Abstract
miR-16-5p is microRNA with important roles in the development of diverse malignancies including neuroblastoma, osteosarcoma, hepatocellular carcinoma, cervical cancer, breast cancer, brain tumors, gastrointestinal cancers, lung cancer and bladder cancer. This miRNA has 22 nucleotides. hsa-miR-16-5p is produced by MIR16-1 gene. First evidence for its participation in the carcinogenesis has been obtained by studies reporting deletion and/or down-regulation of these miRNAs in chronic lymphocytic leukemia. Subsequent studies have shown down-regulation of miR-16-5p in a variety of cancer cell lines and clinical samples. Besides, tumor suppressor role of miR-16-5p has been verified in animal models of different types of cancers. Studies in these models have shown that over-expression of this miRNA or modulation of expression of lncRNAs that sponge this miRNA can block carcinogenic processes. In the current review, we summarize function of miR-16-5p in the development and progression of different cancers.
Similar content being viewed by others
Introduction
MicroRNAs (miRNAs) are small-sized transcripts that regulate expression of genes at post-transcriptional level through specific targeting of mRNAs. With sizes about 21–25 nucleotides, miRNAs are originated from coding and non-coding transcription units in introns, exons or intergenic areas [1]. They are produced in a multi-step process involving both nuclear and cytoplasmic proteins. They are involved in the carcinogenic process, since they can regulate expression of several oncogenes and tumor suppressor genes as well as activities of cancer-associated pathways [2]. Expression pattern and function of several miRNAs have been assessed in different cancer types. Since these small-sized transcripts are stable in the circulation or other biofluids, they represent potential biomarkers for diagnostic and follow-up purposes [3]. Dysregulation of miRNAs has been correlated with evolution of cancers, hence they are regarded as molecular tools for non-invasive assessment of cancer occurrence and its prognosis [4].
miR-16-5p is an example of this class of transcripts with important roles in the development of diverse malignancies including neuroblastoma, osteosarcoma, hepatocellular carcinoma, cervical cancer, breast cancer, brain tumors, gastrointestinal cancers, lung cancer and bladder cancer. This miRNA has 22 nucleotides and is present in Homo sapiens. Homo sapiens hsa-miR-16-5p is produced by MIR16-1 gene.
miR-16-1 is allocated at 13q14.3 along with miR-15a. This miRNA cluster is the target of 13q deletions in chronic lymphocytic leukemia (CLL). miRNAs encoded by this locus have tumor suppressor functions. First evidence for its participation in the carcinogenesis has been obtained by studies reporting deletion and/or down-regulation of these miRNAs in (CLL) [5]. The tumor suppressor functions of miR-15a/16 − 1 are exerted through targeting the BCL2 oncogene. Through a high-throughput study in a leukemic cell line model, Colin et al. have found enrichment in AU-rich elements in the elements of the miR-15a/16 − 1 signature [6].
Subsequently, different studies have assessed role of miR-16-5p in the carcinogenesis using in vitro and in vivo techniques. Moreover, expression pattern of miR-16-5p has been evaluated in clinical samples gathered from patients with diverse malignancies. In the current review, we summarize function of miR-16-5p in the development and progression of different cancers using the above-mentioned lines of evidence. The reason for selection of this miRNA in this review article is the important role of this miRNA in the suppression of carcinogenesis, its down-regulation in a variety of solid and hematological malignancies and its potential as an anti-cancer target. The following strategy was used for selection of papers: publication in full-text English language in a peer-reviewed journal and detailed description of conducted methods. In addition, papers should include in vitro functional studies or expression assays in clinical samples.
Cell line studies
Cell line studies have indicated important roles of miR-16-5p in the carcinogenesis. Moreover, these studies have shown the inhibitory effects of this miRNA on transcription of several genes, particularly a number of known oncogenes. An in vitro study in neuroblastoma has shown interaction between miR-15a, miR‐15b and miR‐16 and MYCN transcript. Based on the results of luciferase reporter assay these miRNAs bind with 3’UTR of MYCN transcript leading to suppression of its expression. Forced up-regulation of these miRNAs has decreased proliferative potential, migratory ability, and invasion of neuroblastoma cells [7]. Another study in neuroblastoma has shown that the oncogenic circular RNA circ-CUX1 enhances tumorigenesis of neuroblastoma and their glycolysis through targeting miR-16-5p. Moreover, miR-16-5p tumor suppressor impact has been partially decreased by transfection of circ-CUX1 overexpressing vectors. DMRT2 has been found to be targeted by miR-16-5p in neuroblastoma cells [8].
