Abstract
Objective
Nasopharyngeal carcinoma (NPC) is prevailing in Southern China, characterized by distinct geographical distribution. Aimed to predict the overall survival (OS) of patients with nasopharyngeal carcinoma, this study developed and validated nomograms considering demographic variables, hematological biomarkers, and oncogenic pathogens in China.
Methods
The clinicopathological and follow-up data of the nasopharyngeal carcinoma patients obtained from a prospective longitudinal cohort study in the Chongqing University Cancer Hospital between Jan 1, 2017 and Dec 31, 2019 (\(\mathrm{n}=1635\)). Cox regression model was used to tested the significance of all available variables as prognostic factors of OS. And independent prognostic factors were identified based on multivariable analysis to model nomogram. Concordance index (C-index), area under the receiver operating characteristic (AUC), calibration curve, and decision curve analysis (DCA) were measured to assess the model performance of nomogram.
Results
Data was randomly divided into a training cohort (1227 observers, about 70% of data) and a validation group (408 observers, about 30% of data). At multivariable analysis, the following were independent predictors of OS in NPC patients and entered into the nomogram: age (hazard ratio [HR]: 1.03), stage (stage IV vs. stage I–II, HR: 4.54), radiotherapy (Yes vs. No, HR: 0.43), EBV (\(\ge 1000\) vs.\(<1000\), HR: 1.92), LAR (\(>6.15\) vs.\(\le 6.15\), HR: 2.05), NLR (\(>4.84\) vs. \(\le 4.84\) HR: 1.54), and PLR (\(>206.33\) vs.\(\le 206.33\), HR: 1.79). The C-indexes for training cohort at 1-, 3- and 5-year were 0.73, 0.83, 0.80, respectively, in the validation cohort, the C-indexes were 0.74 (95% CI 0.63–0.86), 0.80 (95% CI 0.73–0.87), and 0.77 (95% CI 0.67–0.86), respectively. The calibration curve demonstrated that favorable agreement between the predictions of the nomograms and the actual observations in the training and validation cohorts. In addition, the decision curve analysis proved that the nomogram model had the highest overall net benefit.
Conclusion
A new prognostic model to predict OS of patients with NPC was developed. This can offer clinicians treatment making and patient counseling. Furthermore, the nomogram was deployed into a website server for use.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma originating from the nasopharyngeal mucosal tissue, with the characteristic of distinct geographical distribution of occurrence [1, 2]. NPC occurred highly in East and Southeast Asia [1], and it particularly prevalent in Guangdong and Guangxi, the regions of southern China [3]. In 2019 the number of NPC deaths in China reached 28,659, accounting for 40% of NPC deaths worldwide [4]. China accounts for a significant proportion of mortality of NPC over the world, especially in southern China [5, 6].
Epstein-Barr virus (EBV) is one of the most common causative agents, and it can be detected in all types of NPC [7]. Radiotherapy is the primary treatment choice for NPC treatment due to the radiosensitive characteristic of NPC tumor [8]. And precise staging is crucial for reducing mortality in patients with NPC. However, heterogeneities of clinical outcomes of the NPC patients with the same clinical stage and degree of EBV were reported in considerable recent research. Those findings indicated that it is not enough to refine the prediction of outcomes for NPC patients only considering single factors. Recently, a major current focus in the area of prognostic of NPC is to find more risk factors to get a more accurate predictive model. Numerous studies have demonstrated that hematological biomarkers were associated with survival outcomes of NPC patients, such as lymphocyte-albumin ratio (LAR), neutrophil–lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) [9,10,11]. However, the literature related to the survival outcomes of the cohort among NPC patients that take various factors into account in model design are limited. Therefore, in this study, our objective was to develop a clinically useful prognostic model in which demographic variables, hematological biomarkers, and oncogenic pathogens were considered to predict overall survival among NPC patients in the region.
Materials and methods
Data source
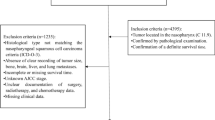
The data set used in the retrospective cohort study was obtained collecting 1635 patients with NPC from the Chongqing University Cancer Hospital tumor database between Jan 1, 2017 and Dec 12, 2019. The inclusion criteria were as follows: (1) age \(\ge 18\) years; (2) histologically confirmed primary NPC; (3) the main treatment occurred in our hospital; (4) completed baseline clinical information and follow-up information; (5) completed the entire course of the treatment of radiotherapy, chemotherapy and targeted therapy. The exclusion criteria for this study were as follows: no follow-up records and a history of cancer treatments. The present study was performed according to the guidelines of the Declaration of Helsinki and was approved by the ethics committee of the Chongqing University Cancer Hospital. Written informed consent was obtained from all subjects.
