Abstract
Cancer is still the leading cause of death globally. The approval of the therapeutic use of monoclonal antibodies against immune checkpoint molecules, notably those that target the proteins PD-1 and PD-L1, has changed the landscape of cancer treatment. In particular, first-line PD-1/PD-L1 inhibitor drugs are increasingly common for the treatment of metastatic cancer, significantly prolonging patient survival. Despite the benefits brought by immune checkpoint inhibitors (ICIs)-based therapy, the majority of patients had their diseases worsen following a promising initial response. To increase the effectiveness of ICIs and advance our understanding of the mechanisms causing cancer resistance, it is crucial to find new, effective, and tolerable combination treatments. In this article, we addressed the potential of ICIs for the treatment of solid tumors and offer some insight into the molecular pathways behind therapeutic resistance to ICIs. We also discuss cutting-edge therapeutic methods for reactivating T-cell responsiveness after resistance has been established.
Similar content being viewed by others
Introduction
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies (mAbs) that target inhibitory checkpoint molecules expressed by cell membrane of antigen presenting cells (APCs) and CD4+ T cells [1, 2]. The development of ICIs has opened a new front in the fight against several types of cancers, including but not limited to melanoma, kidney, and lung cancer, and is expected to change the current conventional interventions for diverse cancers [3].
The activated T cells, B cells, and NK (natural killer) cells can all express PD-1, a protein that belongs to the immunoglobulin superfamily [4, 5]. Since PD-1 and CTLA-4 are expressed on the surface of activated T cells, both of them are recognized as essential regulators of the delicate balance between efficient T-lymphocyte activation and over activation of T-cell functions which may result in deleterious immunopathology [6].
A series of downstream targets are released by PD-1 in response to engagement with one of its ligands, programmed cell death-ligand 1 (PD-L1) or 2 (PD-L2), ultimately resulting in the inhibition of cytotoxic T lymphocytes (CTL) (Figs. 1 and 2). Halting CTL activities is seen as a double-edged sword because it can have both positive and negative consequences on the host immunological surveillance mechanisms. While regulation of CTL activity may operate as a brake to reduce the possibility of autoimmunity against host antigens, suppression of CTLs activation will be used by developing tumor cells to elude the host's immune surveillance, which will result in tumor progression [7].
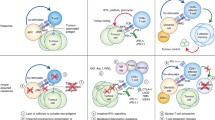
Immune Checkpoint Inhibitor against Tumor Cell. Through the interaction between PD-1 expressed on the surface of T cells and PD-L1 expressed on the surface of tumor cells, the immunological checkpoint prevents T-cell activation. Through contact between PD-1 on the surface of T cells and anti-PD-1 antibodies, T cell activation and immunological attack are enabled
The interaction between the TCR and the tumor-specific antigen shown in the context of MHC II results in T cell activation. Following the activation of a number of downstream targets that PD-1 releases in response to interaction with either of its ligands, programmed cell death-ligand 1 (PD-L1) or 2 (PD-L2), deactivation of T cells takes place, which ultimately leads to the suppression of cytotoxic T lymphocytes (CTL). While controlling CTL activity may work as a brake to lower the likelihood of autoimmunity against host antigens, inhibiting CTL activation will be employed by forming tumor cells to evade the host's immune surveillance, which will lead to the growth of the tumor. The interaction of ICIs mAbs to PD-1, PD-L1, PD-L2, and CTLA4 restores T cell activation and slows the growth of tumors
By employing ICI mAbs to stop the interaction between PD-1 and its ligands (PD-L1 and PD-L2), the PD-1/PD-L1-induced immunosuppression was reversed, this in turn, revived the cytotoxic functions of CTLs against tumor antigens, leading to inhibition of neoplastic growth [8].
The engagement of CTLA-4 with its ligand (CD80/86) may also result in immunosuppression against developing tumor cells, by mediating immune evasion and escape mechanism of tumor cells [9]. Utilizing ICIs to block inhibitory PD-1/PD-L1 and CTLA-4/CD80/86 signaling pathways improves the generation of efficient immune responses against cancer cells, revitalizes tumor antigen recognition, and ultimately leads to tumor death [10]. Tim-3 (T cell immunoglobulin and mucin 3), Lag-3 (lymphocyte activation gene 3), VISTA (programmed death-1 homolog), and Tigit are other immune checkpoint molecules/targets [11].
After the FDA's 2011 approval of the CTLA-4 inhibitor (ipilimumab), six additional ICIs have received FDA clearance [1]. Of those, three (nivolumab, pembrolizumab, and cemiplimab) are PD-1 inhibitors and three (PD-L1 inhibitors) (atezolizumab, avelumab, and durvalumab). Oncologists frequently use these ICIs in their routine treatment for about 15 different tumor types, and they have demonstrated impressive success. For key parameters for use of FDA approved PD-L1 testing for ICIs, the reader can refer to Wang et al. [12]. Beginning in 2014 and continuing into 2018, the FDA approved a number of ICIs targeting PD- 1, and anti-PD-L1 drugs (Table 1). An innovative PD-1 immune checkpoint inhibitor Cemiplimab [1]. The human programmed death receptor-1 (PD-1) monoclonal antibody cemiplimab (LIBTAYO®; cemiplimab-rwlc), which binds to PD-1 and prevents it from interacting with PD-L1 and PD-L2, is being developed by Regeneron Pharmaceuticals and Sanofi Genzyme. The drug was approved in the USA in September 2018 for the treatment of patients with metastatic cutaneous squamous cell carcinoma or locally advanced cutaneous squamous cell carcinoma who are not candidates for curative surgery or curative radiation. The drug is being studied as a treatment for a variety of cancers [13].
Melanoma, non-small cell lung cancer (NSCLC), and glioblastoma (GBM) were among the malignancies for which ICIs were initially licensed [14, 15]. In ovarian cancer patients, a subset of patients with advanced disease and high grade tumors that express PD-L1 may be effectively treated with anti-PD-1 ICI [16]. PD-1/PD-L1 given alone have unknown effects in triple-negative breast cancer (TNBC) patients [17]. The combined use of PD1/PD-L1 inhibitors with chemotherapy significantly boosted the pathologic complete response rates (CR) in TNBC patients, according to an analysis of 4,187 patients [18]. The growing introduction of ICIs into clinical practice is often constrained due to their side effects on immune system, and rarely identified glomerular disorders [19, 20].
Due to different types of resistance to ICIs (primary or intrinsic versus secondary or acquired), most cancer patients receiving ICIs in combination with chemotherapy experience disease progression and mortality [21]. Therefore, new alternative treatment are required to enhance long-term survival in these patients, both as a preventive action and in the event that ICI-based therapy fails [22].When a patient initially responds to ICI therapy for a brief period of time before displaying symptoms of clinical or radiologic disease progression, this is referred to as acquired resistance. Patients with primary resistance do not respond to ICI treatment at all and cancer advances quite rapidly [3, 22, 23].
In this review, we will tackle the potential application of ICIs therapy in various solid tumor types. A list of putative underlying molecular pathways associated to the establishment of ICIs resistance will also be provided. Moreover, we'll take a close look at the state-of-the-art of therapeutic strategies being developed to treat ICI-resistant cancers.
Potential of ICIs in the treatment of different cancer
ICIs have fundamentally altered how cancer is treated clinically. The percentage of patients who can benefit from ICIs is rather low, despite the fact that cancer immunotherapy has so far showed promise in a variety of cancers. Unavoidable issues include immune-related side effects and excessive expense. Hence, there is an urgent need for biomarkers that identify patients who will benefit from ICIs. One reasonable biomarker for predicting how well anti-PD1/PD-L1 immunotherapies will work is the expression of programmed cell death-ligand 1 (PD-L1). Yet, due to its variable definition, threshold, and spatial/temporal variability, its value is currently in question. Recently, it was revealed that certain gene mutations, neoantigen expression, mismatch repair status, deficient mismatch repair (dMMR), high levels of microsatellite instability (MSI-H) across the genome, "cold" vs "hot" and tumor mutational burden (TMB) may all serve as predictors of ICI treatment efficacy [24]. In Table 2 we summarized cancer types and features before diving into the individual cancer description including MSI/dMMR, "cold" vs "hot", clinical trials and outcomes/response, and approved commercial products.
