Abstract
PI3K/AKT pathway is an important pathway in the carcinogenesis since it has central impacts in the regulation of metabolic pathways, cell proliferation and survival, gene expression and protein synthesis. This pathway has been reported to be dysregulated in several types of cancers. In the current review, we summarize the role of this signaling pathway in squamous cell carcinomas (SCCs) originated from different parts of body cervix, oral cavity, head and neck and skin. The data presented in the current review shows the impact of dysregulation of PI3K/AKT pathway in survival of patients with SCC. Moreover, targeted therapies against this pathway have been found to be effective in reduction of tumor burden both in animal models and clinical settings. Finally, a number of molecules that regulate PI3K/AKT pathway can be used as diagnostic markers for different types of SCCs.
Similar content being viewed by others
Introduction
PI3K/AKT pathway has important roles in the carcinogenesis since it has central impact in the regulation of metabolic pathways, cell proliferation and survival, gene expression and protein synthesis [1]. As a multimember family of heterodimeric lipid kinases, PI3Ks are classified into three distinct classes. Class IA PI3Ks are induced by receptor tyrosine kinases such as p110 catalytic subunit as well as p85-like regulatory subunits [1]. Class IB PI3Ks are induced by G protein-coupled receptors and regulatory subunits. Class II PI3Ks includes three proteins, namely PIK3C2A, PIK3C2B and PIK3C2G. Finally PIK3C3 is regarded as the single member of class III PI3Ks. PI3Ks can be induced by several upstream cell-surface receptors. In response to these stimuli, class I proteins catalyze the conversion of PI(4,5)P2 to the second messenger PIP3. AKT and PDK-1 serine/threonine kinases are two proteins that have PIP3-binding Pleckstrin homology (PH) domain and are associated with PI3K in a variety of cells [2, 3]. AKT is an evolutionarily conserved serine protein kinase being attributed to the AGC subfamily. This protein has three structural domains, namely N-terminal PH domain, a short C-terminal tail comprising a regulatory hydrophobic motif (HM) and a linker section with a central kinase catalytic domain [6]. AKT family of proteins includes three homologous subtypes, namely AKT1-AKT3. In response to increase in PI(3,4,5)P3 levels and to a lesser extent accumulation of PI(3, 4)P2, AKT is recruited on the cell membrane through its PH domain and exerts its catalytic roles through activation of a PDK1-induced threonine phosphorylation and mTORC2-mediated serine phosphorylation. These phosphorylation events occur at specific sites of AKT1, AKT2 and AKT3 [4, 5]. The effects of AKT on regulation of important downstream effectors including FOXO, mTOR and GSK3b endows this molecule the ability to influence cell proliferation and survival, genome stability, and metabolic pathways [1]. PI3K/AKT pathway has been reported to be dysregulated in several types of cancers. In the current review, we summarize the role of this signaling pathway in squamous cell carcinomas (SCCs) originated from different parts of body cervix, oral cavity, head and neck and skin.
Cervical cancer
Hou et al. have assessed the clinical outcomes of individuals with metastatic or recurrent cervical cancer during a phase I clinical trial. They have reported longer survival of patients with SCC of the cervix who had PIK3CA mutations compared with those without PIK3CA mutations. In fact, their results have shown that matched therapies against the activated PI3K/AKT/mTOR pathway have significant clinical benefit [6]. Another study in the context of cervical SCC has shown over-expression of the endogenous inhibitor of mTOR complexes DEPTOR in these cells and tissues. DEPTOR silencing has enhanced apoptosis of these cells via increasing expression of p38 MAPK and suppression of PI3K/AKT activity through feed-back suppression from mTORC1-S6K. Moreover, knock down of this gene has led to reduction of levels of nitric oxide synthases iNOS and eNOS, and enhancement of activity of ERK1/2 and p38 MAPKs. Moreover, DEPTOR could affect ERK1/2 expression in through modulation of AKT. Cumulatively, DEPTOR increases survival of cervical SCC cells and its knock down leads to cell apoptosis through distinctive impacts on PI3K/AKT and p38 MAPK [7]. Moreover, the over-expressed receptor for advanced glycation endproducts (RAGE) has been shown to be involved in the pathogenesis of cervical SCC through modulation of PI3K/AKT activity. This protein has been found to promote proliferation of cervical SCC, enhance expression of PCNA, inhibit cell apoptosis along, reduce Bax/Bcl-2 ratio, and induce activity of PI3K/AKT pathway. RAGE silencing has reduced tumor burden in a xenograft model of cervical SCC. Finally, the PI3K inhibitor LY294002 could efficiently inhibit activity of PI3K and AKT, and suppress RAGE-induced pro-proliferative and anti-apoptosis effects [8]. Table 1 shows the role of PI3K/AKT pathway in squamous cell carcinoma of cervix.
Head and neck squamous cell carcinoma
Laryngeal squamous cell carcinoma (LSCC)
Mukonal, the isolated alkaloid from the plant Murraya koenigii has been shown to reduce the viability of laryngeal SCC cells, induce their apoptosis and arrest them at G2/M phase possibly through suppression of activity of PI3K/AKT and MEK/ERK pathways [20]. Moreover, dehydrocostus lactone isolated from Saussurea costus Lipech has been found to exert cytotoxic effects in this type of cancer. This substance could inhibit viability, migration and proliferation of laryngeal SCC cells without affecting viability of normal larynx epithelial cells. Notably, dehydrocostus lactone could promote function of p53 and P21 and induce cells apoptosis through suppression of PI3K/Akt/Bad pathway and stimulation of endoplasmic reticulum stress-mediated apoptotic pathways. In vivo assays have also verified these effects [21].
Another study has shown up-regulation of FGFR1, FGFR3 and PI3K/AKT kinase expression levels in the squamous cell laryngeal cancer samples compared with non-cancerous laryngeal mucosa specimens. Notably, over-expression of PI3K/AKT kinase has been associated with a high tumor front grading. Moreover, levels of the p-PI3K regulatory kinase protein have been associated with survival rate of patients. Taken together, FGFR1, FGFR3, and downstream regulatory kinases from the PI3K/AKT pathway might be regarded as putative markers indicative of invasive properties of laryngeal cancer [22]. Table 2 shows the role of PI3K/AKT pathway in laryngeal squamous cell carcinoma.