Variables
IN this study, we employed demographics, including age, sex (female and male), ethnicity (Han and others), marriage (married and others), and occupation (worker/clerk, self-employed/unemployed, professional and technical personnel, and others). The clinical characteristics were selected, including clinical stage which was classified according to the American Joint Committee on Cancer Staging Manual (8th edition), pathological (non-keratinized differentiation, non-keratinized undifferentiated, and keratinized squamous cell carcinoma and others), and transfer information. We also abstracted therapeutic methods information like radiotherapy, chemical-therapy and targeted-therapy. Finally, we retrieved laboratory variables, which consisted of EBV, LAR, NLR, and PLR and selected the cutoff point using X-tile. Continuous variables in laboratory data were transformed into categorical variables based on cutoff values: EBV (\(<1000\), \(\ge 1000\)), LAR (\(\le 0.13\), \(>0.13\)), NLR (\(\le 4.84\),\(>4.84\)), PLR (\(\le 206.33\), \(>206.33\)).
The endpoint of interest in this study was the overall survival (OS) of NPC patients, calculated from the date of first diagnosed as NPC to the time of death or the last follow-up was set as the end.
Construction of nomogram
Patients were randomly divided into train group (1227 observers, about 70% of data) and a validation group (408 observers, about 30% of data). The nomogram model was developed using training cohort. The univariate Cox regression analysis was performed to verify the prognostic significance of each covariate as factor of OS. And entered the variables with p-value \(<0.05\) to the multivariate Cox regression model to analyze the association between each variable and OS to find independent risk factors. The nomogram was created based on the risk score calculated by the final Cox regression model that was constructed by stepwise process.
Model performance and validation
Concordance index (C-index) and area under the receiver operating characteristic (AUC), calibration curve, and decision curve analysis (DCA) were used to assess the model performance of nomogram. C-index was used to estimate the accuracy of the model calculating the difference between predicted value and actual one. The calibration curve was evaluated using a plot to estimate the performance of accordance of the prediction and e reality. DCA calculates a clinical “net benefit” for one or more prediction models in comparison to default strategies of treating all or no patients.
Statistical analysis
The data analysis was performed using SPSS software version 26.0 (IBM Corp, Armonk, NY) and R software version 4.2.1 (Institute for Statistics and Mathematics, Vienna, Austria) were used. The R packages ‘survival’ (version 3.3-1), ‘foreign’ (version 0.8-82), ‘rms’ (version6.3-1), ‘timeROC’ (version 0.4), ‘rms’ (version 5.0.1) and ‘ggDCA’ (version 5.0.1) were used to develop and evaluate the model. In addition, the R packages ‘rsconnect’ (version 0.8.27) and ‘DynNom’ (version 5.0.1) were employed for developing a webserver of nomogram of NPC. The statistical significance of the two-sided \(p\) was set at \(\le 0.05\).
Results
Characteristics of the training and validation cohorts
Patients with nasopharyngeal carcinoma enrolled in follow-up visit were randomly split between training (n = 1227, 70%) and validation cohorts (n = 408, 30%), from the Chongqing University Cancer Hospital tumor database platform. The median survival time was 27.50 (0.10–135.30) months for the overall cohort. There were 180 deaths over 27.40 (0.10–126.00) months for the training cohort. The validation cohort comprised 69 deaths and the median survival time was 28.00 (0.10–135.30).
The descriptive data of our population are shown in Table 1, and there are no significantly difference in training and testing data set. Overall, the considerable amount of patients were Han (1414, 86.48%), married (1507, 92.17%) male (1189, 72.72), with premetastatic (1493, 91.31%), stage IV (700, 42.81%) and pathological performance status of non-keratinizing differentiation (953, 58.29%). And the mean age of patients was \(51.62\pm 11.15\). With regard to therapy, the majority of patients refused targeted therapy (1197, 73.21%). All enrolled cases received chemotherapy for the major choice (772, 62.92%) and 59.09% of patients treated by radiotherapy.