Head and neck cancer
Table 1 provides a list of few ongoing clinical trials utilizing various ICIs types against various cancer types. Head and neck squamous cell carcinoma (HNSCC) is still the sixth most prevalent cancer worldwide. More than 830,000 new cases and 430,000 fatalities are included in annual reports [25]. More than half of patients with locally advanced HNSCC relapsed despite receiving routine point-of-care therapy [26]. Two monoclonal anti-PD-1 antibodies, nivolumab and pembrolizumab, are the first ICIs approved for the treatment of recurrent HNSCC [27]. Through the PD-1/PD-L1 pathway, these immunotherapeutic drugs suppress inhibitory signals to boost the cellular immune response induced by T cells [28]. Pembrolizumab was approved for patients whose tumors are PD-L1 positive, either alone or in conjunction with chemotherapy [29].
Anti-PD-1 drugs, the current standard of therapy, have changed how HNSCC is managed with chemotherapeutic and targeted therapies [30]. Overall response is still relatively mild despite the fact that anti-PD-1 antibodies are superior to chemotherapy in relation to halting tumor development and survival [31]. The effectiveness of durvalumab for treating HNSCC, either as a single therapy or when combined with the CTLA-4 inhibitor tremelimumab, compared to chemotherapy was studied in phase II and III clinical trials [32,33,34]. A very small percentage of patients in clinical trials looking at ICIs for HNSCC actually benefit from treatment, according to the data, highlighting the importance of patient selection before beginning immunotherapy. Predictive biomarkers are urgently needed to enable more informed therapy selection because not all patients respond to ICIs and others may exhibit more significant tumor responses if treated with chemotherapy or other therapies. One characteristic of a tumor that can predict response to ICI therapy in a variety of cancer types is its tumor mutational burden (TMB). In a pan-cancer investigation involving more than 1600 patients, increased TMB was linked to longer survival and higher ICI therapy response rates. The optimal predictive cut-point varied greatly by histology, indicating that there is unlikely to be a single tissue-neutral definition of high TMB that is useful for predicting ICI response, despite the fact that this effect was observed in the majority of cancer types, indicating that TMB underlies fundamental aspects of immune-mediated tumor rejection. To possibly develop a tissue-agnostic predictor of effectiveness from ICIs, more thorough prediction models combining TMB with additional parameters, such as genetic, immunologic, and clinic-pathologic indicators, will be required [35].
Therefore, there is an urgent need for a fuller knowledge of immune resistance mechanisms, which are likely influenced by the action mode of ICIs. A network meta-analysis (NMA) study compared anti-PD-1 and anti-PD-L1-based therapy for HNSCC patients, proving that there are no differences that are statistically significant between the two groups [27].
Lung cancer
ICIs can now be used in more situations without concurrent chemotherapy or targeted therapy. For NSCLC, ICIs may be utilized either as first-line or secondary treatment [36]. For NSCLC patients treated with PD-1 inhibitors as opposed to chemotherapy, 5-year overall survival (OS) rates ranged from 13 to 25% [47] in the second line and as high as 32% in the first line, according to multiple studies [37].
Validated criteria for long-term immunotherapy survival have so far demonstrated various degrees of accuracy. Although PD-L1 expression, tumor mutational burden (TMB), and interferon gamma (INFγ) are believed to be markers of response rates, these characteristics have not consistently applied to all tumor types, show a range of temporal and spatial variability, and are changeable with different types of therapy [38]. Recently, a variety of cancer types have been subjected to a more extensive evaluation of TMB, and it has been shown that this may not be a good predictor of prognosis [39]. Patients with high TMB who are not treated often have worse prognoses than individuals with low TMB, although the use of ICIs has changed this trend. Patients with non-small cell lung cancer (NSCLC) and melanoma who have higher TMB are more likely to benefit from ICIs than those who have lower TMB, according to numerous studies in particular [39]. Even so, some studies found no association between TMB and the survival of patients receiving ICIs, while others even found the opposite association [40]. Finding relevant predictive biomarkers could also be more challenging by the current propensity to combine ICIs with chemotherapy, targeted drugs, and/or other novel treatments. Numerous studies have highlighted key aspects of ICI-based survival outcomes. Extensive research covering tumor microenvironment (TME) studies, clinical surrogates, and disease mutation burden (TMB), and multi-omics data will be required to ensure the best use of ICIs and combination therapy. Overall survival (OS) for patients with metastatic disease significantly increased after the regulatory approval of PD-1 or PD-L1 inhibitors for NSCLC; currently, the majority of NSCLC patients receive PD-1/PD-L1 inhibitors as part of standard care, typically given as front-line therapy [41, 42]. Other studies showed that combinations of first-line immunotherapy, whether or not including chemotherapy, improved long-term survival, with a 4-year OS of 29% for nivolumab plus ipilimumab and a 2-year OS of 38% for nivolumab plus ipilimumab and chemotherapy [43, 44]. Despite the successful use of ICIs over the past decade in patients with NSCLC, lung cancer remains the most common cause of cancer mortality worldwide [45].
Melanoma
Skin cancer known as melanoma it is well known for having a relatively low survival rate and is caused by the misregulated growth of abnormal melanocytes [46]. Interferon therapy and chemotherapy are ineffective against melanoma, however, relatively recent research into the molecular basis of the disease resulted in novel therapeutic approaches: ICIs and targeted therapies. The first drug for the treatment of melanoma to receive FDA approval was ipilimumab, a CTLA-4 inhibitor. Pembrolizumab was authorized to treat metastatic melanoma just three years later [47]. Nivolumab was the third ICI to get worldwide approval in the same year (Jin et al. 2023).
After 5–10 years of treatment, only 22% of melanoma patients exhibited clinical benefit with ipilimumab, whereas 40–45% of patients with melanoma showed positive efficacy from PD-1 inhibitor therapy. Combination with PD-1 and CTLA-4 inhibitors was more successful than treatment with either drug alone. Consequently, the risks occasionally outweigh the benefits [48].
It was reported that a combination therapy using ICIs had a 5-year OS rate of 52% [49]. Even while ICIs greatly boosted melanoma patients' chances of survival, 40–65 percent of those taking PD-1 inhibitors and more than 70 percent of those taking CTLA-4 inhibitors did not exhibit positive response, primarily because of the emergence of resistance [50]. Additionally, one third of patients that initially had positive clinical outcomes, subsequently developed tumors and acquired drug resistance [51].
Renal cell carcinoma
The treatment of metastatic clear cell renal cell carcinoma (ccRCC) is immunotherapy-based [52].
Examples of broad strategies are ICIs and tyrosine kinase inhibitors (TKIs) that target the vascular endothelial growth factor receptor (VEGFR). Dual ICIs such as ipilimumab and nivolumab (IO/IO) are also exploited for treatment of ccRCC [66,67,68]. Several phase III clinical studies using TKI/IO regimens have reported objective response rates (ORRs) of 58–71% despite the fact that follow-up time is still insufficient to determine whether durable responses would be observed [53,54,55,56]. In contrast, ipilimumab and nivolumab had a 41% ORR, but nearly almost 50% of responders had responses lasting more than 4 years [57]. The best regimen for a given patient is unknown because these regimens have not been properly compared. Furthermore, it is not known if second-line TKI therapy can prolong survival in patients who fail to respond to IO/IO as a first line of treatment. Patients treated with the TKI/IO regimen and ipilimumab/nivolumab had equivalent 12-mo PFS and OS [58]. In cases of metastatic ccRCC after second-line therapy, there was no appreciable difference in PFS between patients receiving ipilimumab plus nivolumab and those receiving TKIs and ICIs [59].
Pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) still has a limited role in immunotherapy. According to reports, PDAC has an immunosuppressive TME and a low TMB, both of which pose challenges for pancreatic cancer immunotherapy [60]. For PDAC patients with microsatellite instability or mismatch repair deficiency (MSI-H/dMMR) who had metastatic or incurable disease, pembrolizumab was approved by FDA in May 2017 [61]. The FDA approval was based on the findings of five clinical trials that evaluated pembrolizumab in patients with incurable solid tumors who had received two prior lines of therapy. Out of 149 MSI-H/dMMR cancer patients studied over the course of the five studies, 59 responded, with an objective response rate (ORR) of 36.9% and a complete response rate (CR) of 7% [76,77,78]. The study by Le et al. reported in eight out of the 86 participants in the trial, the ORR was 62%.
Pembrolizumab was evaluated in a non-randomized, open-label fashion across many centers and cohorts. In 233 patients with 27 different tumor types, the ORR was 34.4%. Despite these positive pooled response rates to pembrolizumab in patients with MSI-H/dMMR cancer who had previously received treatment, the response rate in the subset of patients with MSI-H/dMMR pancreatic tumors was not as high. In the pancreatic cancer subgroup, the median OS was 4.0 months, but the median time to response was 13.4 months. It is difficult to extrapolate these results from a small number of patients with MSI-H/dMMR pancreatic cancers because the rate of mismatch repair deficiency in PDAC has been shown to range from 0.8 to 2% [62,63,64,65].
Long considered to be an immunologically "cold" cancer, PDAC has a number of factors that make it difficult for immunotherapy to be effective [66]. The logical next step is to try to overcome these obstacles by combining anti-PD-1/anti-PD-L1 checkpoint inhibitors with other immunological and targeted therapy (Fig. 3).
Blockade of CTLA-4 or PD-1 Signaling in Tumor Immunotherapy. Dendritic cells (DC) and naive T cells interact in the lymph node during the priming phase. The interaction between the TCR and the tumor-associated antigen shown in the context of MHC II constitutes the activation signals. The interaction between CD28 and B7 expressed on the surface of DC is one of the additional activation signals. The immune system attacks and eliminates tumor cells as a result of the effector phase, which takes place in the peripheral tissue. At this stage, PD-1 and PD-L1 inhibitory signals on T cells are suppressed, effectively activating T cells against the tumor antigen
In the first phase II randomized clinical study investigating dual immune checkpoint therapy with anti-PD-L1 antibody durvalumab with or without anti-CTLA-4 antibody tremelimumab, O'Reilly et al. observed unfavourable outcomes in patients with advanced PDAC [67]. Study participants had previously received a chemotherapy regimen based on fluorouracil or gemcitabine. The ORR was 3.1% for individuals who received durvalumab and tremelimumab together. Effective MSI-H/dMMR immunotherapy for PDAC continues to be elusive, and clinical research in this area is ongoing. Studies combining PD-1/PD-L1 suppression with other PDAC therapies are presently under progress [68].
Breast cancer
Breast cancer can currently be categorized into three primary subtypes based on the expression of the estrogen and progesterone receptors (ER and PR) and HER2 (also known as ERBB2): luminal ER positive and PR positive (further divided into luminal A and B), HER2 positive, and triple-negative breast cancer (TNBC) [69, 70]. Breast cancer has recently surpassed lung cancer as the most common type of cancer in the world, with an estimated 2.3 million new cases annually, or 11.7% of all cancer cases [45].
TNBC and HER2 breast cancer subtypes displayed increased tumor biomarker expression levels in response to immunotherapy, as well as improved immune infiltration and immunogenicity [71], 72]. Treatment with PD-1 checkpoint inhibitors showed good efficacy against these subtypes. For instance, the ORR to atezolizumab therapy for TNBC is 25%, while for pembrolizumab therapy for ER + in tamoxifen-taking patients is 4% [69].
Immunotherapy has several disadvantages when utilized to treat breast cancer due to its significant heterogeneity. Despite this, PD-1/PD-L1 inhibitors can still increase the T cell infiltrate in patients' TME when used in conjunction with other therapies. This prevents tumor immune escape and increases the anti-tumor effects of PD-1/PD-L1 inhibitors [73, 74].
Thirty-two of the 111 patients with metastatic TNBC who tested positive for PD-L1 expression had received weekly intravenous pembrolizumab 10 mg/kg. The median PFS was 1.9 months. One patient (3.7%) experienced complete remission (CR), four (14.8%) experienced partial remission (PR), and seven (24.9%) experienced stable status [75, 76].
Pembrolizumab is administered intravenously every two weeks in a phase II clinical trial to two cohorts of patients with metastatic TNBC: (A) an unselected population of advanced patients, and (B) a first-line cohort of PD-L1-positive tumors. The 170 patients in cohort A had a median OS of 9.0 months, a median PFS of 2.0 months, and an ORR of 5.7% for PD-L1 positive patients [77]. The median PFS, median OS, and ORR for the 84 patients in group B were 2.1 months, 18.0 months, and 21.4%, respectively [78]. The importance of PD-1/PD-L1 inhibitors in early therapy is demonstrated by the fact that various treatment regimens would dramatically impact the response rate of PD-L1-positive patients. For metastatic TNBC, PD-1/PD-L1 inhibitors in combination with other immunotherapies have demonstrated some promising therapeutic effects, such as the ability of atezolizumab and nab-paclitaxel to change patient prognosis. A number of targeted therapies (including radiotherapy, oncolytic virus therapy, CDK4/6 inhibitors, MEK inhibitors, AKT inhibitors, and vaccines) are combined with PD-1/PD-L1 inhibitors. Clinical prognosis improvements for people with metastatic TNBC are currently possible [17].
Prostate cancer
The second most common cancer in the world is prostate cancer (PCa) [79]. Radical prostatectomy or radiation therapy may be used to treat localized PCa, nonetheless, the outlook for advanced or metastatic PCa is dismal [80].
Recent studies have demonstrated excellent responses to ICIs and/or their combinations regimen in a subset of patients with high levels of PD-L1 expression in the tumor, CDK12 mutations, high levels of TMB, or high levels of microsatellite instability (MSI), and low levels of mismatch repair (dMMR). Therefore, to improve the management of this condition, immunotherapy remains a desirable therapeutic choice for prostate cancer and not only [81]. As far as PCa concerns, in a phase I trial, two of fourteen patients with metastatic castration-resistant prostate cancer (mCRPC) showed PSA declines of ≥ 50% after receiving a single intravenous dose of ipilimumab, and the drug was accepted well [82].
In a different Phase I trial using tremelimumab (a humanised anti-CTLA-4 antibody) and androgen deprivation using bicalutamide for recurrent prostate cancer, in three out of eleven patients, the PSA doubling time was prolonged [83].
Ipilimumab was given to 50 patients in a phase I/II research for those with metastatic CRPC (mCRPC), or in combination with radiation. Six patients had stable illness, one had a full response, and eight had PSA decreases of less than 50% [84].
In a phase 3 trial, radiation was followed by ipilimumab or a placebo for 799 individuals with mCRPC [85]. The OS between the ipilimumab and placebo groups did not differ statistically, nevertheless. Instead, PFS showed a statistically significant increase [86, 87].