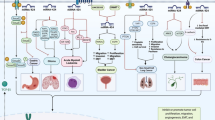
miR-16-5p has been to be down-regulated in osteosarcoma cell lines compared with control cells, parallel with up-regulation of Smad3. Up-regulation of miR-16-5p has suppressed proliferation, migratory potential and invasive features of osteosarcoma cells and increased the cytotoxic effects of cisplatin on these cells. Moreover, miR-16-5p over-expression has led to reduction of Smad3 expression. Notably, cells harboring Smad3 mutation have not responded to miR-16-5p over-expression, indicating that miR-16-5p suppresses invasive properties of osteosarcoma cells through suppressing expression of Smad3 [9]. miR-16-5p effect in suppression of tetraspanin 15 gene has also been involved in the inhibition of osteosarcoma cells proliferation, migration and invasion [10]. Figure 1 shows tumor suppressor role of miR-16-5p in different types of cancer.
The long non-coding RNA (lncRNA) AGAP2-AS1 which targets miR-16-5p has been shown to be up-regulated in hepatocellular carcinoma cell lines. This lncRNA could promote proliferation, migratory aptitude, invasiveness and epithelial-mesenchymal transition (EMT) of these cells through acting as a sponge for miR-16-5p. ANXA11 has been found as a target of miR-16-5p in hepatocellular carcinoma cells, mediating the impacts of miR-16-5p and AGAP2-AS1 in these cells and enhancing activity of AKT signaling. Notably, hypoxia has been shown to increase levels of AGAP2-AS1 in these cells [11]. Another study has confirmed down-regulation of miR-16-5p in hepatocellular cancer cells. Dual-Luciferase reporter gene assay has validated the regulatory role of miR-16-5p on expression of Insulin like growth factor1 receptor (IGF1R). IGF1R down-regulation has decreased the suppressive role of miR-16- 5p on proliferation ability and metastatic potential of hepatocellular cancer cells [12]. Moreover, down-regulation of miR-16-5p by lncRNA TTN-AS1 has been shown to promote resistance to sorafenib through enhancement of expression of cyclin E1 [13]. Finally, another study in hepatocellular carcinoma has shown that SNHG22 increases tumorigenic ability of cancer cells and their angiogenesis though induction of DNA methylation in miR-16-5p [14].
In cervical cancer cells, miR-16‐5p affects radiosensitivity through regulation of expression of coactivator‐associated arginine methyltransferase 1 [15]. Moreover, it can influence metabolic reprogramming and chemoresistance through regulation of Pyruvate Dehydrogenase Kinase 4 (PDK4) expression [16].
In breast cancer cell, down-regulation of miR-16-5p has been associated with high migratory and proliferative potential of cells, induction of cell cycle progression and reduction of cell apoptosis. miR-16-5p could restrain activity of the Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway and reduce expression of AKT3 gene, thus inhibiting development of breast cancer [17]. miR-16-5p could also suppress proliferation of breast cancer cells through down-regulating expression of ANLN [18]. The inhibitory effect of miR-16 -5p in breast cancer cells proliferation and invasiveness can be mediated through regulation of Vascular Endothelial Growth Factor A (VEGFA) expression [19]. Finally, ATXN8OS has been shown to enhance tamoxifen resistance through sponging miR-16-5p [20].
Moreover, miR-16‐5p has been shown to be commonly down‐regulated in astrocytic gliomas. This miRNA could regulate proliferation and apoptosis of these cells as well as effect of cytotoxic agents on these cells [21]. Another study in glioma cells has shown that TIIA could inhibit viability of cells, their migratory potential and invasiveness, and decrease levels of Cyclin D1, Matrix metallopeptidase 9 (MMP-9) and Vimentin via regulation of miR-16-5p/Talin-1 axis [22].
