list of the indicated antibodies was available in Additional file 1: Table S3.
Cell cycle assessment of eHD-NKs and eAML-NKs
Cell cycle assessment of the MNCs (HD-MNCs, AML-MNCs) and the derivatives at the indicated time points was conducted as we reported [19, 23]. In brief, the cells were harvested and suspended in 1 × PBS (Solarbio, China), and then incubated with anti-CD3-PE and anti-CD56-APC antibodies for 20 min in dark. After that, the cells were incubated in 70% (v/v) ethanol (Thermo Fisher Scientific, USA) and fixed for 24 h, and followed by centrifugation at 1000×g for 5 min and resuspended with 1 × PBS (Solarbio, China) at 4 ℃ for twice. Then, the cells were incubated with PI staining solution (BD Pharmigen, USA) for 30 min at 37 ℃ and detected by BD LSR II (BD Biosci, USA) and the ModFit software (Verity Software House Co. Ltd, USA).
Cell apoptosis analysis of eHD-NKs and eAML-NKs
The proportions of apoptotic eHD-NKs and eAML-NKs were assessed with the Annexin V Apoptosis Detection Kit (Tianjin Sungene Biotech, China) according to the manufactures’ instructions as we reported before [19, 24]. In details, 1 × 106 cells at day 14 (eHD-NKs, eAML-NKs) were harvested and resuspended in precooled 1 × PBS (Solarbio, China). Then, the cells were respectively incubated in 100 μl 1 × Binding Buffer, Annexin V-FITC and 7-AAD solution (Tianjin Sungene Biotech, China) in dark. Finally, the proportions of apoptotic cells in eHD-NKs and eAML-NKs were detected by FACS Canto II (BD Biosci, USA) and analyzed by FlowJo 10.0 software (Tree Star, USA).
Cytotoxicity assessment of NK cells
The cytotoxicity of eHD-NKs and eAML-NKs was evaluated as we recently reported [20, 24]. For preparation, the indicated tumor cell lines (K562, human myeloid leukemia cell line; Nalm6, human lymphocytic leukemia cell line; U937, human myeloid leukemia cell line) were co-cultured in RPMI-1640 medium (Gibco, USA) supplemented with 10% FBS (Gibco, USA) at 37 ℃, 5% CO2 as we recently described [20, 25]. In brief, the indicated tumor cell lines were incubated with CellTrace Violet reagent (BV421 laser channel, Invitrogen, USA). Then, the tumor cells were solely (negative control, NC) or co-cultured with eHD-NKs or eAML-NKs at the effector-to-target ratio (E: T = 1: 3) for 8 h. After that, the cells were incubated with the indicated antibodies (anti-CD3-FITC, ant-CD56-APC, anti-CD-107-PE) (BioLegend, USA) and 7-AAD solution (Tianjin Sungene Biotech, China). Then, the cells were resuspended in 200 µl 1 × PBS (Solarbio, China) and 5 µl Precision Count Beads (BioLegend, USA). Finally, the percentage of the cells were detected by FACS Canto II (BD Biosci, USA) and analyzed by FlowJo 7.0 software (Tree Star, USA) as we described before [19, 21]. Cytotoxicity of eHD-NKs or eAML-NKs was calculated according to the formula: Cytotoxicity = (1 − N1/N0) × 100%. N0 and N1 represent the total number of living K562 cells in the negative control group and the experimental group, respectively [19, 20].
RNA-sequencing (RNA-SEQ) and bioinformatics analysis
Total mRNAs were harvested from the indicated eHD-NKs and eAML-NKs by utilizing the TRIZol reagent (ThermoFisher, USA) according to the manufactures’ instructions as we recently reported [19, 26, 27]. For RNA-SEQ analysis, the enriched mRNAs were turned to quality test and sent to BGI Genomics (Shenzhen, China) for sequencing. Multidimensional bioinformatics analyses of the RNA-SEQ data were accomplished with the online database and platforms, including Gene Set Enrichment Analysis (GSEA), Volcano Plot, HeatMap, Venn diagram, Principal Component Analysis (PCA), Gene Ontology (GO), and Kyoto Encyclopedia of Genes and Genomes (KEGG) as we previously described [22, 27, 28].
Statistical analysis
All data were analyzed with the aid of the GraphPad Prism 8.0 software (GraphPad Software Inc., USA) as we recently described [24, 28]. In brief, the data of two unpaired groups were turned to the Student's unpaired t test was utilized for analysis, whereas the data of multiple unpaired groups were analyzed by utilizing the one-way ANOVA method, respectively. All data were shown as mean ± standard error of mean (SEM) (N = 3 independent experiments). Significant statistical difference was considered only when P value was less than 0.05 (P < 0.05). *, P < 0.05; **, P < 0.001; ***, P < 0.001; ****, P < 0.0001; NS, not significant.