Ipilimumab and nivolumab (anti-PD-1) were administered in combination to patients with mCRPC in a phase II clinical trial, which achieved a 25% ORR and was associated with substantial adverse effects [88]. Atezolizumab, avelumab, and durvalumab given alone or in combination regimen are additional treatment options for mCRPC [89]. Since prostate cancer exhibits multiple immunosuppressive characteristics associated with low TMB, low expression of PD-L1, and sparse T-cell infiltration, it has been referred to as an immunologically "cool" tumor. Nevertheless, for some people with prostate cancer, immunotherapy is still a viable option. High MSI/dMMR or CDK12 mutations in prostate cancer may make them more sensitive to ICIs in clinical settings [90].
Glioblastoma
Anti-PD-1/PD-L1 therapy for glioblastoma (GBM) has been shown to be both safe and effective in GBM mice models. Longer life times and a considerable reduction in tumor mass size has been observed. Clinical trials including patients with recurrent GBM are now testing PD-L1 [91]. Despite the fact that ICIs are effective against a number of cancers, the majority of glioblastoma (GBM) patients do not react to ICI therapy [92]. Clinical trials in phase 2/3 have not yet proven that the administration of PD-1 inhibitors to patients with GBM significantly improves overall survival (OS), either when combined with other treatments or when used alone currently considered to be standard of care [93]. Newly diagnosed O6-methylguanine-DNA-methyltransferase (MGMT) methylated GBM, nivolumab was given to upfront radiation and temozolomide in CheckMate 298 [94].
Recurrent GBM patients received either nivolumab or bevacizumab, and the results showed that bevacizumab had a longer PFS of 3.5 months compared to nivolumab and no difference in OS [95]. In a phase 2, there was an advantage in PFS from combination therapy of 4.1 months over pembrolizumab alone [96]. Recurrent GBM patients who received nivolumab together with either bevacizumab (10 mg/kg) or bevacizumab (3 mg/kg) every two weeks showed no change in PFS or OS [97].
Durvalumab’s effects alone or plus radiotherapy in GBM patients [98], or bevacizumab-refractory recurrent GBM have been disappointing. Despite these unpleasant results, there is still more to discover. A small group of GBM patients may benefit from ICI therapy, as evidenced by the fact that the median duration of response for the few nivolumab responders (7.8%) was statistically longer than the bevacizumab cohort (11.1 months versus 5.3 months) [95].
Cloughesy et al. discovered that neoadjuvant anti-PD-1 (pembrolizumab) therapy enhanced CD8 + T cell infiltrate and INFγ-related gene expression in the tumor of recurrent GBM [99]. Instead, a paucity of T cells but a significant infiltration of immunosuppressive CD68 + macrophages were discovered by De Groot et al. in patients' tumor tissue who had already received treatment, which may play a role in the emergence of resistance to anti-PD-1 therapy [100].
The blood–brain barrier (BBB), which will be covered in more depth below, makes treating GBM significantly harder than treating other solid tumors. The BBB creates a selectively permeable barrier across the majority of central nervous system (CNS) blood arteries in order to separate the tumors from therapeutic access [101]. Due to aberrant neovasculature and irregular blood flow, GBM also has a so-called blood–brain tumor barrier (BBTB), which affects the therapy of the tumor when medications are administered systemically and further prevents pharmaceuticals from leaving the circulation [101]. While creating a treatment for GBM, there are a few approaches to get around the BBB. The first step is to create a treatment that is better able to cross the BBB's endothelial cells. An agent's ability to create hydrogen bonds, polarity, or lipophilicity can all be decreased to achieve this [102]. Using the "Trojan horse" technique is a second way to get around the BBB [102]. In this technique, a substance that is typically incapable of entering the brain is coupled to a monoclonal antibody that is directed against one of the BBB's transcytosis receptors. The chemical can enter the brain undetected because the endothelial cell is prompted to permit entry by the binding of the monoclonal antibody to the receptor. Moreover, there has been some success in using nanotechnology to deliver treatments across the BBB. For instance, a medicinal substance can pass through the BBB and infiltrate the tumor when it is carried by a liposome containing an antibody that targets transferrin [103]. A therapeutic drug can be delivered to the brain using inorganic nanoparticles (IONPs) with an iron oxide core while also serving as an imaging agent for MRI. This enables the tracking of the delivery of therapeutic agents to the tumor itself [103]. Other studies have shown that certain peptides can be conjugated with therapeutic molecules to deliver treatment directly to the tumor while sparing the surrounding brain from damage, allowing these peptides to cross through the BBB and home to the tumor [104]. Radiation, electroporation, low intensity ultrasound, among other methods, can physically damage the BBB, allowing medicines to enter the brain [105]. Low-intensity pulsed ultrasound (LIPU) was utilised in a recent Phase I/IIa clinical experiment to damage the BBB and let a medication enter the brain. During the trial, patients had the SonoCloud-1 device implanted into their skull bones so that pulsed sonication could be administered. The research revealed that patients tolerated LIPU well and that carboplatin could penetrate the brain after being sonicated [46]. In a canine model, irreversible electroporation (IRE) has been demonstrated to break the BBB and eradicate tumor cells [106]. The IRE system's testing revealed some shortcomings that this technology has been updated to address; this improved technique is known as high-frequency irreversible electroporation (H-FIRE). Convection enhanced delivery (CED) also avoids the BBB and delivers medication directly to the tumor or to an area around it. ICIs will be better able to target the immunological checkpoint receptors that are expressed in the GBM tumor microenvironment and enable more effector cells to enter the tumor by creating therapies that are more suited to passing through the BBB, disrupting the BBB, or bypassing the BBB entirely. The delivery and concentration of ICI, which interacts with the tumor and may enhance treatment outcomes in GBM patients, could be more precisely controlled with the use of CED.
Mechanisms of ICIs resistance
ICIs have fundamentally changed how cancer is treated for many different tumor types, giving some patients a level of survival that was previously unattainable. However, despite many patients first responding favorably to ICIs, they frequently acquired resistance with time. The effective development of next-generation immunotherapies may be hampered by a lack of understanding of the processes driving acquired resistance to ICIs. The response rate of PD-1 inhibition in many diseases (including melanoma, Merkel cell carcinoma, Hodgkin's lymphoma, and MSI high malignancies) ranges from 40 to 70% in unselected patients [107]. Unfortunately, the majority of other recognized advanced cancers such as advanced non-small-cell lung cancer, advanced or metastatic urothelial cancer (mUC) and advanced renal-cell carcinoma only have response rates of 10% to 25% [108,109,110]. In conclusion, only a small percentage of them actually achieve the long-lasting response.
In contrast to acquired resistance, which typically describes patients who initially respond to therapy for a while before eventually experiencing clinical and/or radiologic disease progression, primary resistance typically describes patients who don't react at all and instead progress quickly or eventually with ICIs. In order to fight the issue of primary resistance, a significant amount of work has gone into designing combination approaches, typically with empiric complementary drugs. For instance, ICIs, multi-target TKIs, and EGFR inhibitors have all been used in conjunction with chemotherapy in the treatment of lung, breast, stomach, and renal cell carcinoma [111]. Additionally, a systematic search for biomarkers that can anticipate the initial ICI response has been conducted. PD-L1 expression, tumor mutational burden, and tumor infiltrating lymphocytes (TILs) have all been explored as potential predictors, and numerous more markers are now being investigated [112]. On the other hand, there haven't been any approved pharmacological advancements for preventing or reversing acquired resistance.
Clinical evidence and molecular mechanisms associated with acquired resistance to ICIs
Neoantigen-specific T cells may have a significant impact on how the body reacts to ICIs. Recently, it has been demonstrated that the long-term effect of ICIs blockage in NSCLC and melanoma correlates with somatic mutational and neoantigen density. Epigenetic suppression, immunological evasion, and clinical advancement may coexist with the absence of somatic mutations encoding putative tumor-specific neoantigens [113]. The absence of many neoantigen-specific T lymphocytes raises the possibility that pressure selection to eradicate these clones led to the development of acquired resistance [114]. No loss-of-function mutations in HLA genes such as B2M, JAK1 or JAK2 were found in a clinical study of 4 patients with NSCLC who had acquired resistance to ICIs (170). Exome analysis of pre vs post-treatment tissue, however, revealed deletion of a large number of mutations that were expected computationally to develop into neoantigens at the time resistance was developed.