Esophageal squamous cell carcinoma (ESCC)
Expression analyses esophageal cancer tissues have shown up-regulation of miR-21, PI3K, and AKT, while down-regulation of PTEN in these tissues compared with adjacent non-cancerous tissues. Notably, samples obtained from patients with lymph node metastases and poor differentiation levels had lower expression of PTEN and higher levels of PI3K and AKT proteins. Suppression of miR-21 levels in esophageal cancer cells has led to up-regulation of PTEN, down-regulation of PI3K and AKT and reduction of proliferation rate, migration, and invasion of cells. This miRNA has been found to target PTEN. Cumulatively, miR-21 has been shown to target important molecules in PTEN/PI3K/AKT signal pathway, enhancing proliferation, migration, invasiveness, and cell cycle transition, and suppressing apoptotic pathways in esophageal SCC cells [47]. Another study in esophageal SCC patients has shown correlation between p-EGFR expression and all of the other phosphorylated biomarkers. Notably, gender, N stage, and expression levels of p-AKT1 have been independently correlated with overall survival of patients. In fact, over-expression of p-AKT1 has been found to be indicative of low survival. However, levels of EGFR and p-EGFR have not been correlated with patients’ survival [48]. Moreover, dysregulation of PAFR via PI3K/AKT pathway has been reported to contribute to the progression of esophageal SCC [49].
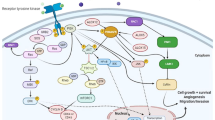
On the other hand, vitamin E succinate could induce apoptosis of esophageal SCC cells through modulation of PI3K/AKT signaling this agent has decreased growth of EC109 cells by approximately 45 and 81% in concentrations of 10 and 100 µM, respectively [50]. Moreover, Dasatinib via suppressing the PI3K/AKT and STST3 pathways could improve sensitivity to cisplatin in esophageal SCC cells [51]. Table 3 shows the role of PI3K/AKT pathway in esophageal SCC. Figure 1 illustrates the aberrant expression of various miRNAs, which contribute to adversely modulating the PI3K/AKT signaling pathway involved in triggering several kinds of squamous cell carcinomas.
A schematic diagram of the role of several miRNAs in triggering the PI3K/AKT signaling cascade in Cervical Cancer, LSCC and ESCC. Mounting studies have revealed that dysregulation of PI3K/AKT signaling pathway can play a crucial role in the carcinogenesis especially in squamous cell carcinomas. A recent study has detected that overexpression of miR-433 could suppress the growth and metastasis of cervical cancer cells via down-regulating the FAK/PI3K/AKT signaling cascade, and could enhance the apoptosis and caspase-3/-9 function. Moreover, miR-433 could promote the expression levels of p53 and Bax, and inhibit that of MDM2 in cervical cancer [18]. Further experiment has validated that miR-132 plays an oncogenic role in laryngeal squamous cell carcinoma by suppressing the expression of FOXO1, p27, and p21. Overexpression of this miRNA could promote cell proliferation and tumor growth via up-regulating cyclin D1 as well as activating the PI3K/AKT pathway in LSCC cells [34]. Another research has pointed out that miR-21 could have a significant role in enhancing cell proliferation, migration, invasion, and cell cycle, and suppressing apoptosis of human esophageal cancer cells via down-regulating the expression of PTEN and activating PI3K/AKT signaling pathway [47]. Green lines indicate the positive regulatory effect among miRNAs and their targets, and red lines depict negative ones among them. All information regarding the role of these miRNAs involved in the modulation of PI3K/AKT signaling cascade in various types of squamous cell carcinomas can be seen in Tables 1–4
Pharyngeal squamous cell carcinoma (PSCC)
In patients with hypopharyngeal SCC, expression of p-Akt and p-Erk has been shown to be remarkably elevated parallel with progression of clinical stage, indicating the possible roils of PI3K/Akt and MAPK/ERK pathways in evolution and progression of this type of cancer. Notably, GDC-0980 and Refametinib have exerted cytotoxic effect on hypopharyngeal SCC cells. These agents could block cell cycle progression in G1 phase, reduce cyclin D1 and p-Rb levels and increase p27 levels. GDC-0980 could also inhibit migratory potential of these cells and reduce levels of p-PKCζ, p-Integrin β1 and uPA metastasis-related proteins. Taken together, dual suppression of PI3K/Akt and MAPK/ERK pathways by mentioned agents can be regarded as a possible strategy for treatment of hypopharyngeal SCC [60]. NVP-BEZ235 when combined with cisplatin could inhibit proliferation of hypopharyngeal SCC cells and arrest cell cycle at G2/M phase via modulation of the PI3K/AKT/mTOR pathway [61].
JARID1B, as a tumor suppressor, via the SHIP1/AKT pathway could improve differentiation of hypopharyngeal SCC cells and suppress their proliferation [62]. On the other hand, S100A11 could play an important role in the migration, carcinogenesis and protection of HPSCC from cell death induced by 5-Fu via the PI3K/AKT pathway [63]. Table 4 shows the role of PI3K/AKT pathway in pharyngeal squamous cell carcinoma.
Oral squamous cell carcinoma (OSCC)/tongue squamous cell carcinoma (TSCC)
Lycopene has been revealed to inhibit proliferation, migration and invasiveness of oral SCC cells as well as in vivo growth of tumors. Moreover, this substance could suppress epithelial–mesenchymal transition and activate apoptotic pathways through decreasing activity of PI3K/AKT/mTOR signaling. These effects are exerted through enhancing expressions of E-cadherin and Bax and decreasing levels of N-cadherin, p-PI3K, p-AKT, p-m-TOR, and bcl-2 [69]. Thymoquinone has also been shown to suppress invasion, proliferation and migration of oral SCC cells and induce their apoptosis via inhibiting the PI3K/AKT pathway [70]. Moreover, Licochalcone A could suppress migration, invasion, and proliferation of oral SCC cells via modulation of the PI3K/AKT pathway [71].
A number of non-coding RNAs have been reported to exert their effects in the pathogenesis of oral SCC through modulation of this pathway. This speculation has been verified by knock-down experiments. For example, suppression of lncRNA MALAT1 could inhibit invasion, migration, and proliferation of TSCC cells via suppressing the PI3K/AKT pathway and down-regulating MMP-9 [72]. Moreover, circCDR1 has been shown to improve the viability of oral SCC cells by promoting autophagy via the AKT/ERK/mTOR pathway [73]. Table 5 shows the role of PI3K/AKT pathway in oral SCC.
Unidentified types of head and neck squamous cell carcinoma (HNSCC)
Expression of FKBP9P1 has been shown to be increased in HNSCC samples and cells. Over-expression of this gene has been correlated with advanced T, N and clinical stages as well as poor prognosis of affected individuals. FKBP9P1 silencing has suppressed proliferation, migratory potential, and invasiveness of these cells, possibly through inhibition of PI3K/AKT signaling [38]. PFN2 is another up-regulated gene in HNSCC and cells. PFN2 silencing has suppressed proliferation, invasiveness, and migratory potential of HNSCC cells, possibly through reduction of Akt and GSK-3β phosphorylation as well as decrease in β-catenin levels. In other words, PFN2 has been shown to promote proliferation and metastatic ability of HNSCC through inducing activity of the PI3K/Akt/β-catenin pathway [39]. Similarly, DKK3 has been shown to increase the malignant properties of HNSCC via the PI3K/AKT/mTOR and MAPK pathways [40].