Summary of studies that evaluated expression of miR-16-5p or its partners in cell lines is presented in Table 1.
Animal studies
The tumor suppressor role of miR-16-5p has been verified in animal models of different types of cancers. Studies in these models have shown that over-expression of this miRNA or modulation of expression of lncRNAs that sponge this miRNA can block carcinogenic processes. For instance, transplantation of miR-15a‐, miR‐15b‐ and miR‐16‐expressing neuroblastoma cells into extremely immunodeficient mice has suppressed formation of tumors as well as expression of MYCN, suggesting that these miRNAs have a tumor suppressor role in neuroblastoma through targeting MYCN [7]. Another study in xenograft model of neuroblastoma has shown that knock down of the miR-16-5p-targeting circ-CUX1 leads to reduction of tumor growth [8].
In animal models of hepatocellular carcinoma, up-regulation of AGAP2-AS1 has enhanced tumor growth via down-regulating miR-16-5p [11]. Moreover, down-regulation of TTN-AS1 decreases tumor size and resistance to sorafenib through enhancement of expression of miR-16-5p [13]. In cervical cancer models, silencing of miR-16-5p target, PDK4 has enhanced efficacy of chemotherapy [16]. Moreover, silencing of DLX6-AS1 which targets miR-16-5p decreases tumor size [25]. Other studies in breast cancer, chordoma/chondrosarcoma, gastric cancer, lung cancer, colorectal cancer, bladder cancer and cholangiocarcinoma have confirmed a tumor suppressor role for miR-16-5p (Table 2).
Human studies
Down-regulation of miR-16-5p has been verified in clinical samples obtained from patients with different malignancies. Moreover, AGAP2-AS1 that decreases miR-16-5p levels has been shown to be up-regulated in hepatocellular carcinoma tissues, particularly in metastatic and recurrent ones. In addition, expression levels of AGAP2-AS1 and miR-16-5p have been correlated with clinical parameters and poor prognosis of patients with this type of cancer [11]. In neuroblastoma, up-regulation in circ-CUX1 that sponges miR-16-5p has been correlated with advanced TNM stage, low differentiation grade and lymph node metastasis [8]. In breast cancer patients, miR-16-5p has been shown to have low expression. Notably, patients with low expression of miR-16-5p have been found to have a lower survival rate compared with those having high expression of miR-16-5p [17].
In the majority of CLL cases, miR-15a and miR-16-1 have been shown to be lost or down-regulated [6]. Moreover, assessment of GO database has led to identification of enrichment of MCL1 Apoptosis Regulator, BCL2 Family Member (MCL1), B-cell lymphoma 2 (BCL2), ETS Proto-Oncogene 1 (ETS1), or Jun Proto-Oncogene, AP-1 Transcription Factor Subunit (JUN) in miR-16 signature. Notably, these genes are involved in the regulation of apoptosis and cell cycle [6].
Several studies have reported down-regulation of this miRNA in nearly all examined malignant tissues except for ovarian cancer tissues. Similarly, lncRNAs or circRNAs that decrease expression of miR-16-5p have been found to be up-regulated in cancer samples compared with non-cancerous controls (Table 3).
Discussion
miR-16-5p is an example of miRNAs with tumor suppressor role in almost all assessed tissues. This speculation is based on the observed down-regulation of this miRNA in nearly all examined malignant tissues except for ovarian cancer tissues. Moreover, a number of studies have reported up-regulation of lncRNAs that target this miRNA or specific targets of this miRNA. This miRNA has been found to be sponged by some lncRNAs and circRNAs, namely LINC00662, LINC00649, LINC00473, LINC00210, PVT1, XIST, AGAP2-AS1, DLX6-AS1, TTN-AS1, circ-CUX1 and hsa_circ_0005721. These observations indicate the complexity of the network through which miR-16-5p exerts its tumor suppressor effects. Moreover, abnormal up-regulation of the mentioned lncRNAs and circRNAs is regarded as a possible mechanism for down-regulation of miR-16-5p along with genomic variations in the genetic locus of this miRNA.