Results
The bone marrow of AML patients revealed sharp decline in resident NK cells and variations in the subsets
To verify the potential variations of NK cells between AML patients and HDs, we isolated MNCs from bone marrow and found that the percentage of total resident AML-NK cells (rAML-NKs) was sharply declined when compared to rHD-NKs (Fig. 1A, B). Furthermore, the proportion of total activated CD3−CD56+ NKs in rAML-NKs was also lower than that in the rHD-NKs group, whereas the NKp44+ subset revealed reverse tendency (Fig. 1C, D). Instead, minimal differences were observed in the content of relative NK cell subpopulations between rHD-NKs and rAML-NKs, including the activated CD25+, and NKG2D+ and NKp46+ subsets (Fig. 1C, D, Additional file 2: Fig. S1A, S1B). Additionally, AML-MNCs showed sharp decline in the proportion of total T cells (CD3+) and CD3+CD56+ NKT cells (Fig. 1E, Additional file 2: Fig. S1C). As to the indicated subsets of T cells, we noticed the diverse variations in contents in AML-MNCs compared to HD-MNCs, including the moderate decrease in CD3+CD8+ T cells, the moderate increase in CD4+FoxP3+ Treg cells, and the minimal differences in CD3+CD4+ T cells (Fig. 1E). Taken together, our data indicated the variations in the content of total rAM-NKs in bone marrow environment.
Comparison of the phenotype and components of rHD-NKs and rAML-NKs. A, B Representative FCM diagrams (A) and statistical analysis (B) of total (CD3−CD56+) NK cells in the rHD-NKs and rAML-NKs groups. C, D Representative FCM diagrams (C) and statistical analysis (D) of total activated (CD3−CD56+CD16+) NK cells and the subsets (NKG2D+, CD25+, NKp44+, NKp46+) in the rHD-NKs and rAML-NKs groups. E Statistical analysis of total CD3+CD56− T lymphocytes, CD3+CD4+ T cells, CD3+CD8+ T cells, CD4+FoxP3+ T cells and CD3+CD56+ NKT cells in the rHD-NKs and rAML-NKs groups. All data were shown as mean ± SEM (N = 3 independent experiments). *P < 0.05; **P < 0.01; ****P < 0.0001; NS: not significant
The expanded AML-NKs showed deficiency in ex vivo amplification and variations in subpopulations
To further dissect the cytophenotypic characteristics of HD-NKs and AML-NKs, we turned to our well-established “3ILs”-based strategy for ex vivo NK cell amplification and activation from HD-MNCs and AML-MNCs [19, 29]. Intuitively, we observed the decrease in cell aggregates and unicellular number in the AML-NKs group compared to the HD-NKs group at day 14 of the NK cell induction (Fig. 2A, B). With the aid of FCM analysis, we found that the percentage of total CD3−CD56+ NK cells in the expanded NK cells (eHD-NKs, eAML-NKs) were promoted substantially improved compared to the corresponding resident NK cells (rHD-NKs, rAML-NKs), whereas the eAML-NKs group showed moderate decline in total NK cells compared to the eHD-NKs group (Fig. 2C, D).
Comparison of the phenotype and components of eHD-NKs and eAML-NKs. A Phase contrast images of HD-NKs and AML-NKs at the indicated time points (day 0, 7, 14) during the 14-days’ in vitro amplification and activation. Scale bar = 100 μm. B Fold change of NK cells in the HD-NKs and AML-NKs groups. C, D Representative FCM diagrams of total (CD3−CD56+) NK cells in expanded NK cells (eHD-NKs, eAML-NKs) (C) and statistical analysis (D) of total (CD3−CD56+) NK cells in resident NK cells (rHD-NKs, rAML-NKs) and expanded NK cells (eHD-NKs, eAML-NKs). E, F FCM diagrams (E) and statistical analysis (F) of total activated NK cells (CD3−CD56+CD16+) and the subsets (NKG2D+, CD25+, NKp44+, NKp46+) in the indicated groups. All data were shown as mean ± SEM (N = 3 independent experiments). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; NS: not significant
Furthermore, we found the percentage of total activated CD3−CD56+ NK cells in the eAML-NKs group was equal to that in the eHD-NK group (Fig. 2E, F). As to the subsets of expanded NK cells, we noticed the significant decrease in the proportions of diverse subpopulations (NKG2D+, CD25+, NKp44+, NKp46+) in the eAML-NKs group, and in particular, the CD25+ cells typically associated with NK cell activation and cytotoxic function revealed sharp decline in eAML-NKs compared to eHD-NKs (Fig. 2E, F, Additional file 3: Fig. S2A). Collectively, these data showed the multidimensional deficiency in ex vivo amplification and activation of eAML-NKs compared to eHD-NKs.
eAML-NKs manifested diversity in cellular vitality and similarity in vitro cytotoxicity with eHD-NKs
Having verified the cellular phenotypes between HD-NKs and AML-NKs, we are aiming to verify the cellular vitality of the indicated NK cells. As shown by Fig. 3A, B, the percentage of 7-AAD− Annexin V+ apoptotic eAML-NKs was increased compared to that in eHD-NKs, whereas minimal differences were observed in the 7-AAD+Annexin V+ and Annexin V+ subsets (also see Additional file 3: Fig. S2B). According to the distributions of sub-stages of cell cycle, a higher percentage of CD3−CD56+ eAML-NKs stayed in G0/G1 stage, whereas a lower percentage of the cells located in the G2/M stage when compared with the eHD-NKs group (Fig. 3C, D).
