Abstract
miR-630 is encoded by MIR630 gene (NC_000015.10) on 15q24.1. This miRNA is mostly associated with cytokine signaling in immune system. Several neoplastic as well as non-neoplastic conditions have been linked with dysregulation of miR-630. It is an oncogenic miRNA in renal cell carcinoma, multiple myeloma, colorectal cancer, acute lymphoblastic leukemia, ovarian cancer and prostate cancer. On the other hand, it is a putative tumor suppressor miRNA in lung, cervical, breast, thyroid and esophageal tissues. In a number of other tissues, data regarding the role of miR-630 in the carcinogenesis is conflicting. Expression levels of miR-630 can be used as markers for prediction of cancer course. Moreover, miR-630 can influence response to chemoradiotherapy. This miRNA is also involved in the pathoetiology of IgA nephropathy, obstructive sleep apnea, age-related nuclear cataract and vitiligo. In the present review, we discuss the role of miR-630 in these conditions.
Similar content being viewed by others
Introduction
MicroRNAs (miRNAs) are small sized transcripts with no capacity of protein coding [1]. miRNAs can bind to coding sequences and miRNA seeds in coding regions are coordinated with regulation/influencing the targets [2,3,4]. Moreover, various molecular techniques like reporter assays confirms that miRNA seeds in coding region can be functional and influence the protein synthesis [5, 6]. Mature miRNAs have sizes about 22 nucleotides and are made from their precursors in a multistep process being catalyzed by Drosha and Dicer RNase III proteins. These transcripts serve as guide molecules for suppression of expression of target RNAs. Since they can target the majority of protein-coding RNAs, miRNAs partake in virtually total physiological and pathological events. miRNA synthesis is tightly controlled in terms of time and location. Not surprisingly, dysregulation of expression of miRNAs is linked with the pathoetiology of several human diseases, above all being cancer [7]. miR-630 is an example of these transcripts which is encoded by MIR630 gene (NC_000015.10) on 15q24.1. This miRNA is mostly associated with cytokine signaling in immune system. Moreover, this miRNA has functional interactions with other non-coding RNAs such as long non-coding RNAs and circular RNAs. Thus, it contributes in the construction of a complex interactive network that is implicated in the pathogenesis of human disorders. Several neoplastic as well as non-neoplastic conditions such as IgA nephropathy, obstructive sleep apnea, cataract, vitiligo and heterotopic ossification have been linked with dysregulation of miR-630. In malignant conditions, both high throughput sequencing methods and candidate gene expression analyses have confirmed association between dysregulation of miR-630 and disease progression. Although there are several miRNAs that play important roles in the pathogenesis of disorders, we have selected miR-630 with respect to being targeted by important upstream factors such as long non-coding RNA H19 and also targeting several important genes and participation in key signaling pathway such as AKT, P53, TGFβ-ERK/SP1, JNK/c-Jun, PI3K/AKT and JAK2/STAT3 which contribute in the development of various malignant and non-malignant diseases. Thus, in the present review, we discuss the role of miR-630 in these conditions.