An in vitro study has shown that the anti-cancer agent osthole induces cell cycle arrest at G2/M phase and blocks proliferation of HNSCC cells via suppressing the PI3K/AKT pathway [41]. Finally, PI3K/AKT pathway has been shown to mediate the adaptive resistance to anti-programmed death-1 (PD1) therapy through upregulating Tim-3 [42]. Table 6 shows the role of PI3K/AKT pathway in head and neck squamous cell carcinoma.
Cutaneous SCC
α-mangostin has been shown to suppress skin tumor formation and growth, decrease levels of pro-inflammatory molecules and increase levels of anti-inflammatory ones both in tumor and circulation. Notably, this substance could induce autophagy of skin cancer cells and regulate expression of autophagy-related proteins. Most notably, α-mangostin can inhibit activity of the PI3K/AKT/mTOR signaling, as demonstrated by down-regulation of p-PI3K, p-Akt and p-mTOR [115]. Moreover, Lapatinib could suppress epithelial-mesenchymal transition in skin SCC via modulation of WNT/ERK/PI3K/AKT axis [116]. The anti-cancer effects of Lactucopicrin in skin cancer is also mediated through modulation of PI3K/AKT/mTOR pathway [103].
A number of non-coding RNAs can also modulate progression of skin SCC through influencing activity of PI3K/AKT pathway. For instance, miR-451a via PDPK1-mediated PI3K/AKT modulation could prevent progression of skin SCC [117]. Moreover, lncRNA LINC00520 via inactivating the PI3K/AKT pathway by downregulating EGFR could prevent the progression of this type of cancer [118]. Table 7 shows the role of PI3K/AKT pathway in skin SCC. Figure 2 represents the role of several ncRNAs in various types of SCCs via regulating the PI3K/AKT/mTOR signaling pathway.
A schematic representation of the role of several ncRNAs in regulating the PI3K/AKT/mTOR signaling pathway in OSCC, TSCC and Cutaneous SCC. Accumulating evidence has revealed that various ncRNAs (lncRNAs, circRNAs, and miRNAs) could be negatively involved in triggering different kinds of SCCs via activating PI3K/AKT/mTOR signaling cascade. As an illustration, previous study has authenticated that up-regulation of lncRNA MALAT1 could promote the proliferation, migration, and invasion of tongue cancer cells via increasing the expression levels of AKT and MMP-9 [72]. Another finding confirms that overexpression of miR-21-5p could inhibit apoptosis via down-regulating the expression levels of PDCD4 as well as pro-apoptotic protein Bax and up-regulating FOXO1 and Bcl2 through directly activating the PI3K/AKT pathway in tongue squamous cell carcinoma [79]. Furthermore, mounting research has demonstrated that miRNA‑451a via directly targeting PDPK1 could suppress cutaneous squamous cell carcinoma development by modulating the PI3K/AKT signaling pathway [117]. Green lines indicate the positive regulatory effect among ncRNAs and their targets, and red lines depict negative one among them. All the information regarding the role of these ncRNAs involved in the regulation of the PI3K/AKT signaling pathway in several kinds of squamous cell carcinomas can be seen in Tables 6, 7
Discussion
PI3K/AKT has essential roles in the development of different types of SCC. Over-expression of PI3K, AKT, and p-mTOR has been reported in SCC tumors in association with down-regulation or absence of PTEN [122]. Gain of function mutations in constituents of this pathway, amplification of PIK3CA and AKT, overexpression of AKT and inactivating mutations or loss of PTEN are involved in the aberrant activity of this signaling pathway and subsequent progression of cancer {Simpson, 2015 #155}. Thus, identification of the underlying mechanism of over-activation of PI3K/AKT pathway in SCC has practical significance in design of novel therapeutic options.
Moreover, a number of anti-cancer drugs such as cisplatin, LY294002, Licochalcone A, Mukonal, Dehydrocostus Lactone, Curcumin, Chloroquine, Osthole, Vitamin E succinate, Dasatinib, Tanshinone IIA, Genipin, Pristimerin, 5 fluorouracil, Sanguinarine, doxorubicin, AD198, Salvanic acid B, Oridonin, Plumbagin, a-mangostin, Lapatinib, Lactucopicrin and caffeic acid n-butyl ester have been found to exert their therapeutic effects in SCC via modulation of this pathway. It is worth mentioning that drug-loaded nanospheres and microspheres as a novel strategy for drug delivery can be used to form a material, mechanism, and cell combination that can not only treat the disease, but also verify the pathway. The possibility of using these systems for delivery of afore-mentioned drugs should be studies in future studies.
In brief, the bulk of evidence shows the impact of dysregulation of PI3K/AKT pathway in the pathogenesis of SCC and determination of survival of patients with this type of cancer. Moreover, targeted therapies against this pathway have been found to be effective in reduction of tumor burden both in animal models and clinical settings. Since this pathway has an established role in the induction of epithelial-mesenchymal transition, these therapies are expected to affect tumor metastasis as well. Besides, therapeutic modalities against PI3K/AKT might act in a synergic manner with other anti-cancer modalities, enhancing the survival of affected individuals. PI3K/AKT pathway can also act as a mediator of HPV-induced cancer stem-like cells features in SCC [59], further highlighting the importance of this pathway in malignant features of SCC.
Finally, a number of molecules that regulate PI3K/AKT pathway can be used as diagnostic markers for different types of SCCs.
Recent studies have also indicated the impact on non-coding RNAs in the regulation of PI3K/AKT pathway in different cancers, including SCC [123]. Thus, when designing novel therapeutic options against this pathway, it is necessary to consider the regulatory roles of these transcripts and their expression levels in these patients. Such approach may lead to establishment of a more effective personalized therapeutic strategy.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
References
Jiang N, Dai Q, Su X, Fu J, Feng X, Peng J. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Mol Biol Rep. 2020;47(6):4587–629.
Fayard E, Moncayo G, Hemmings BA, Holländer GA. Phosphatidylinositol 3-kinase signaling in thymocytes the need for stringent control. Sci Signal. 2010;3(135):re5-re.
Fruman DA, Limon JJ. Akt and mTOR in B cell activation and differentiation. Front Immunol. 2012;3:228.
Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15(23):6541–51.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101.