Independent prognostic factors in the training cohort
In the training cohort (n = 1227), the independent prognostic factors were performed using Cox proportional hazards models and modeled results were reported in Table 2. The following variables were significant as predictors of OS on univariable analysis: age, occupation (only professional and technical personnel), stage, radio therapy, chemical therapy, EBV, LAR, NLR, and PLR (all \(p<0.05\)). Reddy etc. found that keratinization may be a vulnerable aid in predicting response to therapy for NPC [12]. And Luo etc. demonstrated that differentiation is close to EBV, which indicates that it was a link between EBV and NPC [13]. Based on clinical consensus and the previous research, we kept the pathological in the model. On multivariable analysis, age (hazard ratio [HR]: 1.03; 95% confidence interval [CI] 1.01–1.04), stage (stage IV vs. stage I–II, HR: 4.59; CI 2.28–9.25), radio therapy (HR: 0.42; CI 0.27–0.66), EBV (HR: 1.98; CI 1.41–2.79), LAR (HR: 2.01; CI 1.41–2.86), NLR (HR: 1.52; CI 1.02–2.28) and PLR (HR: 1.71; CI 1.20–2.44) were demonstrated to be independent predictors.
Developing the prognostic nomogram model
Independent predictors on multivariable analysis were selected for the development of nomogram model to predict 1-, 3- and 5-year OS in nasopharyngeal carcinoma patients (Fig. 1). Each variable was converted to a point score based on corresponding Cox estimated regression coefficients and the sum of the values was positioned to the total point table to obtain the probability of OS.
Model performance and validation of the nomogram
The C-indexes for training cohort at 1-, 3- and 5-year were 0.73(95% confidence interval [CI] 0.66–0.79), 0.83 (95% CI 0.79–0.86), 0.80 (95% CI 0.75–0.85), respectively. In the validation cohort, the C-indexes was 0.74 (95% CI 0.63–0.86), 0.80 (95% CI 0.73–0.87) and 0.77 (95% CI 0.67–0.86), respectively. And ROC plots presented in Fig. 2. In addition, the calibration curve at 1-, 3-, 5-year survival of the model performed well, showing good agreement between the predictions of the nomograms and the actual observations in the training and validation cohorts (Fig. 3.). Moreover, the decision curve analysis was used to test the predictive ability of the nomograms. The DCA results of the four models showed that, except for a small range of predicted probability threshold between 75 and 90%, the nomogram model displayed a positive net benefit in the train set (Fig. 4.).
The calibration curves for predicting patient OS at 1,3 and 5 years in the training cohort and at 1,3 and 5 years in the validation cohort. Nomogram model-predicted OS is plotted on the x-axis; actual OS is plotted on the y-axis. Closer alignment with the diagonal with the diagonal line represents a better estimation
Risk-stratifying ability of the nomogram
Based on the predictive risk scores calculated by the nomogram model, the study subcategorized the training and validation cohort into low-risk group (the prognostic risk score was less than the threshold) and high-risk group (the prognostic risk score was greater than the threshold). And the Kaplan–Meier survival curves for OS presented significant differences between the two groups in the training and validation cohort (\(p<0.0001\)) (Fig. 5).
Webserver development for the nomogram
We developed an easily accessible webserver for the nomogram model of NPC (https://nomogramwebserverofnpc.shinyapps.io/DynNomapp/). The survival plot and probability of the patient can be displayed by selecting the corresponding indexes and survival time on the left side of the webserver board (Fig. 6). For example, the probability of one patient with the following characteristics at 1-year is 0.82: 65-year-old, stage 3, with non-keratinizing differentiation, no radiotherapy, no chemical-therapy, EBV ≥ 1000, LAR > 6.15, NLR ≤ 4.84, PLR ≤ 206.33, and the probability of the patient with same characteristics at 3-year and 5-year was 0.55, 0.46, respectively.
Discussion
In the present study, we used the follow-up database from the Chongqing University Cancer Hospital to establish a novel nomogram prognostic model of NPC and complete internal verification, by incorporating demographics, hematological biomarkers, and oncogenic pathogens. And a user-friendly online calculator was developed to help clinicians in treatment decision making.
Several previous studies have been published using the nomogram to predict the OS of NPC patients. In 2018, Wu and colleagues evaluated a nomogram for predicting long-term OS for patients wish NPC using demographic variables and TNM stage [14]. And Huang etc. developed a prognostic nomogram to reveal the relationship between EBV and NPC in 2021 [15]. Although the western region is not a high incidence area of nasopharyngeal carcinoma, the survival prediction of its patients should not be ignored. Inflammatory markers have been widely used in various cancers, but rarely in NPC, thus we established a prognostic model considered several systemic inflammation parameters and EBV.