miR-630 in malignancies
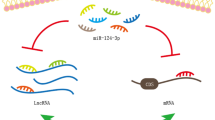
miR-630 has been found to be over-expressed in renal cancer cell lines compared with HK-2 normal renal cells. Inhibition of miR-630 has resulted in suppression of proliferation, migratory aptitude, and invasiveness of 786-O renal cancer cells. Moreover, apoptosis has been induced following miR-630 inhibition, indicating potential of this method for treatment of renal cancer [8]. miR‑630 has also been shown to promote proliferation of HCT116 colorectal cancer cells and inhibit their apoptosis. The impact of miR-630 on apoptosis of HCT116 cells has also been verified through the observed reduction of expressions of p27, BAX (BCL2 Associated X), procaspase‑3 and active caspase‑3. Moreover, miR-630 has enhanced levels of phosphorylated‑AKT and BCL2 (B-Cell CLL/Lymphoma 2). Thus, miR‑630 has an oncogenic role in colorectal cancer through modulation of p27 and AKT pathway [9]. In Jurkat cell line, miR-630 has induced cell proliferation and reduced cell apoptosis through affecting expressions of p53, p21 and BCL2 [10]. Expression of miR-630 has been reported to be increased in epithelial ovarian cancer tissues as compared with normal ovarian tissues. In SKOV3 cells, miR-630 up-regulation has shown pro-proliferative and pro-migratory effects, at least partly through targeting KLF6 (Krüppel-like Factor 6) [11]. Moreover, miR-630 has been shown to affect cell apoptosis and sensitivity of ovarian cancer cells to cisplatin through targeting PTEN (Phosphatase and Tensin Homolog) [12]. In a high throughput study using PCR array and NanoString techniques, miR-630 has been found to be among up-regulated miRNAs in tumoral tissues of young patients compared to normal tissues [13]. In hepatocellular carcinoma (HCC), two different studies have reported contradictory results regarding the role of miR-630. Zhang et al. have shown over-expression of miR-630 in HCC samples and cell lines compared with corresponding controls [14]. miR-630 expression has been found to be significantly elevated at advanced TNM stages [14]. Furthermore, up-regulation of miR-630 in tissue samples of HCC has been associated with elevation in serum levels of AFP (Alpha Fetoprotein), indicating its association with HCC progression [14]. On the other hand, Chen et al. have reported that down-regulation of miR-630 in HCC patients is associated with higher chance of tumor recurrence and shorter survival of patients [15]. Functionally, miR-630 has been shown to attenuate epithelial-mesenchymal transition (EMT) in HCC through targeting Slug [15]. Moreover, TGF-β (Transforming Growth Factor Beta 1)-Erk (Extracellular Signal-Regulated Kinase)/SP1 (Specificity Protein 1) and JNK (Jun N-Terminal Kinase)/c-Jun cascades have been demonstrated to repress transcription of miR-630 via taking the position of transcription factors on promoters. Forced over-expression of miR-630 has reinstated the TGF-β-associated EMT [15]. In non-small cell lung cancer (NSCLC), circMTDH.4 has been shown to regulate expression of AEG-1 (Astrocyte Elevated Gene-1) oncogene through sequestering miR-630 [16]. CircMTDH.4 silencing or miR-630 up-regulation has suppressed resistance of NSCLC cells to chemo/radiotherapy, indicating the importance of circMTDH.4/miR-630/AEG-1 axis in modulation of response of NSCLC cells to these therapeutic options [16]. According to colony formation assays, suppression of circ‐MTDH.4 with sh‐circMTDH.4 or/and miR‐630 up-regulation by miR‐630 mimic significantly increased 5‐FU or cisplatin‐induced cell death in A549 cells [16]. Consistent with this study, miR-630 has been found to be down-regulated in NSCLC tissues and cells [17]. Forced up-regulation of miR-630 could suppress proliferation, migration, and invasiveness of NSCLC cells through targeting LMO3 (LIM Domain Only 3), a gene that encodes a nuclear LIM-only protein [17]. miR-630 acts as up-stream regulator for LMO3 [17]. Restoration of LMO3 significantly reversed the anti-cancerous effects of miR-630 on cell proliferation, migration, and invasion in malignant cells. So, miR-630 inhibited the proliferation, migration, and invasion of NSCLC cells by down-regulation of LMO3 level [17]. Expression of miR-630 has been shown to be decreased in serum of gastric cancer patients compared with controls [18]. Notably, levels of miR-630 have been much lower in those having aggressive tumors [18]. Down-regulation of miR-630 has been correlated with poor prognosis of these patients [18]. The tumor suppressor role of miR-630 in gastric cancer has been further verified through the observed reduction in proliferation ability of SGC-7901 cells following over-expression of this miRNA [18]. Functionally, miR-630 exerts these effects through down-regulating expression of SOX4 (SRY-Box 4) [18]. Conversely, in a study conducted by Zhang et al., expression of miR-630 has been reported to be higher in gastric cancer tissues (intestinal, mixed, and diffuse types) compared to corresponding nearby tissues [19]. The tumor suppressor circular RNA circRNA_100269 has been shown to suppress growth of gastric cancer cells through targeting miR-630 (Fig. 1) [19]. Finally, Feng et al. have reported that miR-630 suppresses EMT, migration and invasive features of gastric cancer cells through regulating FoxM1 (Forkhead Box M1) and decreasing expressions of GTP-Rac1, p-PI3K (Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha), and p-AKT [20]. In the cells treated with TGF-β, miR-630 via blocking of vimentin, slug, snail, and N-cadherin and also by induction of β-catenin, E-cadherin, wnt3a, and wnt5a inhibited cell viability, migration, invasion, and EMT (Fig. 1) [21]. Thus, this miRNA has a role in modulation of canonical Wnt signaling [21].