Hou M-M, Liu X, Wheler J, Naing A, Hong D, Coleman RL, et al. Targeted PI3K/AKT/mTOR therapy for metastatic carcinomas of the cervix: a phase I clinical experience. Oncotarget. 2014;5(22):11168–79.
Srinivas KP, Viji R, Dan VM, Sajitha IS, Prakash R, Rahul PV, et al. DEPTOR promotes survival of cervical squamous cell carcinoma cells and its silencing induces apoptosis through downregulating PI3K/AKT and by up-regulating p38 MAP kinase. Oncotarget. 2016;7(17):24154–71.
Li R, Song Y, Zhou L, Li W, Zhu X. Downregulation of RAGE inhibits cell proliferation and induces apoptosis via regulation of PI3K/AKT pathway in cervical squamous cell carcinoma. Onco Targets Ther. 2020;13:2385.
Prasad SB, Yadav SS, Das M, Modi A, Kumari S, Pandey LK, et al. PI3K/AKT pathway-mediated regulation of p27Kip1 is associated with cell cycle arrest and apoptosis in cervical cancer. Cell Oncol. 2015;38(3):215–25.
Zhang D, Sun G, Zhang H, Tian J, Li Y. Long non-coding RNA ANRIL indicates a poor prognosis of cervical cancer and promotes carcinogenesis via PI3K/Akt pathways. Biomed Pharmacother. 2017;85:511–6.
Wang C, Gu W, Zhang Y, Ji Y, Wen Y, Xu X. Nicotine promotes cervical carcinoma cell line HeLa migration and invasion by activating PI3k/Akt/NF-κB pathway in vitro. Exp Toxicol Pathol. 2017;69(6):402–7.
Shu X-R, Wu J, Sun H, Chi L-Q, Wang J-H. PAK4 confers the malignance of cervical cancers and contributes to the cisplatin-resistance in cervical cancer cells via PI3K/AKT pathway. Diagn Pathol. 2015;10(1):177.
Feng T, Zheng L, Liu F, Xu X, Mao S, Wang X, et al. Growth factor progranulin promotes tumorigenesis of cervical cancer via PI3K/Akt/mTOR signaling pathway. Oncotarget. 2016;7(36):58381–95.
Huang L, Huang Z, Fan Y, He L, Ye M, Shi K, et al. FOXC1 promotes proliferation and epithelial-mesenchymal transition in cervical carcinoma through the PI3K-AKT signal pathway. Am J Transl Res. 2017;9(3):1297–306.
Zhang W, Zhou Q, Wei Y, Da M, Zhang C, Zhong J, et al. The exosome-mediated PI3k/Akt/mTOR signaling pathway in cervical cancer. Int J Clin Exp Pathol. 2019;12(7):2474–84.
Tsai J-P, Lee C-H, Ying T-H, Lin C-L, Lin C-L, Hsueh J-T, et al. Licochalcone A induces autophagy through PI3K/Akt/mTOR inactivation and autophagy suppression enhances Licochalcone A-induced apoptosis of human cervical cancer cells. Oncotarget. 2015;6(30):28851–66.
Li A, Gu Y, Li X, Sun H, Zha H, Xie J, et al. S100A6 promotes the proliferation and migration of cervical cancer cells via the PI3K/Akt signaling pathway. Oncol Lett. 2018;15(4):5685–93.
Xu J, Zhu W, Chen L, Liu L. MicroRNA-433 inhibits cell growth and induces apoptosis in human cervical cancer through PI3K/AKT signaling by targeting FAK. Oncol Rep. 2018;40(6):3469–78.
Lu R, Yang Z, Xu G, Yu S. miR-338 modulates proliferation and autophagy by PI3K/AKT/mTOR signaling pathway in cervical cancer. Biomed Pharmacother. 2018;105:633–44.
Li L, Huizhi L, Binu W, Xinxin D, Longjun W, Liping Y, et al. Anticancer activity of mukonal against human laryngeal cancer cells involves apoptosis, cell cycle arrest, and inhibition of PI3K/AKT and MEK/ERK signalling pathways. Med Sci Monit. 2018;24:7295.
Zhang R, Hao J, Wu Q, Guo K, Wang C, Zhang WK, et al. Dehydrocostus lactone inhibits cell proliferation and induces apoptosis by PI3K/Akt/Bad and ERS signalling pathway in human laryngeal carcinoma. J Cell Mol Med. 2020;24(11):6028–42.
Starska K, Forma E, Lewy-Trenda I, Stasikowska-Kanicka O, Skóra M, Bryś M. Fibroblast growth factor receptor 1 and 3 expression is associated with regulatory PI3K/AKT kinase activity, as well as invasion and prognosis, in human laryngeal cancer. Cell Oncol. 2018;41(3):253–68.
Zhao R, Tian L, Zhao B, Sun Y, Cao J, Chen K, et al. FADS1 promotes the progression of laryngeal squamous cell carcinoma through activating AKT/mTOR signaling. Cell Death Dis. 2020;11(4):1–14.
Gao W, Guo H, Niu M, Zheng X, Zhang Y, Xue X, et al. circPARD3 drives malignant progression and chemoresistance of laryngeal squamous cell carcinoma by inhibiting autophagy through the PRKCI-Akt-mTOR pathway. Mol Cancer. 2020;19(1):1–22.
Gao W, Zhang Y, Luo H, Niu M, Zheng X, Hu W, et al. Targeting SKA3 suppresses the proliferation and chemoresistance of laryngeal squamous cell carcinoma via impairing PLK1–AKT axis-mediated glycolysis. Cell Death Dis. 2020;11(10):1–19.
Tang T, Xiao Z-Y, Shan G, Lei H-B. Descending-SHIP2-mediated radiosensitivity enhancement through PI3K/Akt signaling pathway in laryngeal squamous cell carcinoma. Biomed Pharmacother. 2019;118: 109392.
Ye D, Zhou C, Deng H, Lin L, Zhou S. MicroRNA-145 inhibits growth of laryngeal squamous cell carcinoma by targeting the PI3K/Akt signaling pathway. Cancer Manag Res. 2019;11:3801.
Zhu Y, Yan L, Zhu W, Song X, Yang G, Wang S. MMP2/3 promote the growth and migration of laryngeal squamous cell carcinoma via PI3K/Akt-NF-κB-mediated epithelial–mesenchymal transformation. J Cell Physiol. 2019;234(9):15847–55.
Tian L, Tao Z, Ye H, Li G, Zhan Z, Tuo H. Over-expression of MEOX2 promotes apoptosis through inhibiting the PI3K/Akt pathway in laryngeal cancer cells. Neoplasma. 2018;65(5):745–52.