Several of our findings are worth highlighting. First, age, stage, radio therapy, EBV, LAR, NLR and PLR were recognized as independent prognostic parameters based on the univariate and multivariate cox regression analysis, and the conclusion was in general agreement with previous reports [9, 16,17,18]. EBV infection is the most common causal agent [19] and a useful prognostic factor of NPC [20], and has been used to assess the disease progression and population screening [21]. LAR is a novel independent prognostic risk factor [9] and have a strong survival predictive power for OS in NPC [18]. Li etc. concluded that NLR could be an attractive indicator for evaluating the 5-year OS in NPC patients with stage III [22]. High PLR was associated with poor OS in NPC patients [23]. Notably, chemical therapy was suggested that it did not reach statistical significance to be a prognostic factor in our study, and it was unlike some previous results [24]. Considering the chemotherapy sensitizing the tumor to the toxic effects of the radiotherapy [25] and the choice to chemotherapy depending on clinical risk, for example, the results obtained in the study is reasonable. Radiotherapy is the primary curative treatment of NPC, and combing chemotherapy with radiotherapy is a rational option in the treatment of locoregionally advanced NPC [26]. Therefore, to avoid missing important factors and based on the clinical features, we conduct model incorporating chemotherapy. In addition, it should be noted that through the univariate model, the correlation between pathological and NPC was of no significance, which was contradictory to other researches.
In our study, the calibration curve pointed optimal accordance between predicted survival probability and actual value, which indicated good repeatability and reliability of the model. And the C-index presented the same performance of our model, in the range of 0.72–0.82 (in training and validation cohorts). In addition, the DCA curves illustrated a better performance of survival predictions of nomogram than the models with stage, EBV, and stage + EBV. In conclusion, the results were suggested that our nomogram was a reliable and precise prognostic tool to predict OS in NPC patients.
Our study is not devoid of limitations. First, there may exist a potential source for selection bias based on the serious inclusion and exclusion criteria. Second, our samples were collected from a single center from a non-endemic region in China and lack of external verification. It is necessary for our study, aimed at exploring the performance of combination of OS and disease-free survival, to design a multicenter randomized controlled study in the next step.
Conclusion
Patients with NPC have heterogeneous survival outcomes, which can be predicted using our novel prognostic model. And it can support help in clinicians deciding treatment and patient counseling. Furthermore, the nomogram was deployed into a website server for use.
Data availability
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation, to any qualified researcher.
References
Chen Y-P, Chan ATC, Le Q-T, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80.
Tang L-L, Chen Y-P, Chen C-B, Chen M-Y, Chen N-Y, Chen X-Z, et al. The Chinese society of clinical oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun. 2021;41(11):1195–227.
Yang D, Bin N, Zhou Z, Li Z, Shen M, Yang C, et al. Demographics and economic burden of nasopharyngeal carcinoma inpatients. Biomed Res Int. 2022;2022:6958806.
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, Abdelalim A, Abdollahi M. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England). 2020;396(10258):1204–22.
Wei KR, Zheng RS, Zhang SW, Liang ZH, Li ZM, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China, 2013. Chin J Cancer. 2017;36(1):90.
Carioli G, Negri E, Kawakita D, Garavello W, La Vecchia C, Malvezzi M. Global trends in nasopharyngeal cancer mortality since 1970 and predictions for 2020: Focus on low-risk areas. Int J Cancer. 2017;140(10):2256–64.
Zhang T, Chen Z, Deng J, Xu K, Che D, Lin J, et al. Epstein-Barr virus-encoded microRNA BART22 serves as novel biomarkers and drives malignant transformation of nasopharyngeal carcinoma. Cell Death Dis. 2022;13(7):664.
Xu M, Zang J, Luo S, Wang J, Li X. Long-term survival outcomes and adverse effects of nasopharyngeal carcinoma patients treated with IMRT in a non-endemic region: a population-based retrospective study. BMJ Open. 2021;11(8):e045417.
Peng RR, Liang ZG, Chen KH, Li L, Qu S, Zhu XD. Nomogram based on lactate dehydrogenase-to-albumin ratio (LAR) and platelet-to-lymphocyte ratio (PLR) for predicting survival in nasopharyngeal carcinoma. J Inflamm Res. 2021;14:4019–33.
Gundog M, Basaran H. The prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in nasopharyngeal cancer. J BUON. 2020;25(1):367–75.
Ye L, Oei RW, Kong F, Xu T, Shen C, Wang X, et al. Prognostic values of hematological biomarkers in nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy. Eur Arch Otorhinolaryngol. 2018;275(5):1309–17.
Reddy SP, Raslan WF, Gooneratne S, Kathuria S, Marks JE. Prognostic significance of keratinization in nasopharyngeal carcinoma. Am J Otolaryngol. 1995;16(2):103–8.