In gastric cancer, circRNA-100269 had a negative correlation with miR-630 and inhibited its expression (blue axis) [19]. In the cells treated with TGF-β, miR-630 via blocking of vimentin, slug, snail, and N-cadherin and also by induction of β-catenin, E-cadherin, wnt3a, and wnt5a inhibited cell viability, migration, invasion, and EMT (red axis) [21]. Moreover, miR-630 through inhibition of SOX4 could suppress invasion and proliferation of gastric cancer cells (purple axis) [18]
In nasopharyngeal carcinoma (NPC), miR-630 has been up-regulated in serum samples of patients compared with controls, indicating a biomarker role for this miRNA [22]. On the other hand, its expression has been shown to be down-regulated in NPC samples compared with chronic inflammatory nasopharyngeal epithelium. Functionally, H19 through sponging miR-630 could regulate invasiveness of these cells [23]. Table 1 summarizes the role and expression pattern of miR-630 in cancers.
Expression levels of miR-630 could affect patients' prognosis. In renal cell carcinoma, its expression has been correlated with tumor grade, lymph node metastasis, as well as distant metastasis [33]. In NSCLC, low level of miR-630 and high level of BCL2 have predicted poor outcomes in the patients [34]. In HCC, miR-630 up-regulation has been correlated with advanced stage, micro and macro-vascular invasion in a single study [14], while in another study its down-regulation has been associated with high metastasis probability, incomplete encapsulation, high tumor number, and vascular invasion possibility [15]. In gastric cancer, Zhou et al. [18] and Chu et al. [35] have reported totally contradicted results. Table 2 summarizes the impact of miR-630 expression on patients’ survival in different types of cancers.
miR-630 has also been found to affect response of cancer cells to chemo/radiotherapy. Pre-miR-630 has been shown to decrease cisplatin (CDDP)-induced cell death in NSCLC cells. Pre-miR-630 could modulate several phase of the intrinsic pathway of apoptosis, such as oligomerization of BAX, dissipation of transmembrane potential in the mitochondria, and processing of caspases-9 and 3. Furthermore, pre-miR-630 has been found to obstruct early signs of the DNA damage responses, such as ATM (Ataxia Telangiectasia Mutated) phosphorylation (Fig. 2) [40].
In non-small cell lung cancer (NSCLC) cells were treated with cisplatin (CDDP), miR-181a promoted cell death via BAX oligomerization and enhancement of procaspase-3 and procaspase-9 maturation, but miR-630 had an opposite effect. Also, miR-630 via blocking phosphorylation of ATM, H2AX, and P53 inhibited apoptosis. Moreover, miR-630 through enhancement of p27 expression, promoted cell cycle arrest in G0/G1 resulting in significant decrease in sensitivity to CDDP [40]
In renal cancer cells, miR-630 up-regulation has been found to inhibit uptake of oxaliplatin by cancer cells through targeting organic cation transporter OCT2 (Organic Cation Transporter 2) [36]. On the other hand, expression of miR-630 has been demonstrated to be associated with higher response of colon cancer cells to radiotherapy. miR-630 has been shown to induce apoptosis in these cells following ionic radiation through targeting BCL2L2 (BCL2 Like 2) and TP53RK (TP53 Regulating Kinase). Moreover, CREB (CAMP Responsive Element Binding Protein 1) could regulate expression of miR-630, and demethylation could enhance expression of miR-630 [41]. In ovarian cancer cells, miR-630 inhibition could increase sensitivity to cisplatin [12], decline cell proliferation, invasion, and motility and improves apoptosis and sensitivity to paclitaxel [42]. miR-630 has also been shown to target IGF1R (Insulin Like Growth Factor 1 Receptor) to influence response of breast cancer cells to HER2-targeting agents [43]. Finally, in 4-aminobiphenyl-treated HCC cells, the level of miR-630 has been increased, leading to suppression of genes involved in DNA repair [44]. Table 3 shows the effect of miR-630 in the response of tumor cells to chemotherapy, radiotherapy and carcinogens.