Ni HS, Hu SQ, Chen X, Liu YF, Ni TT, Cheng L. Tra2β silencing suppresses cell proliferation in laryngeal squamous cell carcinoma via inhibiting PI3K/AKT signaling. Laryngoscope. 2019;129(9):E318–28.
Zhu X, Zhu R. Curcumin suppresses the progression of laryngeal squamous cell carcinoma through the upregulation of miR-145 and inhibition of the PI3K/Akt/mTOR pathway. Onco Targets Ther. 2018;11:3521.
Si F, Sun J, Wang C. MicroRNA-138 suppresses cell proliferation in laryngeal squamous cell carcinoma via inhibiting EZH2 and PI3K/AKT signaling. Exp Ther Med. 2017;14(3):1967–74.
Wang B, Lv K, Chen W, Zhao J, Luo J, Wu J, et al. miR-375 and miR-205 regulate the invasion and migration of laryngeal squamous cell carcinoma synergistically via AKT-mediated EMT. BioMed Res Int. 2016;2016:11.
Lian R, Lu B, Jiao L, Li S, Wang H, Miao W, et al. MiR-132 plays an oncogenic role in laryngeal squamous cell carcinoma by targeting FOXO1 and activating the PI3K/AKT pathway. Eur J Pharmacol. 2016;792:1–6.
Zhu H, Lv Z, An C, Shi M, Pan W, Zhou L, et al. Onco-lncRNA HOTAIR and its functional genetic variants in papillary thyroid carcinoma. Sci Rep. 2016;23(6):31969.
Wang B, Qin H, Wang Y, Chen W, Luo J, Zhu X, et al. Effect of DJ-1 overexpression on the proliferation, apoptosis, invasion and migration of laryngeal squamous cell carcinoma SNU-46 cells through PI3K/AKT/mTOR. Oncol Rep. 2014;32(3):1108–16.
Lu B, Di W, Wang H, Ma H, Li J, Zhang Q. Tumor suppressor TSLC1 is implicated in cell proliferation, invasion and apoptosis in laryngeal squamous cell carcinoma by regulating Akt signaling pathway. Tumor Biol. 2012;33(6):2007–17.
Yang Y-F, Feng L, Shi Q, Ma H-Z, He S-Z, Hou L-Z, et al. Silencing novel long non-coding RNA FKBP9P1 represses malignant progression and inhibits PI3K/AKT signaling of head and neck squamous cell carcinoma in vitro. Chin Med J. 2020;133(17):2037.
Zhou K, Chen J, Wu J, Xu Y, Wu Q, Yue J, et al. Profilin 2 promotes proliferation and metastasis of head and neck cancer cells by regulating pi3k/akt/β-catenin signaling pathway. Oncol Res. 2019;27(9):1079–88.
Katase N, Nishimatsu SI, Yamauchi A, Yamamura M, Fujita S. DKK3 knockdown confers negative effects on the malignant potency of head and neck squamous cell carcinoma cells via the PI3K/Akt and MAPK signaling pathways. Int J Oncol. 2019;54(3):1021–32.
Yang J, Zhu X-J, Jin M-Z, Cao Z-W, Ren Y-Y, Gu Z-W. Osthole induces cell cycle arrest and apoptosis in head and neck squamous cell carcinoma by suppressing the PI3K/AKT signaling pathway. Chem Biol Interact. 2020;316:108934.
Shayan G, Srivastava R, Li J, Schmitt N, Kane LP, Ferris RL. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology. 2017;6(1): e1261779.
Bernard M, Cardin GB, Cahuzac M, Ayad T, Bissada E, Guertin L, et al. Dual inhibition of autophagy and PI3K/AKT/MTOR pathway as a therapeutic strategy in head and neck squamous cell carcinoma. Cancers. 2020;12(9):2371.
Yang S, Ji Q, Chang B, Wang Y, Zhu Y, Li D, et al. STC2 promotes head and neck squamous cell carcinoma metastasis through modulating the PI3K/AKT/Snail signaling. Oncotarget. 2017;8(4):5976.
Lee S, Kopp F, Chang T-C, Sataluri A, Chen B, Sivakumar S, et al. Noncoding RNA NORAD regulates genomic stability by sequestering PUMILIO proteins. Cell. 2016;164(1–2):69–80.
Zhu G-C, Yu C-Y, She L, Tan H-L, Li G, Ren S-L, et al. Metadherin regulation of vascular endothelial growth factor expression is dependent upon the PI3K/Akt pathway in squamous cell carcinoma of the head and neck. Medicine. 2015;94(6): e502.
Wu Y-R, Qi H-J, Deng D-F, Luo Y-Y, Yang S-L. MicroRNA-21 promotes cell proliferation, migration, and resistance to apoptosis through PTEN/PI3K/AKT signaling pathway in esophageal cancer. Tumor Biol. 2016;37(9):12061–70.
Zhu Z, Yu W, Fu X, Sun M, Wei Q, Li D, et al. Phosphorylated AKT1 is associated with poor prognosis in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2015;34(1):1–8.
Chen J, Lan T, Zhang W, Dong L, Kang N, Zhang S, et al. Platelet-activating factor receptor-mediated PI3K/AKT activation contributes to the malignant development of esophageal squamous cell carcinoma. Oncogene. 2015;34(40):5114–27.
Yang P, Zhao J, Hou L, Yang L, Wu K, Zhang L. Vitamin E succinate induces apoptosis via the PI3K/AKT signaling pathways in EC109 esophageal cancer cells. Mol Med Rep. 2016;14(2):1531–7.
Chen J, Lan T, Zhang W, Dong L, Kang N, Fu M, et al. Dasatinib enhances cisplatin sensitivity in human esophageal squamous cell carcinoma (ESCC) cells via suppression of PI3K/AKT and Stat3 pathways. Arch Biochem Biophys. 2015;575:38–45.
Zhang W, Lei C, Fan J, Wang J. miR-18a promotes cell proliferation of esophageal squamous cell carcinoma cells by increasing cylin D1 via regulating PTEN-PI3K-AKT-mTOR signaling axis. Biochem Biophys Res Commun. 2016;477(1):144–9.
Tian B, Chen X, Zhang H, Li X, Wang J, Han W, et al. Urokinase plasminogen activator secreted by cancer-associated fibroblasts induces tumor progression via PI3K/AKT and ERK signaling in esophageal squamous cell carcinoma. Oncotarget. 2017;8(26):42300.
Lu H, Jiang T, Ren K, Li ZL, Ren J, Wu G, et al. RUNX2 plays an oncogenic role in esophageal carcinoma by activating the PI3K/AKT and ERK signaling pathways. Cell Physiol Biochem. 2018;49(1):217–25.