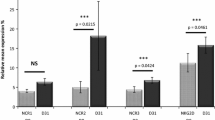
Comparison of the cellular vitality and cytotoxicity of eHD-NKs and eAML-NKs upon tumor cell lines. A, B FCM diagrams (A) and statistical analysis (B) of the apoptotic cells (7-AAD−Annexin V+, 7-AAD+Annexin V+, Annexin V+) in eHD-NKs and eAML-NKs. C, D FCM diagrams (C) and statistical analyses (D) of the distribution of the indicated sub-stages (G0/G1, S, G2/M) in cell cycle. E, F FCM diagrams (E) and statistical analysis (F) of CD107a+ NK cells in the Ctr group (monoculture) and the experimental groups cocultured with diverse tumor cell lines (K562, Nalm6, U937) at the effector-to-target ratio (E: T = 1:3). G, H Comparison of the ex vivo cytotoxicity of eHD-NKs and eAML-NKs against the indicated tumor cell lines (K562, Nalm6, U937) (E: T = 1:3). All data were shown as mean ± SEM (N = 3 independent experiments). All data were shown as mean ± SEM (N = 3 independent experiments). *P < 0.05; **P < 0.01; NS: not significant
To further explore the in vitro cytotoxic activity of eAML-NKs and eHD-NKs, we turned to ex vivo tumor-killing model by coculturing the aforementioned eNK cells with diverse targeted tumor cell lines, including K562 cells (human chronic myeloid leukemia cell line), Nalm6 cells (human B lympholeukemia cell line), and U937 cells (human histiocytic lymphoma cells). As shown by the FCM diagrams and statistical analysis, the percentages of eAML-NKs with CD107a expression at the effector-to-target ratio (E: T = 1:3) was comparable to those in the eHD-NKs groups (Fig. 3E, F). Meanwhile, we consistently observed the minimal differences between eAML-NKs and eHD-NKs in killing capacity against the indicated tumor cell lines based on the calculation of the residual living tumor cells (Fig. 3G, H). Taken together, these data indicated the diverse variations in cellular vitality and conservations in cytotoxicity between eAML-NKs and eHD-NKs.
The multifaceted variations in the landscape of gene expression profile between HD-NKs and AML-NKs
Having clarified the cytophenotypic characteristics, we next turned to dissect the similarities and differences between HD-NKs and AML-NKs at omics features. For the purpose, we took advantage of RNA-SEQ and multidimensional bioinformatics analyses, and found that HD-NKs and AML-NKs revealed variations in general gene expression pattern according to the box plot and violin plot of gene expression (Fig. 4A, B). With the aid of principal component analysis (PCA), we intuitively observed the distant genetic affiliation between the indicated groups, which was further confirmed by Pearson correlation analysis (Fig. 4C, D).
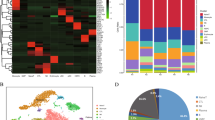
The landscape of gene expression pattern of eHD-NKs and eAML-NKs. A, B Box plot (A) and Violin plot (B) showed the distribution of genes expression pattern in eHD-NKs (HD-NK-1, HD-NK-2, HD-NK-3) and eAML-NKs (AML-NK-1, AML-NK-2, AML-NK-3). C, D Principal component analysis (PCA) and correlation analysis (D) of the aforementioned NK cells (eHD-NKs, eAML-NKs). E, F Volcano plot diagram (E) and bar chart (F) revealed the up-regulated (Up) and downregulated (Down) DEGs, together with the non DEGs (no-DEG) between eHD-NKs and eAML-NKs. G HeatMap diagram showed the hierarchical cluster pattern and GO terms of DEGs between the indicated groups (eHD-NKs, eAML-NKs). H, I Gene Ontology Biological Process (GOBP) analysis (H) and KEGG signaling pathway analysis (I) of the DEGs between eHD-NKs and eAML-NKs
Simultaneously, a cohort of upregulated (Up) and downregulated (Down) differentially expressed genes (DEGs) were enriched according to the log10 (Q value) of the indicated genes in HD-NKs and AML-NKs (Fig. 4E, F). In details, as shown by the hierarchical cluster analysis of HeatMap diagram, the DEGs were enriched in diverse gene ontology (GO) terms such as cargo receptor activity, translation regulator activity and molecular function regulator (Fig. 4G). Furthermore, by conducting GOBP analysis, we found the aforementioned DEGs were involved in biological processes, including positive regulation of cell migration, negative regulation of cell proliferation, cytokine-mediated signaling pathway, JAK-STAT cascade and inflammatory response (Fig. 4H). According to KEGG analysis, the DEGs between HD-NKs and AML-NKs mainly participated in pathways in cancer, cytokine-cytokine receptor interaction, immune response (e.g., Th17 cell differentiation, antigen processing and presentation, chemokine signaling pathway) (Fig. 4I). Taken together, AML-NKs revealed multifaceted variations with HD-NKs in gene expression profiling and especially the DEGs related with cellular vitality- and immune response-associated biological processes.