Antigen-presenting cells' Major Histocompatibility Complexes (MHCs) are required for the activation of T-cell mediated immunity (Fig. 4). The coordinated expression of several genes is what allows MHC class I to deliver tumor antigens [115,116,117].
Combination Treatment of Pancreatic Cancer. In PDAC, the autophagy cargo receptor NBR1 directs an autophagy-dependent pathway that targets MHC-I molecules for lysosomal degradation. MHC-I is more frequently identified inside autophagosomes and lysosomes than on the cell surfaces of PDAC cells. Notably, restoring surface levels of MHC-I in syngeneic host mice results in improved antigen presentation, increased anti-tumor T cell responses, and inhibition of tumor growth. To enhance the anti-tumor immune response, dual ICI therapy (anti-PD1 and anti-CTLA4 antibodies) is used in conjunction with autophagy suppression, either genetically or pharmacologically with chloroquine
The beta-2-microglobulin (B2M) gene is required for both the stability of the MHC class 1 molecule at the cell surface and the facilitation of peptide loading (173). Loss of function mutations in B2M have previously been demonstrated to cause MHC I loss and serve as a biological pathway for tumor escape from immune detection [118].
One of the common findings in acquired resistance to ICIs has recently been identified as truncating changes in B2M. Using data from 4 melanoma patients who had developed immunotherapy resistance. One patient was found to have a homozygous acquired truncating B2M mutation, according to Zaretsky et al. [119]. In melanoma and other tumor forms, acquired defects in antigen presentation are observed. One patient exhibited B2M loss of heterozygosity (LOH) and two frameshift mutations, and another melanoma patient had two frameshifts B2M changes at the time of disease progression [120]. B2M changes were shown to be more prevalent in non-responders to anti-CTLA4 therapy [121].
In several studies of acquired resistance to immune checkpoints inhibitors in lung cancer, and MMR-d cancers were described homozygous deletion of B2M and alterations of B2M respectively [24]. Additionally, 4 of 9 patients and 3 of 9 patients, respectively, had considerably lower levels of the B2M protein and the MHC class 1 protein without any corresponding B2M molecular changes, according to Gettinger et al. [122]. Other unknown genomic or nongenomic variables may change MHC class 1 expression and affect resistance to ICIs under these circumstances (Fig. 4).
Role of IFN signaling in ICIs resistance
The JAK-STAT pathway, which regulates the expression of MHC class I and PD-L1 in tumor cells, is activated by the release of IFNγ from effector T cells. This signalling chain reaction may result in tumor cell death [123]. Both the JAK1 (JAK1 Q503*) and JAK2 genes have acquired loss-of-function mutations in two individuals who had side effects with ICIs after 1 and 2 years (JAK2 F547 splice-site mutation) [119].
Increased surface HLA class 1 and PD-L1 expression as well as considerable IFNγ pathway activation are seen in patients with JAK1/2 heterozygous mutations [124].
Genomics modifications in JAK1, JAK2, or IFNGR1 has been linked to primary immunotherapy resistance [125]. Ipilimumab, a medication that targets the CTLA-4 protein, was ineffective against melanomas that had deletions (copy number loss) of crucial IFNγ pathway genes including IRF1, IFT1/2, and amplifications (copy number increase) of IFNγ-related pathway inhibitors like SOCS1 and PIAS4 [125]. It is unclear how much other IFNγ pathway chromosomal aberrations besides JAK1 and JAK2 affect the development of acquired resistance to ICIs, and there are few clinical reports on the acquisition of these alterations.
Immunosuppression/exclusion caused by tumors
Loss of the tumor suppressor PTEN raises the expression of immunosuppressive cytokines and lowers T-cell effectors, which limits T-cell-driven infiltration and immunity. PTEN is crucial for regulating PI3K activity in preclinical models [126]. A patient with metastatic uterine leiomyosarcoma who had previously shown a virtually full response to pembrolizumab for more than 2 years exhibited PTEN deletion, according to a recent study [127]. Similarly, melanoma patients who had acquired resistance to immune therapies, reported PTEN loss [23]. The generation of immunosuppressive cytokines, modifications in dendritic cell priming, activation of regulatory T cells, and a lack of significant T cell infiltration in melanoma have all been associated with Wnt/-catenin pathway activity [128].
Other inhibitory checkpoints
When resistance is gained, the expression of TIM3, LAG3, and V-domain Ig suppressor of T cell activation (VISTA) increases, however it is unclear whether these modifications are directly linked to resistance [129]. Such checkpoints may occasionally be linked to T cell depletion and terminal malfunction, but in other contexts they may also be linked to T cell activation (Blank et al., 2019). Even with many of the research mentioned above, it can be challenging to confirm or identify a specific resistance mechanism. VISTA is a type I transmembrane protein. In particular for triple-negative breast cancer, VISTA is a potential immunological therapeutic target because to its association with immunotherapy resistance. It is found in regulatory T cells and myeloid-derived suppressor cells in large concentrations, and functional inhibition of it is proven to slow tumor growth [130]. It is still unclear how common acquired resistance to ICIs actually is because some authors deduce the resistance mechanism from circumstantial evidence.
Therapeutic strategies for disrupting acquired resistance
Several therapeutic strategies that target one or more of the major biological pathways, including the IFNγ pathway, other immunological checkpoints, the tumor microenvironment, and epigenetic modification, have been developed to combat acquired resistance to ICIs.
Numerous clinical trials focusing on JAK1/2 and STAT are currently being conducted. In a phase 1/2 research, advanced NSCLC patients received either osimertinib alone or in conjunction with the JAK1-selective inhibitor AZD4205 (NCT03450330). SC-43, a SHP-1 agonist that inhibits STAT3, is undergoing a phase 1/2 clinical trial for NSCLC when combined with cisplatin (NCT04733521).
The stimulation of IFN genes (STING) showed an increase in anti-tumor immunity through the production of proinflammatory chemokines and cytokines, including type I IFNs [131]. STING agonists like E7766, GSK3745417, and MIW815 are now undergoing clinical studies (NCT04144140, NCT03843359, and NCT03172936, respectively).
Patients with PD-L1 + NSCLC were enrolled in the phase 2 clinical study (CITYSCAPE) to compare the anti-TIGIT antibody tiragolumab with atezolizumab versus placebo plus atezolizumab. Overall response rates improved (Rodriguez-Abreu et al., 2020). Additional drugs that target the tumor microenvironment have been explored such as inhibitors of CSF1R, TGFβ, VEGF, IL-1/6, A2AR, CD73, IDO1, and B7-H4. DNA methylation and histone alterations are examples of epigenetic changes [132]. The enzyme DNA methyltransferase (DNMT), which controls the silence of genes and non-coding genomic regions, mediates DNA methylation. Histone modification enzymes like histone methyltransferase (HMT) and histone deacetylase alter the structure of chromatin, which affects how genes are regulated (HDAC) (Kanwal and Gupta, 2012). Immunotherapy resistance may be treated with epigenetic modification enzyme inhibitors, such as DNA methyltransferase inhibitors (DNMTis), histone methyltransferase inhibitors (HMTis), and histone deacetylase inhibitors (HDACis) [133].
According to preclinical research, HDACi and DNMTi both improve the responsiveness to anti-PD-1 therapy in a variety of malignancies [134]. Enhancer of zeste homolog 2 (EZH2), one of the histone methyltransferase enzymes, is associated to the expansion, migration, and invasion of malignant cells, such as glioblastoma, ovarian, and prostate cancer. Inhibiting EZH2 along with anti-CTLA-4 and IL-2 immunotherapy had silencing effects on antigen presentation and immune response [135].