In lung cancer cells after DNA damage, miR-630 could inhibit proliferation and promote apoptosis by CDC7 (Cell Division Cycle 7) targeting, but so it could suppress apoptosis via other targets. In other words, miR-630 could have dual effect on apoptosis process and this issue goes back to its downstream targets [45].
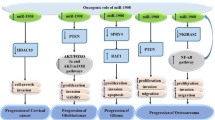
According to Rupaimoole et al. study, in ovarian and breast cancer cell lines, hypoxia condition led to miR-630 enhancement and targeting of Dicer by this miRNA [49]. This approach caused more tumor growth and metastasis. Moreover, the level of miR-630 expression affected the overall survival of patients, so that in patients who had a higher level of this miRNA, overall survival was more unfavorable [49]. The participation of miR-630 in various signaling pathways in different malignancies was shown in Fig. 3.
miR-630 in non-malignant conditions
Expression of miR-630 has also been appraised in the context of non-neoplastic conditions (Table 4). For instance, this miRNA has been found to be up-regulated in human milk exosomes of HIV (Human Immunodeficiency Virus)-1 infected mothers compared with uninfected controls [50]. Differentially expressed miRNAs between these two groups have been enriched in pathways related with cell cycle transition, cancer, TGF-β pathway, FoxO pathway, fatty acid biosynthesis, p53 pathway and apoptosis [50]. Besides, the area under receiver operating characteristics curve of miR-630 has been measured to be 0.82, indicating its potential to detect HIV-1 infection in mothers [50]. In another study, miR-630 has been identified as a putative biomarker for prediction of AIDS (Acquired Immunodeficiency Syndrome) progression [51]. A high throughput method has shown down-regulation of miR-630 levels in palatal tonsils from IgA nephropathy patients compared with chronic tonsillitis. miR-630 has been shown to decrease expression of TLR4 (Toll-like Receptor 4), thus reducing levels of secreted IgA1 and increasing galactosylation of the IgA1 hinge region. Furthermore, TLR4 can influence expression levels of IL (Interleukin 1)-1β and IL-8 via NF-κB signaling to control both IgA1 levels and its glycosylation [52]. miR-630 has also been among miRNAs whose expressions have been correlated with the severity of nuclear opacity in age-related nuclear cataract [53]. This miRNA has been found to regulate expression of MAPK14 (Mitogen-Activated Protein Kinase 14) and a number of other genes [53].
Discussion
miR-630 has different gene targets among them are those associated with cancer phenotype such as BCL2 [50], YAP-1 [27] and Slug [15]. Expression of this miRNA has been best assessed in the context of malignant conditions. However, the results of conducted studies in this field are contradictory. It is an oncogenic miRNA in renal cell carcinoma [8], multiple myeloma [24], colorectal cancer [9], acute lymphoblastic leukemia [10], ovarian cancer [11] and prostate cancer [13]. On the other hand, it is a putative tumor suppressor miRNA in lung [17], cervical [26], breast [28], thyroid [30] and esophageal tissues [31]. In a number of other tissues, data regarding the role of miR-630 in the carcinogenesis is conflicting [18, 19].
Circulating levels of miR-630 can be used as marker for separation of cancer patients from controls [22]. Moreover, expression profile of this miRNA has the potential to predict course of malignancy in several types of cancers (summarized in Table 2).
In addition, miR-630 can affect response of cancer cells to ionizing radiation, oxaliplatin and cisplatin. Thus, prior identification of miR-630 levels in tumoral tissues or circulation of patients might help in choosing the best efficient anticancer regimen in a personalized manner.
miR-630 is functionally linked with AKT, P53, TGFβ-ERK/SP1, JNK/c-Jun, PI3K/AKT and JAK2/STAT3 pathways which are among the mostly dysregulated pathways in cancers. Similar to other miRNAs, miR-630 has functional interactions with other classes of non-coding RNAs including long non-coding RNAs and circRNAs. H19, circMTDH.4 and circRNA_100269 are among transcripts whose interactions with miR-630 have been verified so far. High throughput evaluation of expression profiles of different classes of RNAs and additional functional analyses are needed for identification of other interacting molecules with miR-630.