Javid H, Asadi J, Avval FZ, Afshari AR, Hashemy SI. The role of substance P/neurokinin 1 receptor in the pathogenesis of esophageal squamous cell carcinoma through constitutively active PI3K/Akt/NF-κB signal transduction pathways. Mol Biol Rep. 2020;47(3):2253–63.
Zhang H-F, Alshareef A, Wu C, Li S, Jiao J-W, Cao H-H, et al. Loss of miR-200b promotes invasion via activating the Kindlin-2/integrin β1/AKT pathway in esophageal squamous cell carcinoma: an E-cadherin-independent mechanism. Oncotarget. 2015;6(30):28949.
Wu N, Du Z, Zhu Y, Song Y, Pang L, Chen Z. The expression and prognostic impact of the PI3K/AKT/mTOR signaling pathway in advanced esophageal squamous cell carcinoma. Technol Cancer Res Treat. 2018;17:1533033818758772.
Kodama T, Koma Y-I, Arai N, Kido A, Urakawa N, Nishio M, et al. CCL3–CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab Invest. 2020;100(9):1140–57.
Xi R, Pan S, Chen X, Hui B, Zhang L, Fu S, et al. HPV16 E6–E7 induces cancer stem-like cells phenotypes in esophageal squamous cell carcinoma through the activation of PI3K/Akt signaling pathway in vitro and in vivo. Oncotarget. 2016;7(35):57050.
Peng X, Liu Y, Zhu S, Peng X, Li H, Jiao W, et al. Co-targeting PI3K/Akt and MAPK/ERK pathways leads to an enhanced antitumor effect on human hypopharyngeal squamous cell carcinoma. J Cancer Res Clin Oncol. 2019;145(12):2921–36.
Hsu C-M, Lin P-M, Tsai Y-T, Tsai M-S, Tseng C-H, Lin S-F, et al. NVP-BEZ235, a dual PI3K-mTOR inhibitor, suppresses the growth of FaDu hypopharyngeal squamous cell carcinoma and has a synergistic effect with Cisplatin. Cell Death Discov. 2018;4(1):1–10.
Zhang J, An X, Han Y, Ma R, Yang K, Zhang L, et al. Overexpression of JARID1B promotes differentiation via SHIP1/AKT signaling in human hypopharyngeal squamous cell carcinoma. Cell Death Dis. 2016;7(9):e2358-e.
Wang C, Lin C, Tao Q, Zhao S, Liu H, Li L. Evaluation of calcium-binding protein A11 promotes the carcinogenesis of hypopharygeal squamous cell carcinoma via the PI3K/AKT signaling pathway. Am J Transl Res. 2019;11(6):3472.
Zhang Y, Wang B, Chen X, Li W, Dong P. AGO2 involves the malignant phenotypes and FAK/PI3K/AKT signaling pathway in hypopharyngeal-derived FaDu cells. Oncotarget. 2017;8(33):54735.
Zhang Y, Cong L, He J, Wang Y, Zou Y, Yang Z, et al. Photothermal treatment with EGFRmAb–AuNPs induces apoptosis in hypopharyngeal carcinoma cells via PI3K/AKT/mTOR and DNA damage response pathways. Acta Biochim Biophys Sin. 2018;50(6):567–78.
Horn D, Freudlsperger C, Holzinger D, Kunzmann K, Plinkert P, Dyckhoff G, et al. Upregulation of pAKT (Ser473) expression in progression of HPV-positive oropharyngeal squamous cell carcinoma. Head Neck. 2017;39(12):2397–405.
Won HS, Jung C-K, Chun SH, Kang J-H, Kim Y-S, Sun D-I, et al. Difference in expression of EGFR, pAkt, and PTEN between oropharyngeal and oral cavity squamous cell carcinoma. Oral Oncol. 2012;48(10):985–90.
Choi MS, Moon SM, Lee SA, Park BR, Kim JS, Kim DK, et al. Adenosine induces intrinsic apoptosis via the PI3K/Akt/mTOR signaling pathway in human pharyngeal squamous carcinoma FaDu cells Corrigendum in/https://doi.org/10.3892/ol.2019.10014. Oncol Lett. 2018;15(5):6489–96.
Wang R, Lu X, Yu R. Lycopene inhibits epithelial-mesenchymal transition and promotes apoptosis in oral cancer via PI3K/AKT/m-TOR signal pathway. Drug Des Dev Ther. 2020;14:2461.
Ren X, Luo W. Exploration of pro-apoptotic effect of Thymoquinone on oral squamous cell carcinoma cells through PI3K/Akt signaling pathway. Cell Mol Biol (Noisy-le-grand). 2019;65(1):61–4.
Hao Y, Zhang C, Sun Y, Xu H. Licochalcone A inhibits cell proliferation, migration, and invasion through regulating the PI3K/AKT signaling pathway in oral squamous cell carcinoma. Onco Targets Ther. 2019;12:4427.
Yuan J, Xu X, Lin Y, Chen Q, Sun W, Tang L, et al. LncRNA MALAT1 expression inhibition suppresses tongue squamous cell carcinoma proliferation, migration and invasion by inactivating PI3K/Akt pathway and downregulating MMP-9 expression. Eur Rev Med Pharmacol Sci. 2019;23(1):198–206.
Li Z, Liu J, Que L, Tang X. The immunoregulatory protein B7–H3 promotes aerobic glycolysis in oral squamous carcinoma via PI3K/Akt/mTOR pathway. J Cancer. 2019;10(23):5770.
Li C, Lin X, Wang J, Ren X. FBXW7 inhibited cell proliferation and invasion regulated by miR-27a through PI3K/AKT signaling pathway and epithelial-to-mesenchymal transition in oral squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2020;24(7):3701–9.
Wang H, Wang L, Zhou X, Luo X, Liu K, Jiang E, et al. OSCC exosomes regulate miR-210–3p targeting EFNA3 to promote oral cancer angiogenesis through the PI3K/AKT pathway. BioMed Res Int. 2020;2020:13.
Fan Q-C, Tian H, Wang Y, Liu X-B. Integrin-α5 promoted the progression of oral squamous cell carcinoma and modulated PI3K/AKT signaling pathway. Arch Oral Biol. 2019;101:85–91.
Jing Y, Jin Y, Wang Y, Chen S, Zhang X, Song Y, et al. SPARC promotes the proliferation and metastasis of oral squamous cell carcinoma by PI3K/AKT/PDGFB/PDGFRβ axis. J Cell Physiol. 2019;234(9):15581–93.
Kowshik J, Nivetha R, Ranjani S, Venkatesan P, Selvamuthukumar S, Veeravarmal V, et al. Astaxanthin inhibits hallmarks of cancer by targeting the PI3K/NF-κΒ/STAT3 signalling axis in oral squamous cell carcinoma models. IUBMB Life. 2019;71(10):1595–610.