The PD-1/PD-L1 pathway is not the only mechanism slowing down antitumor immunity in the majority of cancer patients, and inhibiting the PD-1/PD-L1 axis does not enough stimulate an efficient antitumor immune response. Certain combinations of treatments, such as -PD-1/PD-L1 plus radiotherapy, chemotherapy, angiogenesis inhibitors, targeted therapy, other immune checkpoint inhibitors, agonists of the co-stimulatory molecule, stimulators of interferon genes, faecal microbiota transplantation, epigenetic modulators, or metabolic modulators, have been shown to have superior antitumor efficacies and higher response rates. Moreover, -PD-1/PD-L1 moiety-containing bifunctional or bispecific antibodies also induced stronger antitumor activity. These combination techniques eliminate immunosuppressive brakes, promote numerous cancer-immunity cycle activities at once, and manipulate an immunosupportive tumor microenvironment. We outlined the synergistic antitumor efficacies and mechanisms of -PD-1/PD-L1 in this review when used in conjunction with other treatments [136].
Future perspective
Eventually, immunotherapy took a while to break through a wall of active cancer medications. In the past ten years, ICI have been developed and approved at an extraordinary rate for a number of cancer types. ICI has made great strides, yet the problem of cancer treatment remains. Immune-checkpoint immunotherapy has unlocked a door, but the case is still open. In the coming ten years, we want to identify pharmacodynamics characteristics and biomarkers for ICI efficacy and toxicity prediction in order to optimize ICI regimens and develop novel combinations.
Conclusions
Clinical research for the next generation of immunotherapies for patients with primary and acquired resistance is ongoing despite the lack of notable results. A deeper comprehension of the underlying biology may allow for more specific application of immunotherapies other than immune checkpoint inhibitors, leading to more effective therapeutic choices. The development of drugs and cellular therapies to prevent, avoid, or overcome ICI resistance will eventually be made possible by this advancement. In order to provide cancer patients a variety of therapeutic options, it is critical to understand the mechanisms underlying acquired resistance. In particular, the activation of the IFNγ pathway, inhibition of TGFβ, and co-suppression of immunological checkpoints like TIGIT have attracted interest as fascinating potential therapeutic strategies and are awaiting results.
Availability of data and materials
All data are included in the manuscript.
References
Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):1–3.
Bai R, Chen N, Li L, Du N, Bai L, Lv Z, et al. Mechanisms of cancer resistance to immunotherapy. Front Oncol. 2020;10:1290.
Peters S, Paz-Ares L, Herbst RS, Reck M. Addressing CPI resistance in NSCLC: targeting TAM receptors to modulate the tumor microenvironment and future prospects. J Immunother Cancer. 2022;10:7.
Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S. Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am Soc Clin Oncol Educ Book. 2019;39:147–64.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–61.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26(1):677–704.
Webb ES, Liu P, Baleeiro R, Lemoine NR, Yuan M, Wang Y. Immune checkpoint inhibitors in cancer therapy. J Biomed Res. 2018;32(5):317.
Seto T, Sam D, Pan M. Mechanisms of primary and secondary resistance to immune checkpoint inhibitors in cancer. Med Sci. 2019;7(2):14.
Mitsuiki N, Schwab C, Grimbacher B. What did we learn from CTLA-4 insufficiency on the human immune system? Immunol Rev. 2019;287(1):33–49.
Andrews LP, Yano H, Vignali DA. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat Immunol. 2019;20(11):1425–34.
Granier C, De Guillebon E, Blanc C, Roussel H, Badoual C, Colin E, et al. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open. 2017;2(2): e000213.
Wang Y, Tong Z, Zhang W, Zhang W, Buzdin A, Mu X, et al. FDA-approved and emerging next generation predictive biomarkers for immune checkpoint inhibitors in cancer patients. Front Oncol. 2021;11: 683419.
Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841–6.
Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29–39.
Hodi FS, Oday SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23.
Drakes ML, Mehrotra S, Aldulescu M, Potkul RK, Liu Y, Grisoli A, et al. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand- 1 (PD-L1) in ovarian cancer. J Ovarian Res. 2018;11(1):43.
He R, Yuan X, Chen Z, Zheng Y. Combined immunotherapy for metastatic triple-negative breast cancer based on PD-1/PD-L1 immune checkpoint blocking. Int Immunopharmacol. 2022;113: 109444.
Cui W, Luo Q, Sun SL, Xu JL, Gu H. Efficacy and safety of PD-1/PD-L1 inhibitors plus chemotherapy for triple-negative breast cancer: a systematic review and meta-analysis. Recenti Prog Med. 2018;12:67.
Gupta S, Short SA, Sise ME, Prosek JM, Madhavan SM, Soler MJ, et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9(10).
Kitchlu A, Jhaveri KD, Wadhwani S, Deshpande P, Harel Z, Kishibe T, et al. A systematic review of immune checkpoint inhibitor–associated glomerular disease. Kidney Int Rep. 2021;6(1):66–77.
Pérez-Ruiz E, Melero I, Kopecka J, Sarmento-Ribeiro AB, García-Aranda M, De Las RJ. Cancer immunotherapy resistance based on immune checkpoints inhibitors: Targets, biomarkers, and remedies. Drug Resist Updat. 2020;53: 100718.
Schoenfeld AJ, Antonia SJ, Awad MM, Felip E, Gainor J, Gettinger SN, et al. Clinical definition of acquired resistance to immunotherapy in patients with metastatic non-small-cell lung cancer. Ann Oncol. 2021;32(12):1597–607.
Schoenfeld AJ, Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. 2020;37(4):443–55.
Otoshi T, Nagano T, Tachihara M, Nishimura Y. Possible biomarkers for cancer immunotherapy Cancers. 2019;11(7):935.
Lang Y, Dong D. Cetuximab plus chemotherapy versus chemotherapy alone in recurrent or metastatic head and neck squamous cell carcinoma: a cost-effectiveness analysis. Cancer Manag Res. 2020;12:11383.
Price KA, Cohen EE. Current treatment options for metastatic head and neck cancer. Curr Treat Options Oncol. 2012;13(1):35–46.
Botticelli A, Cirillo A, Strigari L, Valentini F, Cerbelli B, Scagnoli S, et al. Anti–PD-1 and Anti–PD-L1 in Head and Neck Cancer: A Network Meta-Analysis. Front Immunol. 2021;12: 705096.
Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A, et al. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J Cell Physiol. 2019;234(10):16824–37.
Fashoyin-Aje L, Donoghue M, Chen H, He K, Veeraraghavan J, Goldberg KB, et al. FDA approval summary: pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist. 2019;24(1):103–9.
Cohen EE, Bell RB, Bifulco CB, Burtness B, Gillison ML, Harrington KJ, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J Immunother Cancer. 2019;7(1):1–31.
Yang B, Liu T, Qu Y, Liu H, Zheng SG, Cheng B, et al. Progresses and perspectives of anti-PD-1/PD-L1 antibody therapy in head and neck cancers. Front Oncol. 2018;8:563.
Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31(7):942–50.
Siu LL, Even C, Mesía R, Remenar E, Daste A, Delord JP, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1–low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 2019;5(2):195–203.
Seiwert TY, Weiss J, Baxi SS, Ahn MJ, Fayette J, Gillison ML, et al. A phase 3, randomized, open-label study of first-line durvalumab (MEDI4736)±tremelimumab versus standard of care (SoC; EXTREME regimen) in recurrent/metastatic (R/M) SCCHN: KESTREL. American Society of Clinical Oncology; 2016.
Lee M, Samstein RM, Valero C, Chan TA, Morris LG. Tumor mutational burden as a predictive biomarker for checkpoint inhibitor immunotherapy. Hum Vaccines Immunother. 2020;16(1):112–5.