Conclusion
Cumulatively, miR-630 is involved in the pathoetiology of several malignant and non-malignant conditions. Yet, its role in the carcinogenesis is so complicated that it is not possible to assign a tumor suppressor or oncogene role for it in all tissue contexts. In spite of the presence of vast body of literature on the role of this miRNA in malignant conditions, few studies have addressed its contribution in non-malignant conditions. Among non-malignant conditions, the pathogenesis of IgA nephropathy, obstructive sleep apnea, age-related nuclear cataract, vitiligo and heterotopic ossification is related with levels of miR-630. Moreover, this miRNA has been found in exosome secreted in the human milk, possibly reflecting the disease status of the mother. This miRNA can be used as a potential biomarker for cancerous conditions. However, since its levels are different in diverse malignancies, it can be better used for patients’ follow-up rather than initial diagnosis. The presence of this miRNA in exosomes potentiates its applications in non-invasive diagnostic methods. However, future studies are necessary for validation of this hypothesis. A major limitation of studies that assessed the diagnostic or prognostic impact of miR-630 in human disorders is lack of validation in independent cohorts.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Abbreviations
- miRNA:
-
MicroRNA
- circRNA:
-
Circular RNA
- ANCT:
-
Adjacent non-cancerous tissue
- NPC:
-
Nasopharyngeal carcinoma
- EMT:
-
Epithelial–mesenchymal transition
- RCC:
-
Renal cell carcinoma
- MM:
-
Multiple myeloma
- CRC:
-
Colorectal cancer
- ALL:
-
Acute lymphoblastic leukemia
- OC:
-
Ovarian cancer
- PCa:
-
Prostate cancer
- HCC:
-
Hepatocellular carcinoma
- PTC:
-
Papillary thyroid carcinoma
- NSCLC:
-
Non-small cell lung cancer
- GC:
-
Gastric cancer
- ESCC:
-
Esophageal squamous cell carcinoma
- CC:
-
Cervical cancer
- CDDP:
-
Cisplatin
- PC:
-
Pancreatic cancer
- BC:
-
Breast cancer
- IgAN:
-
IgA nephropathy
- HO:
-
Heterotopic ossification
- ED:
-
Endothelial dysfunction
- NEF:
-
Normal endothelial function
- OB:
-
Obese
- OSA:
-
Obstructive sleep apnea
- GEO:
-
Gene expression omnibus
- BCLC:
-
Barcelona-clinic liver cancer
References
Hussen BM, Hidayat HJ, Salihi A, Sabir DK, Taheri M, Ghafouri-Fard S. MicroRNA: a signature for cancer progression. Biomed Pharmacother. 2021;138:111528.
Ghafouri-Fard S, Shoorei H, Bahroudi Z, Abak A, Majidpoor J, Taheri M. An update on the role of miR-124 in the pathogenesis of human disorders. Biomed Pharmacother. 2021;135:111198.
Ghafouri-Fard S, Bahroudi Z, Shoorei H, Abak A, Ahin M, Taheri M. microRNA-140: a miRNA with diverse roles in human diseases. Biomed Pharmacother. 2021;135:111256.
Ghafouri-Fard S, Khoshbakht T, Hussen BM, Taheri M, Samadian M. A review on the role of miR-1290 in cell proliferation, apoptosis and invasion. Front Mol Biosci. 2021;8:763338.
Macfarlane L-A, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Curr Genomics. 2010;11(7):537–61.
Ghafouri-Fard S, Khoshbakht T, Hussen BM, Kadkhoda S, Taheri M, Tafrishinejad A. A review on the role of miR-149-5p in the carcinogenesis. Int J Mol Sci. 2021;23(1):415.
Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–24.
Zhao JJ, Chen PJ, Duan RQ, Li KJ, Wang YZ, Li Y. miR-630 functions as a tumor oncogene in renal cell carcinoma. Arch Med Sci. 2016;12(3):473–8.
Zhang L, Feng G, Zhang X, Ding Y, Wang X. microRNA-630 promotes cell proliferation and inhibits apoptosis in the HCT116 human colorectal cancer cell line. Mol Med Rep. 2017;16(4):4843–8.