Phosphoinositide 3-kinase (PI3K)/AKT, Phosphatase and tensin homolog (PTEN)/AKT, NF-κB, Hippo and E1-pRb-E2F1 pathways are among signaling pathways being affected by dysregulation of miR-16-5p. Thus, down-regulation of miR-16-5p can lead to over-activity of cancer-related signals enhancing cell survival.
Down-regulation of miR-16-5p or up-regulation of lncRNAs/circRNAs that sponge this miRNA has been shown to be associated with malignant features of different cancers such as neuroblastoma, osteosarcoma, renal cell carcinoma and colorectal cancer, indicating a role for miR-16-5p as a prognostic marker in human cancers. In fact, down-regulation of this miRNA has been detected in samples with low level of differentiation and high propensity to local and distant metastases. Thus, patient with low levels of expression of this miRNA has exhibited poor clinical outcomes.
Since this miRNA can be detected in the peripheral blood, it represents a novel non-invasive strategy for early detection of cancer. However, since it is down-regulated in several types of cancers, the type of cancer cannot be detected through this route. Moreover, evaluation of levels of miR-16-5p in cancer patients can be used for follow-up after removal of primary tumor.
The mechanism behind down-regulation of miR-16-5p in malignant tissues is not investigated thoroughly, although deletion in the genomic region coding this miRNA is a putative mechanism. Moreover, up-regulation of lncRNAs/circRNAs that sponge this miRNA is a well-established mechanism for its down-regulation in different cancers. Induction of DNA methylation in miR-16-5p is another mechanism of down-regulation of this miRNA in cancers [14]. Future studies are needed to find possible epigenetic alterations that affect transcription of precursor of miR-16-5p.
Different studies have shown the effects of miR-16-5p in regulation of chemosensitivity, radiosensitivity as well as response to the targeted therapy by sorafenib. From a clinical point of view, up-regulation of miR-16-5p is a potentially effective modality for suppression of tumor growth and defeating chemotherapy resistance. However, introduction of miR-16-5p mimic into cancerous cells needs a specific strategy to shield the miRNA mimics from self-hydrolysis or degradation by RNases. Without these considerations, the short half-life of naked RNA mimics reduces the potential effects of miRNAs [51]. Moreover, issues regarding the toxicity or nonspecific cell-targeting nature of miRNA carriers should be solved. These issues have attenuated the pace of entering miRNA mimics into the clinical setting.
Cumulatively, miR-16-5p is a putative tumor suppressor miRNA that can be used as a therapeutic modality in different cancers. However, the biosafety and bioavailability issues should be solved before introduction of this modality in clinical settings.
Data availability
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
References
Wahid F, Shehzad A, Khan T, Kim YY. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2010;1803(11):1231–43.
Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target therapy. 2016;1(1):1–9.
Galvão-Lima LJ, Morais AH, Valentim RA, Barreto EJ. miRNAs as biomarkers for early cancer detection and their application in the development of new diagnostic tools. Biomed Eng Online. 2021;20(1):1–20.
Filipów S, Łaczmański Ł. Blood Circulating miRNAs as Cancer Biomarkers for Diagnosis and Surgical Treatment Response. Front Genet. 2019;10:169-. PubMed PMID: 30915102. eng.
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the national academy of sciences. 2002;99(24):15524-9.
Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proceedings of the National Academy of Sciences. 2008;105(13):5166-71.
Chava S, Reynolds CP, Pathania AS, Gorantla S, Poluektova LY, Coulter DW, et al. miR-15a‐5p, miR‐15b‐5p, and miR‐16‐5p inhibit tumor progression by directly targeting MYCN in neuroblastoma. Mol Oncol. 2020;14(1):180–96.
Zhang X, Zhang J, Liu Q, Zhao Y, Zhang W, Yang H. Circ-CUX1 accelerates the progression of neuroblastoma via miR-16-5p/DMRT2 axis. Neurochem Res. 2020;45(12):2840–55.
Gu Z, Li Z, Xu R, Zhu X, Hu R, Xue Y, et al. miR-16-5p Suppresses Progression and Invasion of Osteosarcoma via Targeting at Smad3. Front Pharmacol. 2020;11:1324.