Mamdani H, Matosevic S, Khalid AB, Durm G, Jalal SI. Immunotherapy in lung cancer: Current landscape and future directions. Front Immunol. 2022;13: 823618.
Xu Y, Wan B, Chen X, Zhan P, Zhao Y, Zhang T, et al. The association of PD-L1 expression with the efficacy of anti-PD-1/PD-L1 immunotherapy and survival of non-small cell lung cancer patients: a meta-analysis of randomized controlled trials. Transl Lung Cancer Res. 2019;8(4):413.
Rossi G, Russo A, Tagliamento M, Tuzi A, Nigro O, Vallome G, et al. Precision medicine for NSCLC in the era of immunotherapy: new biomarkers to select the most suitable treatment or the most suitable patient. Cancers. 2020;12(5):1125.
Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–6.
Chae YK, Davis AA, Agte S, Pan A, Simon NI, Iams WT, et al. Clinical implications of circulating tumor DNA tumor mutational burden (ctDNA TMB) in non-small cell lung cancer. Oncologist. 2019;24(6):820–8.
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92.
Planchard D, Popat ST, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:192–237.
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211.
Reck M, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open. 2021;6(5): 100273.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Basurto-Lozada P, Molina-Aguilar C, Castaneda-Garcia C, Vázquez-Cruz ME, Garcia-Salinas OI, Álvarez-Cano A, et al. Acral lentiginous melanoma: Basic facts, biological characteristics and research perspectives of an understudied disease. Pigment Cell Melanoma Res. 2021;34(1):59–71.
Franklin C, Livingstone E, Roesch A, Schilling B, Schadendorf D. Immunotherapy in melanoma: recent advances and future directions. Eur J Surg Oncol EJSO. 2017;43(3):604–11.
Yun S, Vincelette ND, Green MR, Wahner Hendrickson AE, Abraham I. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: a systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Med. 2016;5(7):1481–91.
Fujisawa Y, Ito T, Kato H, Irie H, Kaji T, Maekawa T, et al. Outcome of combination therapy using BRAF and MEK inhibitors among Asian patients with advanced melanoma: an analysis of 112 cases. Eur J Cancer. 2021;145:210–20.
Zeng N, Ma L, Cheng Y, Xia Q, Li Y, Chen Y, et al. Construction of a ferroptosis-related gene signature for predicting survival and immune microenvironment in melanoma patients. Int J Gen Med. 2021;64:23–38.
Yardley DA. Drug resistance and the role of combination chemotherapy in improving patient outcomes. Int J Breast Cancer. 2013;2013:1–15.
Fitzgerald KN, Lee CH. Personalizing First-Line Management of Metastatic Renal Cell Carcinoma: Leveraging Current and Novel Therapeutic Options. J Natl Compr Canc Netw. 2022;1:1–9.
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829–41.
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289–300.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–15.
Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21(12):1563–73.
Albiges L, Tannir NM, Burotto M, McDermott D, Plimack ER, Barthélémy P, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6): e001079.
Fitzgerald KN, Duzgol C, Knezevic A, Shapnik N, Kotecha R, Aggen DH, et al. Progression-free survival after second line of therapy for metastatic clear cell renal cell carcinoma in patients treated with first-line immunotherapy combinations. Eur Urol. 2023;83(3):195–9.
Izumi K, Inoue M, Washino S, Shirotake S, Kagawa M, Takeshita H, et al. Clinical outcomes of nivolumab plus ipilimumab in patients with metastatic non-clear cell renal cell carcinoma: Real-world data from a Japanese multicenter retrospective study. Int J Urol. 2022. https://doi.org/10.1111/iju.15128.
Yang J, Zeng L, Chen R, Huang L, Wu Z, Yu M, et al. Leveraging tumor microenvironment infiltration in pancreatic cancer to identify gene signatures related to prognosis and immunotherapy response. Cancers. 2023;15(5):1442.
Krantz BA, O’Reilly EM. How I Treat Metastatic Pancreatic Cancer With Chemotherapy. In: Handbook of Gastrointestinal Cancers: Evidence-Based Treatment and Multidisciplinary Patient Care. 2019. p. 4.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–13.
Hu ZI, Shia J, Stadler ZK, Varghese AM, Capanu M, Salo-Mullen E, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations evaluating mismatch repair deficiency in pancreatic cancer. Clin Cancer Res. 2018;24(6):1326–36.
Humphris JL, Patch AM, Nones K, Bailey PJ, Johns AL, McKay S, et al. Hypermutation in pancreatic cancer. Gastroenterology. 2017;152(1):68–74.
Lupinacci RM, Goloudina A, Buhard O, Bachet JB, Maréchal R, Demetter P, et al. Prevalence of microsatellite instability in intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2018;154(4):1061–5.
Balachandran VP, Beatty GL, Dougan SK. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology. 2019;156(7):2056–72.
O’Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(10):1431–8.
Zhang X, Lao M, Xu J, Duan Y, Yang H, Li M, et al. Combination cancer immunotherapy targeting TNFR2 and PD-1/PD-L1 signaling reduces immunosuppressive effects in the microenvironment of pancreatic tumors. J Immunother Cancer. 2022;10:3.
Majidpoor J, Mortezaee K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin Immunol. 2021;226: 108707.
Denkert C, Liedtke C, Tutt A, von Minckwitz G. Molecular alterations in triple-negative breast cancer—the road to new treatment strategies. The Lancet. 2017;389(10087):2430–42.
Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117.
Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–87.
Stapleton S, Jaffray D, Milosevic M. Radiation effects on the tumor microenvironment: Implications for nanomedicine delivery. Adv Drug Deliv Rev. 2017;109:119–30.
Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. 2016;44(2):343–54.
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460.
Nanda R, Specht J, Dees C, Berger R, Gupta S, Geva R, et al. Abstract P6–10–03: KEYNOTE-012: long-lasting responses in a phase Ib study of pembrolizumab for metastatic triple-negative breast cancer (mTNBC). Cancer Res. 2017;77(4):P6.
Uliano J, Nicolò E, Corvaja C, Taurelli Salimbeni B, Trapani D, Curigliano G. Combination immunotherapy strategies for triple-negative breast cancer: current progress and barriers within the pharmacological landscape. Expert Rev Clin Pharmacol. 2022;15(12):1399–413.
Adams S, Loi S, Toppmeyer D, Cescon D, De Laurentiis M, Nanda R, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):405–11.
Zhong W, Shen Z, Wu Y, Mao X, Kong J, Wu W. Knowledge mapping and current trends of immunotherapy for prostate cancer: A bibliometric study. Front Immunol. 2022;6512.
Moul JW. The evolving definition of advanced prostate cancer. Rev Urol. 2004;6(Suppl 8):S10.
Fellner C. Ipilimumab (yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its use. Pharm Ther. 2012;37(9):503.
Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13(6):1810–5.
McNeel DG, Smith HA, Eickhoff JC, Lang JM, Staab MJ, Wilding G, et al. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother. 2012;61(7):1137–47.
Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7):1813–21.
Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, Van den Eertwegh AJ, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–12.
Gao J, Ward JF, Pettaway CA, Shi LZ, Subudhi SK, Vence LM, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med. 2017;23(5):551–5.
Sharma P, Pachynski RK, Narayan V, Fléchon A, Gravis G, Galsky MD, et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the CheckMate 650 trial. Cancer Cell. 2020;38(4):489–99.
Caruso C. Anti–PD-1–CTLA4 Combo Hits Prostate Cancer. AACR; 2019.
Taghizadeh H, Marhold M, Tomasich E, Udovica S, Merchant A, Krainer M. Immune checkpoint inhibitors in mCRPC-rationales, challenges and perspectives. Oncoimmunology. 2019;8(11): e1644109.