Gharehdaghi Z, Obeidi N, Rostami yasuj S, Khamisipour G. Overexpression of microRNA-630 in acute leukemic T-cell line. Iran J Pediatr Hematol Oncol. 2020;10(3):159–72.
Zhang S, Zhang JY, Lu LJ, Wang CH, Wang LH. MiR-630 promotes epithelial ovarian cancer proliferation and invasion via targeting KLF6. Eur Rev Med Pharmacol Sci. 2017;21(20):4542–7.
Zou YT, Gao JY, Wang HL, Wang Y, Wang H, Li PL. Downregulation of microRNA-630 inhibits cell proliferation and invasion and enhances chemosensitivity in human ovarian carcinoma. Genet Mol Res. 2015;14(3):8766–77.
Valera VA, Parra-Medina R, Walter BA, Pinto P, Merino MJ. microRNA expression profiling in young prostate cancer patients. J Cancer. 2020;11(14):4106–14.
Zhang JW, Li Y, Zeng XC, Zhang T, Fu BS, Yi HM, et al. miR-630 overexpression in hepatocellular carcinoma tissues is positively correlated with alpha-fetoprotein. Med Sci Monit. 2015;21:667–73.
Chen WX, Zhang ZG, Ding ZY, Liang HF, Song J, Tan XL, et al. MicroRNA-630 suppresses tumor metastasis through the TGF-β- miR-630-Slug signaling pathway and correlates inversely with poor prognosis in hepatocellular carcinoma. Oncotarget. 2016;7(16):22674–86.
Li YH, Xu CL, He CJ, Pu HH, Liu JL, Wang Y. circMTDH.4/miR-630/AEG-1 axis participates in the regulation of proliferation, migration, invasion, chemoresistance, and radioresistance of NSCLC. Mol Carcinog. 2020;59(2):141–53.
Song YF, Hong JF, Liu DL, Lin QA, Lan XP, Lai GX. miR-630 targets LMO3 to regulate cell growth and metastasis in lung cancer. Am J Transl Res. 2015;7(7):1271–9.
Zhou L, Zhang H, Li C, Zhang G, Song X, Liu J. MicroRNA-630 acts as a prognostic marker in gastric cancer and its role in cell migration and invasion. Int J Clin Exp Pathol. 2017;10:4969–78.
Zhang Y, Liu H, Li W, Yu J, Li J, Shen Z, et al. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Aging (Albany NY). 2017;9(6):1585–94.
Feng J, Wang X, Zhu W, Chen S, Feng C. MicroRNA-630 suppresses epithelial-to-mesenchymal transition by regulating FoxM1 in gastric cancer cells. Biochemistry. 2017;82(6):707–14.
Lin Y, Leng Q, Zhan M, Jiang F. A plasma long noncoding RNA signature for early detection of lung cancer. Transl Oncol. 2018;11(5):1225–31.
Zhuo X, Zhou W, Li D, Chang A, Wang Y, Wu Y, et al. Plasma microRNA expression signature involving miR-548q, miR-630 and miR-940 as biomarkers for nasopharyngeal carcinoma detection. Cancer Biomark. 2018;23(4):579–87.
Li X, Lin Y, Yang X, Wu X, He X. Long noncoding RNA H19 regulates EZH2 expression by interacting with miR-630 and promotes cell invasion in nasopharyngeal carcinoma. Biochem Biophys Res Commun. 2016;473(4):913–9.
Qu X, Zhao M, Wu S, Yu W, Xu J, Xu J, et al. Circulating microRNA 483-5p as a novel biomarker for diagnosis survival prediction in multiple myeloma. Med Oncol (Northwood, London, England). 2014;31(10):219.
Zhao X, Ren Y, Cui N, Wang X, Cui Y. Identification of key microRNAs and their targets in exosomes of pancreatic cancer using bioinformatics analysis. Medicine. 2018;97(39):e12632.
Lyu Y-Y, Wang F-M, Yang X-M, Zhang C-C, Gu F, Luo T-J, et al. MiR-630 acts as a tumor suppressor in cervical cancer and inhibits epithelial–mesenchymal transition in cervical cancer. Int J Clin Exp Pathol. 2017;10:972–83.