Yu J, Zhang H, Yan L, Chang F, Jia Z, Yang X. microRNA-16-5p targeted tetraspanin 15 gene to inhibit the proliferation, migration and invasion of osteosarcoma cell through phospoinositide 3-kinase/protein kinase B signaling pathway. Zhonghua yi xue za zhi. 2020;100(21):1668–75.
Liu Z, Wang Y, Wang L, Yao B, Sun L, Liu R, et al. Long non-coding RNA AGAP2-AS1, functioning as a competitive endogenous RNA, upregulates ANXA11 expression by sponging miR-16-5p and promotes proliferation and metastasis in hepatocellular carcinoma. J Experimental Clin Cancer Res. 2019;38(1):1–15.
Cheng B, Ding F, Huang C, Xiao H, Fei F, Li J. Role of miR-16-5p in the proliferation and metastasis of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2019;23(1):137–45.
Zhou Y, Huang Y, Dai T, Hua Z, Xu J, Lin Y, et al. LncRNA TTN-AS1 intensifies sorafenib resistance in hepatocellular carcinoma by sponging miR-16-5p and upregulation of cyclin E1. Biomed Pharmacother. 2021;133:111030.
Zhang Y, Lu C, Cui H. Long non-coding RNA SNHG22 facilitates hepatocellular carcinoma tumorigenesis and angiogenesis via DNA methylation of microRNA miR-16-5p. Bioengineered. 2021;12(1):7446–58.
Zhang S, Wang W, Wu X, Liu W, Ding F. miR-16‐5p modulates the radiosensitivity of cervical cancer cells via regulating coactivator‐associated arginine methyltransferase 1. Pathol Int. 2020;70(1):12–20.
Zhao Z, Ji M, Wang Q, He N, Li Y. miR-16-5p/PDK4-mediated metabolic reprogramming is involved in chemoresistance of cervical cancer. Mol Therapy-Oncolytics. 2020;17:509–17.
Ruan L, Qian X. MiR-16-5p inhibits breast cancer by reducing AKT3 to restrain NF-κB pathway. Biosci Rep. 2019;39(8):BSR20191611.
Wang Z, Hu S, Li X, Liu Z, Han D, Wang Y, et al. MiR-16-5p suppresses breast cancer proliferation by targeting ANLN. BMC Cancer. 2021;21(1):1–12.
Qu Y, Liu H, Lv X, Liu Y, Wang X, Zhang M, et al. MicroRNA-16-5p overexpression suppresses proliferation and invasion as well as triggers apoptosis by targeting VEGFA expression in breast carcinoma. Oncotarget. 2017;8(42):72400.
Zhang H, Zhang J, Dong L, Ma R. LncRNA ATXN8OS enhances tamoxifen resistance in breast cancer. Open Med. 2021;16(1):68–80.
Krell A, Wolter M, Stojcheva N, Hertler C, Liesenberg F, Zapatka M, et al. MiR-16‐5p is frequently down‐regulated in astrocytic gliomas and modulates glioma cell proliferation, apoptosis and response to cytotoxic therapy. Neuropathol Appl Neurobiol. 2019;45(5):441–58.
You S, He X, Wang M, Mao L, Zhang L. Tanshinone IIA. Suppresses Glioma Cell Proliferation, Migration and Invasion Both in vitro and in vivo Partially Through miR-16-5p/Talin-1 (TLN1) Axis. Cancer Manage Res. 2020;12:11309.
Yu M, Lu W, Cao Z, Xuan T. LncRNA LINC00662 Exerts an Oncogenic Effect on Osteosarcoma by the miR-16-5p/ITPR1 Axis. Journal of oncology. 2021;2021.
Xu M, Sun X, Liu Y, Chang L, Te Wang H, Wang S. hsa_circ_0005721 triggers proliferation, migration and invasion of osteosarcoma by upregulating the linear transcript TEP1. J BU ON: Official J Balkan Union Oncol. 2021;26(4):1588–94.
Xie F, Xie G, Sun Q. Long noncoding RNA DLX6-AS1 promotes the progression in cervical cancer by targeting miR-16-5p/ARPP19 axis. Cancer Biother Radiopharm. 2020;35(2):129–36.