Wang I, Song L, Wang BY, Kalebasty AR, Uchio E, Zi X. Prostate cancer immunotherapy: a review of recent advancements with novel treatment methods and efficacy. Am J Clin Exp Urol. 2022;10(4):210.
Litak J, Mazurek M, Grochowski C, Kamieniak P, Roliński J. PD-L1/PD-1 axis in glioblastoma multiforme. Int J Mol Sci. 2019;20(21):5347.
Garg AD, Vandenberk L, Van Woensel M, Belmans J, Schaaf M, Boon L, et al. Preclinical efficacy of immune-checkpoint monotherapy does not recapitulate corresponding biomarkers-based clinical predictions in glioblastoma. Oncoimmunology. 2017;6(4): e1295903.
Duerinck J, Schwarze JK, Awada G, Tijtgat J, Vaeyens F, Bertels C, et al. Intracerebral administration of CTLA-4 and PD-1 immune checkpoint blocking monoclonal antibodies in patients with recurrent glioblastoma: a phase I clinical trial. J Immunother Cancer. 2021;9(6).
Corso CD, Bindra RS. Success and failures of combined modalities in glioblastoma multiforme: old problems and new directions. New York: Elsevier; 2016. p. 281–98.
Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(7):1003–10.
Nayak L, Molinaro AM, Peters K, Clarke JL, Jordan JT, de Groot J, et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent GlioblastomaPembrolizumab±Bevacizumab for Recurrent GBM. Clin Cancer Res. 2021;27(4):1048–57.
Sahebjam S, Forsyth PA, Tran ND, Arrington JA, Macaulay R, Etame AB, et al. Hypofractionated stereotactic re-irradiation with pembrolizumab and bevacizumab in patients with recurrent high-grade gliomas: results from a phase I study. Neuro-Oncol. 2021;23(4):677–86.
McAleavey PG, Walls GM, Chalmers AJ. Radiotherapy-drug combinations in the treatment of glioblastoma: a brief review. CNS Oncol. 2022;11(02):CNS86.
Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med. 2019;25(3):477–86.
De Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro-Oncol. 2010;12(3):233–42.
Debinski W, Priebe W, Tatter S. Maximizing local access to therapeutic deliveries in glioblastoma. Part I: targeted cytotoxic therapy. Exon Publ. 2017. p. 341–58.
Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13(6):1663–74.
Michael JS, Lee BS, Zhang M, John SY. Nanotechnology for treatment of glioblastoma multiforme. J Transl Intern Med. 2018;6(3):128–33.
Raucher D. Tumor targeting peptides: novel therapeutic strategies in glioblastoma. Curr Opin Pharmacol. 2019;47:14–9.
Brem H, Piantadosi S, Burger P, Walker M, Selker R, Vick N, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Lancet. 1995;345(8956):1008–12.
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123–35.
Sunshine J, Taube JM. Pd-1/pd-l1 inhibitors. Curr Opin Pharmacol. 2015;23:32–8.
Sacher AG, Gandhi L. Biomarkers for the clinical use of PD-1/PD-L1 inhibitors in non–small-cell lung cancer: a review. JAMA Oncol. 2016;2(9):1217–22.
Roviello G, Catalano M, Nobili S, Santi R, Mini E, Nesi G. Focus on biochemical and clinical predictors of response to immune checkpoint inhibitors in metastatic urothelial carcinoma: Where do we stand? Int J Mol Sci. 2020;21(21):7935.
Yin X, Wang Z, Wang J, Xu Y, Kong W, Zhang J. Development of a novel gene signature to predict prognosis and response to PD-1 blockade in clear cell renal cell carcinoma. Oncoimmunology. 2021;10(1):1933332.
Han B, Zhang B, Zhong H, Shi C, Gao Z, Zhong R, et al. 134P Safety and efficacy of multi-target TKI combined with nivolumab in checkpoint inhibitor-refractory advanced NSCLC patients: A prospective, single arm, two stage study. Immuno-Oncol Technol. 2022;16:7.
Shi Y, Lei Y, Liu L, Zhang S, Wang W, Zhao J, et al. Integration of comprehensive genomic profiling, tumor mutational burden, and PD-L1 expression to identify novel biomarkers of immunotherapy in non-small cell lung cancer. Cancer Med. 2021;10(7):2216–31.
Wang Q, Wu X. Primary and acquired resistance to PD-1/PD-L1 blockade in cancer treatment. Int Immunopharmacol. 2017;46:210–9.
Restifo NP, Smyth MJ, Snyder A. Acquired resistance to immunotherapy and future challenges. Nat Rev Cancer. 2016;16(2):121–6.
Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975.
Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443.
Hulpke S, Tampé R. The MHC I loading complex: a multitasking machinery in adaptive immunity. Trends Biochem Sci. 2013;38(8):412–20.
Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional beta2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. JNCI J Natl Cancer Inst. 1996;88(2):100–8.
Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–29.
Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017;8(1):1136.
Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017;8(1):1–11.
Gettinger S, Choi J, Mani N, Sanmamed M, Datar I, Sowell R, et al. A dormant TIL phenotype defines non-small cell lung carcinomas sensitive to immune checkpoint blockers. Nat Commun. 2018;9(1):3196.
Bach EA, Aguet M, Schreiber RD. The IFNγ receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15(1):563–91.
Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7(2):188–201.
Keenan TE, Burke KP, Van Allen EM. Genomic correlates of response to immune checkpoint blockade. Nat Med. 2019;25(3):389–402.
Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer. 2018;18(3):139–47.
George S, Miao D, Demetri GD, Adeegbe D, Rodig SJ, Shukla S, et al. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity. 2017;46(2):197–204.
Gajos-Michniewicz A, Czyz M. WNT signaling in melanoma. Int J Mol Sci. 2020;21(14):4852.
Murga-Zamalloa CA, Brown NA, Wilcox RA. Expression of the checkpoint receptors LAG-3, TIM-3 and VISTA in peripheral T cell lymphomas. J Clin Pathol. 2020;73(4):197–203.
Muneer I, Ahmad S, Naz A, Abbasi SW, Alblihy A, Aloliqi AA, et al. Discovery of novel inhibitors from medicinal plants for v-domain ig suppressor of t-cell activation. Front Mol Biosci. 2021;951:8.
Su T, Zhang Y, Valerie K, Wang XY, Lin S, Zhu G. STING activation in cancer immunotherapy. Theranostics. 2019;9(25):7759.
Kim D, Lee YS, Kim DH, Bae SC. Lung cancer staging and associated genetic and epigenetic events. Mol Cells. 2020;43(1):1.
Da Costa EM, McInnes G, Beaudry A, Raynal NJM. DNA methylation-targeted drugs. Cancer J. 2017;23(5):270–6.
Zheng H, Zhao W, Yan C, Watson CC, Massengill M, Xie M, et al. HDAC Inhibitors Enhance T-Cell Chemokine Expression and Augment Response to PD-1 Immunotherapy in Lung AdenocarcinomaHDAC Inhibitors Augment PD-1 Immunotherapy. Clin Cancer Res. 2016;22(16):4119–32.
Zingg D, Arenas-Ramirez N, Sahin D, Rosalia RA, Antunes AT, Haeusel J, et al. The histone methyltransferase Ezh2 controls mechanisms of adaptive resistance to tumor immunotherapy. Cell Rep. 2017;20(4):854–67.
Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):1–27.
Acknowledgements
All figures were obtained from Biorender templates.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
HEM wrote the manuscript, designed the figures using Bio render templates; AH critically revised the manuscript. CC revised the manuscript, GP replied to reviewer’s comments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
not applicable.
Competing interests
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marei, H.E., Hasan, A., Pozzoli, G. et al. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int 23, 64 (2023). https://doi.org/10.1186/s12935-023-02902-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-023-02902-0