The variations in enriched gene sets and the spectrum of the genetic spectrum between HD-NKs and AML-NKs
To further illuminate the omics features of HD-NKs and AML-NKs, we turned to gene set enrichment analysis (GSEA) and noticed the specific enrichment of the significantly different gene sets associated with interferon-α response (P < 0.0023) and inflammatory response (P = 0.0299) rather than inflammatory response (P = 0.0530) (Fig. 5A). Meanwhile, gene sets involved in multiple biofunctions were also enriched, including heme metabolism, hypoxia and epithelial mesenchymal transition (EMT) (P < 0.0001) (Fig. 5B). As to those with signaling pathways, we observed the gene sets with significant differences between HD-NKs and AML-NKs were mainly related with IL6-JAK-STAT3 signaling and KRAS signaling rather than the P53 signaling or m TORC1 signaling instead (Fig. 5C).
Comparison of the gene sets and somatic variations between eHD-NKs and eAML-NKs. A–C Gene set enrichment analysis (GSEA) sowed the differentially expressed gene sets, including those involved in representative immune response (A), biological processes (B), and signaling pathways (C). D, E The distribution of genes with somatic variations, including the variable shear events (VSE) (D) and the differentially VSEs (DVSE) (E). F Circos diagrams revealed the distribution of genes with the indicated somatic variations in the chromosomes (SNPs, INDELs, and gene fusion events)
Subsequently, we compared the spectrum of genes with genetic modifications, we meticulously dissected the overview of the indicated variable shear events (VSE), and verified the conservations in the distribution of as_se, as_ri, as_mxe, as_a3ss, and as_a5ss between HD-NKs and AML-NKs (Fig. 5D). Of them, the as_se subset is the dominant differentially VSEs (DVSE) between HD-NKs and AML-NKs (Fig. 5E). In addition, according to the Circos diagrams, there were minimal differences between HD-NKs and AML-NKs in the loci regional distribution of the aforementioned somatic variations in the chromosomes, including SNPs, INDELs, and gene fusion events (Fig. 5F). Collectively, these data indicated the variations in diverse gene sets and conservations in genetic spectrum between HD-NKs and AML-NKs.
Discussion
As the most common malignant disease of myeloid precursors in adult, AML with various genetic abnormalities has caused great difficulty for accurate risk stratification and treatment intensity [30]. Despite the progress of novel agents for the treatment paradigm, yet the optimal regimens for AML patients remain absent due to the limited guidance and the deficiency of pathogenesis [31]. For the purpose, in this study, we prepared resident and expanded AML-NKs and HD-NKs, and verified the alterations in the content of total NK cells and the subpopulations, together with the cellular vitality and cytotoxic activity. Furthermore, by conducting omics analysis, we illuminated the gene expression spectrum and somatic variations between AML-NKs and HD-NKs. Collectively, our data would collectively supply new references for further dissecting the pathogenesis and clinical diagnosis of AML from the viewport of NK cells in bone marrow environment.