Luo WJ, He SW, Zou WQ, Zhao Y, He QM, Yang XJ, et al. Epstein-Barr virus microRNA BART10-3p promotes dedifferentiation and proliferation of nasopharyngeal carcinoma by targeting ALK7. Exp Biol Med (Maywood). 2021;246(24):2618–29.
Wu J, Zhou Q, Pan Z, Wang Y, Hu L, Chen G, et al. Development and validation of a nomogram for predicting long-term overall survival in nasopharyngeal carcinoma: a population-based study. Medicine. 2020;99(4): e18974.
Huang ZZ, Wen W, Hua X, Song CG, Bi XW, Huang JJ, et al. Establishment and validation of nomogram based on combination of pretreatment C-reactive protein/albumin ratio-EBV DNA Grade in nasopharyngeal carcinoma patients who received concurrent chemoradiotherapy. Front Oncol. 2021;11: 583283.
Huang SJ, Tang YY, Liu HM, Tan GX, Wang X, Zhang H, et al. Impact of age on survival of locoregional nasopharyngeal carcinoma: an analysis of the surveillance, epidemiology, and end results program database, 2004–2013. Clin Otolaryngol. 2018;43(5):1209–18.
Wang J, Huang X, Sun S, Wang K, Qu Y, Chen X, et al. Stage-dependent conditional survival and failure hazard of non-metastatic nasopharyngeal carcinoma after intensity-modulated radiation therapy: clinical implications for treatment strategies and surveillance. Cancer Med. 2021;10(11):3613–21.
Zhao R, Liang Z, Chen K, Zhu X. Nomogram based on inflammatory biomarkers and nutritional indicators for predicting overall survival in locoregionally advanced nasopharyngeal carcinoma. J Inflamm Res. 2022;15:2971–81.
Ooft ML, van Ipenburg JA, Braunius WW, Zuur CI, Koljenović S, Willems SM. Prognostic role of tumor infiltrating lymphocytes in EBV positive and EBV negative nasopharyngeal carcinoma. Oral Oncol. 2017;71:16–25.
Ooft ML, van Ipenburg JA, Sanders ME, Kranendonk M, Hofland I, de Bree R, et al. Prognostic role of tumour-associated macrophages and regulatory T cells in EBV-positive and EBV-negative nasopharyngeal carcinoma. J Clin Pathol. 2018;71(3):267–74.
Wei WI, Sham JST. Nasopharyngeal carcinoma. Lancet. 2005;365(9476):2041–54.
Li J, Wu YL, Li WF, Ma J. Neutrophil to apolipoprotein A-I ratio as an independent indicator of locally advanced nasopharyngeal carcinoma. Laryngoscope Investig Otolaryngol. 2021;6(5):1049–61.
Li Q, Yu L, Yang P, Hu Q. Prognostic value of inflammatory markers in nasopharyngeal carcinoma patients in the intensity-modulated radiotherapy era. Cancer Manag Res. 2021;13:6799–810.
Reffai A, Mesmoudi M, Derkaoui T, Ghailani Nourouti N, Barakat A, Sellal N, et al. Epidemiological profile and clinicopathological, therapeutic, and prognostic characteristics of nasopharyngeal carcinoma in Northern Morocco. Cancer Control. 2021;28:10732748211050588.
Zhang Y, Chen L, Hu G-Q, Zhang N, Zhu X-D, Yang K-Y, et al. Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med. 2019;381(12):1124–35.
Ma BBY, Chen YP, Hui EP, Liu X, Chan AKC, Chan ATC, et al. Recent advances in the development of biomarkers and chemoradiotherapeutic approaches for nasopharyngeal carcinoma. Am Soc Clin Oncol Educ Book. 2020;40:1–11.
Funding
Support for this work was provided by the Chongqing Performance Incentive and Guidance Project for Scientific Research Institutions (cstc2020jxjl130016), Chongqing Key Disease Prevention and Control Technology Project (2019ZX002).
Author information
Authors and Affiliations
Contributions
SM and HL conceived and designed the study. Analysis and interpretation of statistics were performed by XL, WZ. SM wrote initial drafts of the paper. XL and HL handled the data collection and statistical analysis. GW, AS, YW and YW made revisions to the article. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
We followed the ethical principles for medical research involving human subjects as laid down in the Declaration of Helsinki. The studies involving human participants were reviewed and approved by The Ethics Committee of Chongqing University Cancer Hospital.
Competing interests
There are no potential conflicts of interest among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Miao, S., Lei, H., Li, X. et al. Development and validation of a risk prediction model for overall survival in patients with nasopharyngeal carcinoma: a prospective cohort study in China. Cancer Cell Int 22, 360 (2022). https://doi.org/10.1186/s12935-022-02776-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02776-8