Liu C, Tong Z, Tan J, Xin Z, Wang Z, Tian L. MicroRNA-21-5p targeting PDCD4 suppresses apoptosis via regulating the PI3K/AKT/FOXO1 signaling pathway in tongue squamous cell carcinoma. Exp Ther Med. 2019;18(5):3543–51.
Liu H, Gong X, Yang K. Overexpression of the clock gene Per2 suppresses oral squamous cell carcinoma progression by activating autophagy via the PI3K/AKT/mTOR pathway. J Cancer. 2020;11(12):3655.
Qu Z, Zhang R, Su M, Liu W. USP13 serves as a tumor suppressor via the PTEN/AKT pathway in oral squamous cell carcinoma. Cancer Manag Res. 2019;11:9175.
Guo L, Yang G, Kang Y, Li S, Duan R, Shen L, et al. Construction and analysis of a ceRNA network reveals potential prognostic markers in colorectal cancer. Front Genet. 2020;11:418.
Tang K-L, Tang H-Y, Du Y, Tian T, Xiong S-J. PAR-2 promotes cell proliferation, migration, and invasion through activating PI3K/AKT signaling pathway in oral squamous cell carcinoma. 2019. Biosci Rep. https://doi.org/10.1042/BSR20182476.
Tang G, Tang Q, Jia L, Chen Y, Lin L, Kuai X, et al. TROP2 increases growth and metastasis of human oral squamous cell carcinoma through activation of the PI3K/Akt signaling pathway. Int J Mol Med. 2019;44(6):2161–70.
Tomita R, Sasabe E, Tomomura A, Yamamoto T. Macrophage-derived exosomes attenuate the susceptibility of oral squamous cell carcinoma cells to chemotherapeutic drugs through the AKT/GSK-3β pathway. Oncol Rep. 2020;44(5):1905–16.
Tsai C-N, Tsai C-L, Yi J-S, Kao H-K, Huang Y, Wang C-I, et al. Activin A regulates the epidermal growth factor receptor promoter by activating the PI3K/SP1 pathway in oral squamous cell carcinoma cells. Sci Rep. 2019;9(1):1–14.
Velu P, Vijayalakshmi A, Vinothkumar V. Inhibiting the PI3K/Akt, NF-κB signalling pathways with syringic acid for attenuating the development of oral squamous cell carcinoma cells SCC131. J Pharm Pharmacol. 2020;72(11):1595–606.
Wang H, Wu B, Wang H. Alpha-hederin induces the apoptosis of oral cancer SCC-25 cells by regulating PI3K/Akt/mTOR signaling pathway. Electron J Biotechnol. 2019;38:27–31.
Wang L, Song Y, Wang H, Liu K, Shao Z, Shang Z. MiR-210-3p-EphrinA3-PI3K/AKT axis regulates the progression of oral cancer. J Cell Mol Med. 2020;24(7):4011–22.
Wang J, Jiang C, Li N, Wang F, Xu Y, Shen Z, et al. The circEPSTI1/mir-942-5p/LTBP2 axis regulates the progression of OSCC in the background of OSF via EMT and the PI3K/Akt/mTOR pathway. Cell Death Dis. 2020;11(8):1–18.
Wei M, Wu Y, Liu H, Xie C. Genipin induces autophagy and suppresses cell growth of oral squamous cell carcinoma via PI3K/AKT/MTOR pathway. Drug Des Dev Ther. 2020;14:395.
Wu H, Li L, Ai Z, Yin J, Chen L. Pristimerin induces apoptosis of oral squamous cell carcinoma cells via G1 phase arrest and MAPK/Erk1/2 and Akt signaling inhibition. Oncol Lett. 2019;17(3):3017–25.
Wu J, Wang X, Shang A, Vella G, Sun Z, Ji P, et al. PLAC8 inhibits oral squamous cell carcinogenesis and epithelial-mesenchymal transition via the Wnt/β-catenin and PI3K/Akt/GSK3β signaling pathways. Oncol Lett. 2020;20(5):1.
Yang Y, Chen D, Liu H, Yang K. Increased expression of lncRNA CASC9 promotes tumor progression by suppressing autophagy-mediated cell apoptosis via the AKT/mTOR pathway in oral squamous cell carcinoma. Cell Death Dis. 2019;10(2):1–16.
Yang G, Yang Y, Tang H, Yang K. Loss of the clock gene Per1 promotes oral squamous cell carcinoma progression via the AKT/mTOR pathway. Cancer Sci. 2020;111(5):1542.
Zhang X, Ding H, Lu Z, Ding L, Song Y, Jing Y, et al. Increased LGALS3BP promotes proliferation and migration of oral squamous cell carcinoma via PI3K/AKT pathway. Cell Signal. 2019;63: 109359.
Zheng Y, Wang Z, Xiong X, Zhong Y, Zhang W, Dong Y, et al. Membrane-tethered Notch1 exhibits oncogenic property via activation of EGFR–PI3K–AKT pathway in oral squamous cell carcinoma. J Cell Physiol. 2019;234(5):5940–52.
Zhou Y-M, Yao Y-L, Liu W, Shen X-M, Shi L-J, Wu L. MicroRNA-134 inhibits tumor stem cell migration and invasion in oral squamous cell carcinomas via downregulation of PI3K-Akt signaling pathway by inhibiting LAMC2 expression. Cancer Biomark. 2020. https://doi.org/10.3233/CBM-191362.
Liu M, Gao X, Liu C. Increased expression of lncRNA FTH1P3 promotes oral squamous cell carcinoma cells migration and invasion by enhancing PI3K/Akt/GSK3b/Wnt/beta-catenin signaling. Eur Rev Med Pharmacol Sci. 2018;22(23):8306–14.
Chi H. miR-194 regulated AGK and inhibited cell proliferation of oral squamous cell carcinoma by reducing PI3K-Akt-FoxO3a signaling. Biomed Pharmacother. 2015;71:53–7.
Ferreira DM, Neves TJ, Lima LGC, Alves FA, Begnami MD. Prognostic implications of the phosphatidylinositol 3-kinase/Akt signaling pathway in oral squamous cell carcinoma: overexpression of p-mTOR indicates an adverse prognosis. Appl Cancer Res. 2017;37(1):1–8.
Gao P, Li C, Chang Z, Wang X, Xuan M. Carcinoma associated fibroblasts derived from oral squamous cell carcinoma promote lymphangiogenesis via c-Met/PI3K/AKT in vitro. Oncol Lett. 2018;15(1):331–7.