To overcome the poor prognosis of AML, we and other investigators in the field have devoted to verifying the pathogenesis. For instance, Gruszka et al. reported the hyperactivation of Wnt signaling cascades (e.g., β-catenin, phosphorylated-GSK3β) in haematopoietic stem/progenitor cells (HSPCs) in AML patients, and highlighted the necessity for the maintenance of leukemic stem cells (LSCs) and the prognostic value for tumor eradication [32]. Nilsson et al. verified the worse prognosis of therapy-related AML (t-AML) with mutated NPM1 over the relative subsets in cytogenetically intermediate- and adverse-risk [33]. Instead, our group illuminated the involvement of transcriptomic alterations and cellular vitality of bone marrow-derived mesenchymal stem/stromal cells (BM-MSCs), and in particular, the hyperactivation of JAK-STAT signaling pathway in the pathogenesis of AML [22]. In this study, we further put forward the potential pathogenicity of AML-NKs with multidimensional alterations in the cellular and omics characteristics, especially the sharp decline in the content of total NK cells (CD3−CD56+) and the activated CD25+ NK cell subset. In consistence, in this study, we also observed the variations in the aforementioned activated subsets of NK cells, together with the specific enrichment of gene sets involved in JAK-STAT cascade and inflammatory response (e.g., Treg, Th17, IL-6, IFN-α) in HD-NKs and AML-NKs, which collectively indicated the pathogenicity of JAK-STAT hyperactivation in AML. However, the systemic and detailed information of JAK-STAT signaling cascades in mediating AML-NK-related immunodysfunction and alterations in cellular viability (e.g., cell cycle, cell apoptosis) still need to be further illuminated. For instance, Wang and the colleagues reported the bone marrow NK (BM-NK) cell dysfunction by GARP-mediated TGF-β activation in AML patients, which indicated the pathogenicity of BM-NK cell-associated immunodysfunction in AML [34]. Interestingly, Haroun-Izquierdo et al. and Dong et al. respectively reported the adaptive single-KIR+NKG2C+ NK cells and CAR-transduced memory-like NK cells with potent missing-self reactivity upon HLA-mismatched AML and potent activity against NMP1 mutated AML, which collectively highlighted the feasibility of AML treatment by modulating specific NK cell subsets [35, 36]. Additionally, Paczulla et al. observed LSCs with absence of NKG2D ligands revealed immune evasion in AML, whereas Romee et al. and Parihar verified the therapeutic effect of memory NK cells for relapsed AML [37,38,39]. Therewith, it’s of interesting to further explore the biofunction and omics signatures of the aforementioned NK cell subsets for developing novel NK cell- and CAR-NK cell-based immunotherapy.
With the aid of transcriptomic analysis, we further observed that the DEGs between AML-NKs and HD-NKs were involved in immunoregulatory processes (e.g., IL-6 biosynthesis, immune response, antigen processing and presentation, graft-versus-host disease, inflammatory response, interferon response) and signaling cascades (e.g., IL6-JAK-STAT, KRAS signaling). Very recently, D'Silva et al. reviewed the implication of NK cell defects in AML progression and discussed the disease-associated mechanisms, and in particular the expression profiling of different cell surface markers of AML-NK cells [40]. The further exploration of the alterations in AML-NK cells would benefit our understanding upon pathogenesis and therapeutic regimens of AML from the aspect of numerical, receptor expression, and maturation defects of NK cells, along with checkpoint overexpression inhibitors and epigenetic modifications [40]. Of note, it’s of great interesting to further explore the potential variations of NK cells in bone marrow environment of AML patients before treatment and remission, which will help illuminate the biofunction and omics features of AML and benefit the development of targeted therapy in clinical practice.
State-of-the-art literatures have highlighted the involvement of dysimmunity in the pathogenesis and drug resistance of AML patients. For instance, Zhang et al. verified the multiple chemoresistant properties in LSCs and the oxidative phosphorylation (OXPHOS) signatures in pediatric AML [41]. Very recently, Naldini et al. reported the longitudinal single-cell profiling and functional validation of chemotherapy response in AML patients, and identified the variations in the stemness and quiescence signatures of the OxPhoslow miR-126high LSCs [42]. Of note, Abbas et al. outlined the T cell receptor repertoire profiling and the T cell landscape in AML patients with PD-1 blockade therapy [43]. Instead, we conducted systematic and detailed characterization of the multifaceted signatures of NK cells and the variations in subsets and gene expression pattern between resident and expanded AML-NKs and HD-NKs. To our knowledge, there were minimal reports upon the specific subsets (e.g., CD3−CD56+CD25+) of AML-NKs in AML patients. Similarly, Stringaris and the colleagues verified the upregulation of NKG2A (a inhibitory receptor) and the downregulation of NKp46 in NK cells of AML patients, which indicated the long-lasting changes and effector function impairment in AML-NK cells induced by AML blasts [44]. Interestingly, Crinier and the colleagues revealed the trajectories and the stress signature of NK cell differentiation in bone marrow, which further indicated the involvement of AML-NKs in the pathogenesis of AML [45]. Therefore, it is of great interesting to further dissect the single-cell mutation and gene expression profiling of clonal evolution as well as cellular hierarchy in AML patients, which will collectively benefit the development of accurate diagnosis and targeted therapy [46, 47].
Conclusion
Overall, in this study, we systematically and meticulously dissected the biological phenotypes, cellular vitality and cytotoxicity of both resident and expanded NK cells between AML-NKs and HD-NKs from the landscape of gene expression pattern and somatic variations. Our findings put forward the potential pathogenic role of AML-NK cells in bone marrow microenvironment (BME) in AML patients, which could be conducive to the further exploration of the pathogenesis of AML and the development of NK cell-based targeted therapy in future.
Availability of data and materials
All data of this study are included in the published article. Meanwhile, the datasets analyzed during this study are available from corresponding author upon reasonable request.