Jo H, Park Y, Kim J, Kwon H, Kim T, Lee J, et al. Elevated miR-16-5p induces somatostatin receptor 2 expression in neuroendocrine tumor cells. PLoS ONE. 2020;15(10):e0240107.
Zhang H, Yang K, Ren T, Huang Y, Tang X, Guo W. miR-16-5p inhibits chordoma cell proliferation, invasion and metastasis by targeting Smad3. Cell Death Dis. 2018;9(6):1–13.
Wang C, Wang Y, Wang J, Guo X. LINC00662 triggers malignant progression of chordoma by the activation of RNF144B via targeting miR-16-5p. Eur Rev Med Pharmacol Sci. 2020;24(3):1007–22.
Li Z, Suo B, Long G, Gao Y, Song J, Zhang M, et al. Exosomal miRNA-16-5p derived from M1 macrophages enhances T cell-dependent immune response by regulating PD-L1 in gastric cancer. Front Cell Dev Biology. 2020;8:1362.
Zhu C, Huang Q, Zhu H. Melatonin inhibits the proliferation of gastric cancer cells through regulating the miR-16-5p-Smad3 pathway. DNA Cell Biol. 2018;37(3):244–52.
Wang H, Di X, Bi Y, Sun S, Wang T. Long non-coding RNA LINC00649 regulates YES-associated protein 1 (YAP1)/Hippo pathway to accelerate gastric cancer (GC) progression via sequestering miR-16-5p. Bioengineered. 2021;12(1):1791–802.
Zhuo S, Sun M, Bai R, Lu D, Di S, Ma T, et al. Long intergenic non-coding RNA 00473 promotes proliferation and migration of gastric cancer via the miR-16-5p/CCND2 axis and by regulating AQP3. Cell Death Dis. 2021;12(5):1–14.
Wang Q, Chen Y, Lu H, Wang H, Feng H, Xu J, et al. Quercetin radiosensitizes non-small cell lung cancer cells through the regulation of miR‐16‐5p/WEE1 axis. IUBMB Life. 2020;72(5):1012–22.
Peng Q, Chen Y, Li C. Long noncoding RNA Linc00210 promotes non-small cell lung cancer progression via sponging miR-16-5p/PTK2 axis. Eur Rev Med Pharmacol Sci. 2020;24(18):9438–52.
Du R, Jiang F, Yin Y, Xu J, Li X, Hu L, et al. Knockdown of lncRNA X inactive specific transcript (XIST) radiosensitizes non-small cell lung cancer (NSCLC) cells through regulation of miR-16-5p/WEE1 G2 checkpoint kinase (WEE1) axis. Int J ImmunoPathol Pharmacol. 2021;35:2058738420966087.
Wu H, Wei M, Jiang X, Tan J, Xu W, Fan X, et al. lncRNA PVT1 promotes tumorigenesis of colorectal cancer by stabilizing miR-16-5p and interacting with the VEGFA/VEGFR1/AKT axis. Mol Therapy-Nucleic Acids. 2020;20:438–50.
Huang X, Hou Y, Weng X, Pang W, Hou L, Liang Y, et al. Diethyldithiocarbamate-copper complex (CuET) inhibits colorectal cancer progression via miR-16-5p and 15b-5p/ALDH1A3/PKM2 axis-mediated aerobic glycolysis pathway. Oncogenesis. 2021;10(1):1–16.
Xu Y, Shen L, Li F, Yang J, Wan X, Ouyang M. microRNA-16‐5p‐containing exosomes derived from bone marrow‐derived mesenchymal stem cells inhibit proliferation, migration, and invasion, while promoting apoptosis of colorectal cancer cells by downregulating ITGA2. J Cell Physiol. 2019;234(11):21380–94.
Papagiannopoulos CI, Theodoroula NF, Vizirianakis IS. miR-16-5p Promotes Erythroid Maturation of Erythroleukemia Cells by Regulating Ribosome Biogenesis. Pharmaceuticals. 2021;14(2):137.
Wang F, Wang W, Lu L, Xie Y, Yan J, Chen Y, et al. MicroRNA–16–5p regulates cell survival, cell cycle and apoptosis by targeting AKT3 in prostate cancer cells. Oncol Rep. 2020;44(3):1282–92.