Gu Y, Liu H, Kong F, Ye J, Jia X, Zhang Z, et al. miR-22/KAT6B axis is a chemotherapeutic determiner via regulation of PI3k-Akt-NF-kB pathway in tongue squamous cell carcinoma. J Exp Clin Cancer Res. 2018;37(1):1–14.
Lee TK, Park C, Jeong SJ, Jeong MJ, Kim GY, Kim WJ, et al. Sanguinarine induces apoptosis of human oral squamous cell carcinoma KB cells via inactivation of the PI3K/Akt signaling pathway. Drug Dev Res. 2016;77(5):227–40.
Matsuo FS, Andrade MF, Loyola AM, da Silva SJ, Silva MJB, Cardoso SV, et al. Pathologic significance of AKT, mTOR, and GSK3β proteins in oral squamous cell carcinoma-affected patients. Virchows Arch. 2018;472(6):983–97.
Smolensky D, Rathore K, Bourn J, Cekanova M. Inhibition of the PI3K/AKT pathway sensitizes oral squamous cell carcinoma cells to anthracycline-based chemotherapy in vitro. J Cell Biochem. 2017;118(9):2615–24.
Yu C-C, Hung S-K, Lin H-Y, Chiou W-Y, Lee M-S, Liao H-F, et al. Targeting the PI3K/AKT/mTOR signaling pathway as an effectively radiosensitizing strategy for treating human oral squamous cell carcinoma in vitro and in vivo. Oncotarget. 2017;8(40):68641.
Wang H, Deng X, Zhang J, Ou Z, Mai J, Ding S, et al. Elevated expression of zinc finger protein 703 promotes cell proliferation and metastasis through PI3K/AKT/GSK-3β signalling in oral squamous cell carcinoma. Cell Physiol Biochem. 2017;44(3):920–34.
Wang Q, Zhang X, Song X, Zhang L. Overexpression of T-cadherin inhibits the proliferation of oral squamous cell carcinoma through the PI3K/AKT/mTOR intracellular signalling pathway. Arch Oral Biol. 2018;96:74–9.
Wei J, Wu J, Xu W, Nie H, Zhou R, Wang R, et al. Salvianolic acid B inhibits glycolysis in oral squamous cell carcinoma via targeting PI3K/AKT/HIF-1α signaling pathway. Cell Death Dis. 2018;9(6):1–16.
Yang H, Wen L, Wen M, Liu T, Zhao L, Wu B, et al. FoxM1 promotes epithelial–mesenchymal transition, invasion, and migration of tongue squamous cell carcinoma cells through a c-Met/AKT-dependent positive feedback loop. Anticancer Drugs. 2018;29(3):216.
Yang J, Ren X, Zhang L, Li Y, Cheng B, Xia J. Oridonin inhibits oral cancer growth and PI3K/Akt signaling pathway. Biomed Pharmacother. 2018;100:226–32.
Pan S-T, Qin Y, Zhou Z-W, He Z-X, Zhang X, Yang T, et al. Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK-and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells. Drug Des Dev Ther. 2015;9:1601.
Zhu L, Shen Y, Sun W. Paraoxonase 3 promotes cell proliferation and metastasis by PI3K/Akt in oral squamous cell carcinoma. Biomed Pharmacother. 2017;85:712–7.
Wang F, Ma H, Liu Z, Huang W, Xu X, Zhang X. α-Mangostin inhibits DMBA/TPA-induced skin cancer through inhibiting inflammation and promoting autophagy and apoptosis by regulating PI3K/Akt/mTOR signaling pathway in mice. Biomed Pharmacother. 2017;92:672–80.
Yao M, Shang Y-Y, Zhou Z-W, Yang Y-X, Wu Y-S, Guan L-F, et al. The research on lapatinib in autophagy, cell cycle arrest and epithelial to mesenchymal transition via Wnt/ErK/PI3K-AKT signaling pathway in human cutaneous squamous cell carcinoma. J Cancer. 2017;8(2):220.
Fu J, Zhao J, Zhang H, Fan X, Geng W, Qiao S. MicroRNA-451a prevents cutaneous squamous cell carcinoma progression via the 3-phosphoinositide-dependent protein kinase-1-mediated PI3K/AKT signaling pathway. Exp Therapeutic Med. 2021;21(2):1.
Mei X-L, Zhong S. Long noncoding RNA LINC00520 prevents the progression of cutaneous squamous cell carcinoma through the inactivation of the PI3K/Akt signaling pathway by downregulating EGFR. Chin Med J. 2019;132(4):454.
Zeng N, Hongbo T, Xu Y, Wu M, Wu Y. Anticancer activity of caffeic acid n-butyl ester against A431 skin carcinoma cell line occurs via induction of apoptosis and inhibition of the mTOR/PI3K/AKT signaling pathway. Mol Med Rep. 2018;17(4):5652–7.
Ci C, Wu C, Lyu D, Chang X, He C, Liu W, et al. Downregulation of kynureninase restrains cutaneous squamous cell carcinoma proliferation and represses the PI3K/AKT pathway. Clin Exp Dermatol. 2020;45(2):194–201.
Sun Y, Tu Y, He L, Ji C, Cheng B. High mobility group box 1 regulates tumor metastasis in cutaneous squamous cell carcinoma via the PI3K/AKT and MAPK signaling pathways. Oncol Lett. 2016;11(1):59–62.
Marques AEM, Borges GA, Viesi CH, Vianna LMS, Ramos D, Castilho RM, et al. Expression profile of the PI3K-AKT-mTOR pathway in head and neck squamous cell carcinoma: data from Brazilian population. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133(4):453–61.
Ghafouri-Fard S, Abak A, Tondro Anamag F, Shoorei H, Majidpoor J, Taheri M. The emerging role of non-coding RNAs in the regulation of PI3K/AKT pathway in the carcinogenesis process. Biomed pharmacother. 2021;137:111279.
Acknowledgements
The authors would like to thank the clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their support, cooperation and assistance throughout the period of study (Grant Number 43002643).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
SGF wrote the manuscript and revised it. MT and EJ supervised and designed the study. TK, AA and BMH collected the data and designed the figures and tables. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent forms were obtained from all study participants. The study protocol was approved by the ethical committee of Shahid Beheshti University of Medical Sciences. All methods were performed in accordance with the relevant guidelines and regulations.
Consent of publication
Not applicable.
Competing interests
The authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ghafouri-Fard, S., Noie Alamdari, A., Noee Alamdari, Y. et al. Role of PI3K/AKT pathway in squamous cell carcinoma with an especial focus on head and neck cancers. Cancer Cell Int 22, 254 (2022). https://doi.org/10.1186/s12935-022-02676-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02676-x