Abbreviations
- BM-NKs:
-
Natural killer cells from bone marrow
- MNCs:
-
Mononuclear cells
- GvHD:
-
Graft-versus-host disease
- HSC:
-
Hematopoietic stem cell
- PBS:
-
Phosphate buffer solution
- PCA:
-
Principal component analysis
- GSEA:
-
Gene set enrichment analysis
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- EMT:
-
Epithelial-mesenchymal transition
- NKCE:
-
NK cell engager
- ADCC:
-
Antibody-dependent cytotoxicity
- BME:
-
Bone marrow microenvironment
- VSEs:
-
Variable shear events
- DEGs:
-
Differentially expressed genes
- OXPHOS:
-
Oxidative phosphorylation
- BM-MSCs:
-
Bone marrow-derived mesenchymal stem/stromal cells
- t-AML:
-
Therapy-related AML
- LSCs:
-
Leukemic stem cells
- HSPCs:
-
Haematopoietic stem/progenitor cells
- ILCs:
-
Innate lymphoid cells
References
Saleh K, Khalifeh-Saleh N, Kourie HR. Acute myeloid leukemia transformed to a targetable disease. Future Oncol. 2020;16(14):961–72.
De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7): e441.
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–74.
Prada-Arismendy J, Arroyave JC, Rothlisberger S. Molecular biomarkers in acute myeloid leukemia. Blood Rev. 2017;31(1):63–76.
Li Y, Xu Q, Lv N, Wang L, Zhao H, Wang X, Guo J, Chen C, Li Y, Yu L. Clinical implications of genome-wide DNA methylation studies in acute myeloid leukemia. J Hematol Oncol. 2017;10(1):41.
Kayser S, Levis MJ. The clinical impact of the molecular landscape of acute myeloid leukemia. Haematologica. 2023;108(2):308–20.
Zhang C, Hu Y, Shi C. Targeting natural killer cells for tumor immunotherapy. Front Immunol. 2020;11:60.
Zhang L, Liu M, Yang S, Wang J, Feng X, Han Z. Natural killer cells: of-the-shelf cytotherapy for cancer immunosurveillance. Am J Cancer Res. 2021;11(4):1770–91.
Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19(1):120.
Crinier A, Narni-Mancinelli E, Ugolini S, Vivier E. SnapShot: natural killer cells. Cell. 2020;180(6):1280–1280.
Bjorkstrom NK, Ljunggren HG, Michaelsson J. Emerging insights into natural killer cells in human peripheral tissues. Nat Rev Immunol. 2016;16(5):310–20.
Rahmani S, Yazdanpanah N, Rezaei N. Natural killer cells and acute myeloid leukemia: promises and challenges. Cancer Immunol Immunother. 2022;71(12):2849–67.
Zhang L, Meng Y, Yao H, Zhan R, Chen S, Miao W, Ma S, Xu X, Li Y, Yu M, et al. CAR-NK cells for acute myeloid leukemia immunotherapy: past, present and future. Am J Cancer Res. 2023;13(11):5559–76.
Terren I, Orrantia A, Astarloa-Pando G, Amarilla-Irusta A, Zenarruzabeitia O, Borrego F. Cytokine-induced memory-like NK cells: from the basics to clinical applications. Front Immunol. 2022;13: 884648.
Albinger N, Pfeifer R, Nitsche M, Mertlitz S, Campe J, Stein K, Kreyenberg H, Schubert R, Quadflieg M, Schneider D, et al. Primary CD33-targeting CAR-NK cells for the treatment of acute myeloid leukemia. Blood Cancer J. 2022;12(4):61.
Soldierer M, Bister A, Haist C, Thivakaran A, Cengiz SC, Sendker S, Bartels N, Thomitzek A, Smorra D, Hejazi M, et al. Genetic of engineering and enrichment human NK cells for CAR-enhanced immunotherapy of hematological malignancies. Front Immunol. 2022;13: 847008.
Xu J, Niu T. Natural killer cell-based immunotherapy for acute myeloid leukemia. J Hematol Oncol. 2020;13(1):167.
Gauthier L, Virone-Oddos A, Beninga J, Rossi B, Nicolazzi C, Amara C, Blanchard-Alvarez A, Gourdin N, Courta J, Basset A, et al. Control of acute myeloid leukemia by a trifunctional NKp46-CD16a-NK cell engager targeting CD123. Nat Biotechnol. 2023;41(9):1296–306.
Xie B, Zhang L, Gao J, Wang T, Liu M, Feng X, Xu X, Ma S, Cai H, Guo T, et al. Decoding the biological properties and transcriptomic landscapes of human natural killer cells derived from bone marrow and umbilical cord blood. Am J Cancer Res. 2023;13(5):2087–103.
Liu M, Meng Y, Zhang L, Han Z, Feng X. High-efficient generation of natural killer cells from peripheral blood with preferable cell vitality and enhanced cytotoxicity by combination of IL-2, IL-15 and IL-18. Biochem Biophys Res Commun. 2021;534:149–56.
Zhang L, Yang S, Chen H, Xue C, Wang T, Chen S, Xu X, Ma S, Yu M, Guo T, et al. Characterization of the biological and transcriptomic signatures of natural killer cells derived from cord blood and peripheral blood. Am J Cancer Res. 2023;13(8):3531–46.