Wang F, Mao A, Tang J, Zhang Q, Yan J, Wang Y, et al. microRNA-16‐5p enhances radiosensitivity through modulating Cyclin D1/E1–pRb–E2F1 pathway in prostate cancer cells. J Cell Physiol. 2019;234(8):13182–90.
Chen S-S, Tang C-H, Chie M-J, Tsai C-H, Fong Y-C, Lu Y-C, et al. Resistin facilitates VEGF-A-dependent angiogenesis by inhibiting miR-16-5p in human chondrosarcoma cells. Cell Death Dis. 2019;10(1):1–12.
Sang S, Zhang Z, Qin S, Li C, Dong Y. MicroRNA-16-5p inhibits osteoclastogenesis in giant cell tumor of bone. BioMed research international. 2017;2017.
Feng X, Dong X, Wu D, Zhao H, Xu C, Li H. Long noncoding RNA small nucleolar RNA host gene 12 promotes papillary thyroid carcinoma cell growth and invasion by targeting miR-16-5p. Histol Histopathol. 2019;35(2):217–24.
Ren Y, Huang W, Weng G, Cui P, Liang H, Li Y. lncrna PVT1 promotes proliferation, invasion and epithelial–mesenchymal transition of renal cell carcinoma cells through downregulation of mir-16-5p. OncoTargets and therapy. 2019;12:2563.
He J, Qiu Z, Zhang H, Gao Z, Jiang Y, Li Z, et al. MicroRNA–16–5p/BIMP1/NF–κB axis regulates autophagy to exert a tumor–suppressive effect on bladder cancer. Mol Med Rep. 2021;24(2):1–10.
Liu Y, Huang X, Guo L, Luo N. LINC00649 Facilitates the Cellular Process of Bladder Cancer Cells via Signaling Axis miR-16-5p/JARID2. Urologia Internationalis. 2021:1–9.
Gao Y, Ouyang X, Zuo L, Xiao Y, Sun Y, Chang C, et al. R-2HG downregulates ERα to inhibit cholangiocarcinoma via the FTO/m6A-methylated ERα/miR16-5p/YAP1 signal pathway. Mol Therapy-Oncolytics. 2021;23:65–81.
Casabonne D, Benavente Y, Seifert J, Costas L, Armesto M, Arestin M, et al. Serum levels of hsa-miR‐16‐5p, hsa‐miR‐29a‐3p, hsa‐miR‐150‐5p, hsa‐miR‐155‐5p and hsa‐miR‐223‐3p and subsequent risk of chronic lymphocytic leukemia in the EPIC study. Int J Cancer. 2020;147(5):1315–24.
Saral MA, Tuncer SB, Odemis DA, Erdogan OS, Erciyas SK, Saip P, et al. New biomarkers in peripheral blood of patients with ovarian cancer: high expression levels of miR-16-5p, miR-17-5p, and miR-638. Archives of Gynecology and Obstetrics. 2021:1–9.
Lee TJ, Yuan X, Kerr K, Yoo JY, Kim DH, Kaur B, et al. Strategies to Modulate MicroRNA Functions for the Treatment of Cancer or Organ Injury. Pharmacol Rev. 2020 Jul;72(3):639–67. PubMed PMID: 32554488. Pubmed Central PMCID: PMC7300323. Epub 2020/06/20. eng.
Acknowledgements
The authors would like to thank the clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their support, cooperation and assistance throughout the period of study
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SGF wrote the manuscript and revised it. MT supervised and designed the study. TK, MS, STA and BMH collected the data and designed the figures and tables. All authors read and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participant
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent forms were obtained from all study participants. The study protocol was approved by the ethical committee of Shahid Beheshti University of Medical Sciences. All methods were performed in accordance with the relevant guidelines and regulations.
Consent of publication.
Not applicable.
Competing interests
The authors declare they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghafouri-Fard, S., Khoshbakht, T., Hussen, B.M. et al. A review on the role of mir-16-5p in the carcinogenesis. Cancer Cell Int 22, 342 (2022). https://doi.org/10.1186/s12935-022-02754-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02754-0