Zhang L, Chi Y, Wei Y, Zhang W, Wang F, Zhang L, Zou L, Song B, Zhao X, Han Z. Bone marrow-derived mesenchymal stem/stromal cells in patients with acute myeloid leukemia reveal transcriptome alterations and deficiency in cellular vitality. Stem Cell Res Ther. 2021;12(1):365.
Huo J, Zhang L, Ren X, Li C, Li X, Dong P, Zheng X, Huang J, Shao Y, Ge M, et al. Multifaceted characterization of the signatures and efficacy of mesenchymal stem/stromal cells in acquired aplastic anemia. Stem Cell Res Ther. 2020;11(1):59.
Zhang L, Liu M, Song B, Miao W, Zhan R, Yang S, Han Z, Cai H, Xu X, Zhao Y, et al. Decoding the multidimensional signatures of resident and expanded natural killer cells generated from perinatal blood. Am J Cancer Res. 2022;12(5):2132–45.
Zhang L, Zou L, Ma Y, Feng C, Zhan R, Yang H, Song B, Han Z. Multifaceted modifications for a cell size-based circulating tumor cell scope technique hold the prospect for large-scale application in general populations. Cell Biol Int. 2021;45(2):345–57.
Wei Y, Zhang L, Chi Y, Ren X, Gao Y, Song B, Li C, Han Z, Zhang L, Han Z. High-efficient generation of VCAM-1(+) mesenchymal stem cells with multidimensional superiorities in signatures and efficacy on aplastic anaemia mice. Cell Prolif. 2020;53(8): e12862.
Zhang L, Wei Y, Chi Y, Liu D, Yang S, Han Z, Li Z. Two-step generation of mesenchymal stem/stromal cells from human pluripotent stem cells with reinforced efficacy upon osteoarthritis rabbits by HA hydrogel. Cell Biosci. 2021;11(1):6.
Zhang L, Wang H, Liu C, Wu Q, Su P, Wu D, Guo J, Zhou W, Xu Y, Shi L, et al. MSX2 initiates and accelerates mesenchymal stem/stromal cell specification of hPSCs by regulating TWIST1 and PRAME. Stem Cell Rep. 2018;11(2):497–513.
Gao H, Liu M, Zhang Y, Zhang L, Xie B. Multifaceted characterization of the biological and transcriptomic signatures of natural killer cells derived from cord blood and placental blood. Cancer Cell Int. 2022;22(1):291.
Shiba N. Comprehensive molecular understanding of pediatric acute myeloid leukemia. Int J Hematol. 2023;117(2):173–81.
Ochs MA, Marini BL, Perissinotti AJ, Foucar CE, Pettit K, Burke P, Bixby DL, Benitez LL. Oncology stewardship in acute myeloid leukemia. Ann Hematol. 2022;101(8):1627–44.
Gruszka AM, Valli D, Alcalay M. Wnt signalling in acute myeloid leukaemia. Cells. 2019;8(11):1403.
Nilsson C, Linde F, Hulegardh E, Garelius H, Lazarevic V, Antunovic P, Cammenga J, Deneberg S, Eriksson A, Jadersten M, et al. Characterization of therapy-related acute myeloid leukemia: increasing incidence and prognostic implications. Haematologica. 2023;108(4):1015–25.
Wang D, Sun Z, Zhu X, Zheng X, Zhou Y, Lu Y, Yan P, Wang H, Liu H, Jin J, et al. GARP-mediated active TGF-beta1 induces bone marrow NK cell dysfunction in AML patients with early relapse post-allo-HSCT. Blood. 2022;140(26):2788–804.
Dong H, Ham JD, Hu G, Xie G, Vergara J, Liang Y, Ali A, Tarannum M, Donner H, Baginska J, et al. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc Natl Acad Sci U S A. 2022;119(25): e2122379119.
Haroun-Izquierdo A, Vincenti M, Netskar H, van Ooijen H, Zhang B, Bendzick L, Kanaya M, Momayyezi P, Li S, Wiiger MT, et al. Adaptive single-KIR(+)NKG2C(+) NK cells expanded from select superdonors show potent missing-self reactivity and efficiently control HLA-mismatched acute myeloid leukemia. J Immunother Cancer. 2022;10(11): e005577.
Parihar R. Memory NK cells to forget relapsed AML. Blood. 2022;139(11):1607–8.
Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357): 357ra123.
Paczulla AM, Rothfelder K, Raffel S, Konantz M, Steinbacher J, Wang H, Tandler C, Mbarga M, Schaefer T, Falcone M, et al. Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature. 2019;572(7768):254–9.
D’Silva SZ, Singh M, Pinto AS. NK cell defects: implication in acute myeloid leukemia. Front Immunol. 2023;14:1112059.
Zhang Y, Jiang S, He F, Tian Y, Hu H, Gao L, Zhang L, Chen A, Hu Y, Fan L, et al. Single-cell transcriptomics reveals multiple chemoresistant properties in leukemic stem and progenitor cells in pediatric AML. Genome Biol. 2023;24(1):199.
Naldini MM, Casirati G, Barcella M, Rancoita PMV, Cosentino A, Caserta C, Pavesi F, Zonari E, Desantis G, Gilioli D, et al. Longitudinal single-cell profiling of chemotherapy response in acute myeloid leukemia. Nat Commun. 2023;14(1):1285.
Abbas HA, Hao D, Tomczak K, Barrodia P, Im JS, Reville PK, Alaniz Z, Wang W, Wang R, Wang F, et al. Single cell T cell landscape and T cell receptor repertoire profiling of AML in context of PD-1 blockade therapy. Nat Commun. 2021;12(1):6071.
Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R, Pavlu J, Brisley G, de Lavallade H, Sarvaria A, et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica. 2014;99(5):836–47.
Crinier A, Dumas PY, Escaliere B, Piperoglou C, Gil L, Villacreces A, Vely F, Ivanovic Z, Milpied P, Narni-Mancinelli E, et al. Single-cell profiling reveals the trajectories of natural killer cell differentiation in bone marrow and a stress signature induced by acute myeloid leukemia. Cell Mol Immunol. 2021;18(5):1290–304.
Wu J, Xiao Y, Sun J, Sun H, Chen H, Zhu Y, Fu H, Yu C, Weigao E, Lai S, et al. A single-cell survey of cellular hierarchy in acute myeloid leukemia. J Hematol Oncol. 2020;13(1):128.
Miles LA, Bowman RL, Merlinsky TR, Csete IS, Ooi AT, Durruthy-Durruthy R, Bowman M, Famulare C, Patel MA, Mendez P, et al. Single-cell mutation analysis of clonal evolution in myeloid malignancies. Nature. 2020;587(7834):477–82.
Acknowledgements
The co-authors would like to thank the members in The Fourth People’s Hospital of Jinan (The Teaching Hospital of Shandong First Medical University), The Second Hospital of Shandong University, Peking University Cancer Hospital Yunnan & Yunnan Cancer Hospital & The Third Affiliated Hospital of Kunming Medical University, Chinese Academy of Medical Sciences and Peking Union Medical College, The First Affiliated Hospital of Soochow University, and Gansu Provincial Hospital for their assistance. We also thank Professor Lugui Qiu in Chinese Academy of Medical Sciences and Peking Union Medical College for scientific suggestions.
Funding
The work was supported by the project Youth Fund supported by the National Natural Science Foundation of China (82260031), SDU-KI Collaborative Research Project of Qilu Medical College, Shandong University (SDU-KI-2019-15), Natural Science Foundation of Shandong Province (ZR2023MH341), Fujian Provincial Ministerial Finance Special Project (2021XH018), Postdoctoral Program of Natural Science Foundation of Gansu Province (23JRRA1319), Joint Major Project of Science and Technology Innovation in Fujian Province (2021Y9083), Natural Science Foundation of Fujian Province (2022J01266), Natural Science Foundation of Jiangxi Province (20224BAB206077), Science and Technology Research and Development Project of Langfang City (2023013147), Natural Science Foundation of Gansu Province (21JR7RA594), Gansu Provincial Hospital Intra-Hospital Research Fund Project (22GSSYB-6), The 2022 Master/Doctor/Postdoctoral program of NHC Key Laboratory of Diagnosis and Therapy of Gastrointestinal Tumor (NHCDP2022004, NHCDP2022008), project funded by China Postdoctoral Science Foundation (2023M730723), project funded by Lugui Qiu Yunnan Expert Workstation (202105AF150051).
Author information
Authors and Affiliations
Contributions
Leisheng Zhang, Yunyan Sun, and Chun-e Xue: performed the experiments, collection and assembly of data, manuscript writing; Yunyan Sun, Chengyun Zheng, Shuling Wang, and Xianghong Xu: helped with collection or assembly of data; Leisheng Zhang, Cunrong Chen and Dexiao Kong: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted with the approval of the Ethics Committee of Gansu Provincial Hospital and the guideline of Helsinki Declaration (2023-120), and informed consent were obtained from the participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. The detail data of AML. Table S2. The cytokines used in this study. Table S3. Antibodies for flow cytometry assay in the study.
Additional file 2: Figure S1.
Representative FCS raw data files for rHD-NKs and rAML-NKs.
Additional file 3: Figure S2.
Antibody matching tables and representative FCS Raw Data files for cell apoptosis analysis of eHD-NKs and eAML-NKs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, L., Sun, Y., Xue, Ce. et al. Uncovering the cellular and omics characteristics of natural killer cells in the bone marrow microenvironment of patients with acute myeloid leukemia. Cancer Cell Int 24, 106 (2024). https://doi.org/10.1186/s12935-024-03300-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-024-03300-w