Ge B, Liu H, Liang Q, Shang L, Wang T, Ge S. Oxytocin facilitates the proliferation, migration and osteogenic differentiation of human periodontal stem cells in vitro. Arch Oral Biol. 2019;99:126–33.
Zhou C-X, Wang C-L, Yu A-L, Wang Q-Y, Zhan M-N, Tang J, et al. MiR-630 suppresses breast cancer progression by targeting metadherin. Oncotarget. 2016;7(2):1288.
Li GW, Yan X. Lower miR-630 expression predicts poor prognosis of osteosarcoma and promotes cell proliferation, migration and invasion by targeting PSMC2. Eur Rev Med Pharmacol Sci. 2019;23(5):1915–25.
Pan XM, He XY, Yang YL, Jia WJ, Yang ZQ, Yan D, et al. MiR-630 inhibits papillary thyroid carcinoma cell growth, metastasis, and epithelial–mesenchymal transition by suppressing JAK2/STAT3 signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(6):2453–60.
Liu X, Wu W, Zhang S, Tan W, Qiu Y, Liao K, et al. Effect of miR-630 expression on esophageal cancer cell invasion and migration. J Clin Lab Anal. 2021;35(6):e23815.
Jin L, Yi J, Gao Y, Han S, He Z, Chen L, et al. MiR-630 inhibits invasion and metastasis in esophageal squamous cell carcinoma. Acta Biochim Biophys Sin. 2016;48(9):810–9.
Zhao JJ, Chen PJ, Duan RQ, Li KJ, Wang YZ, Li Y. Up-regulation of miR-630 in clear cell renal cell carcinoma is associated with lower overall survival. Int J Clin Exp Pathol. 2014;7(6):3318–23.
Chen MJ, Wu DW, Wang GC, Wang YC, Chen CY, Lee H. MicroRNA-630 may confer favorable cisplatin-based chemotherapy and clinical outcomes in non-small cell lung cancer by targeting Bcl-2. Oncotarget. 2018;9(17):13758–67.
Chu D, Zhao Z, Li Y, Li J, Zheng J, Wang W, et al. Increased microRNA-630 expression in gastric cancer is associated with poor overall survival. PLoS ONE. 2014;9(3):e90526.
Chen L, Chen L, Qin Z, Lei J, Ye S, Zeng K, et al. Upregulation of miR-489-3p and miR-630 inhibits oxaliplatin uptake in renal cell carcinoma by targeting OCT2. Acta Pharm Sin B. 2019;9(5):1008–20.
Wu DW, Wang YC, Wang L, Chen CY, Lee H. A low microRNA-630 expression confers resistance to tyrosine kinase inhibitors in EGFR-mutated lung adenocarcinomas via miR-630/YAP1/ERK feedback loop. Theranostics. 2018;8(5):1256–69.
Wang ZY, Zhang W, Yang JJ, Song DK, Wei JX. Expression of miRNA-630 in bladder urothelial carcinoma and its clinical significance. J Huazhong Univ Sci Technol Med Sci. 2016;36(5):705–9.
Chu D, Zheng J, Li J, Li Y, Zhang J, Zhao Q, et al. MicroRNA-630 is a prognostic marker for patients with colorectal cancer. Tumour Biol. 2014;35(10):9787–92.
Galluzzi L, Morselli E, Vitale I, Kepp O, Senovilla L, Criollo A, et al. miR-181a and miR-630 regulate cisplatin-induced cancer cell death. Cancer Res. 2010;70(5):1793–803.
Zhang Y, Yu J, Liu H, Ma W, Yan L, Wang J, et al. Novel epigenetic CREB-miR-630 signaling axis regulates radiosensitivity in colorectal cancer. PLoS ONE. 2015;10(8):e0133870.
Eoh KJ, Lee SH, Kim HJ, Lee JY, Kim S, Kim SW, et al. MicroRNA-630 inhibitor sensitizes chemoresistant ovarian cancer to chemotherapy by enhancing apoptosis. Biochem Biophys Res Commun. 2018;497(2):513–20.
Corcoran C, Rani S, Breslin S, Gogarty M, Ghobrial IM, Crown J, et al. miR-630 targets IGF1R to regulate response to HER-targeting drugs and overall cancer cell progression in HER2 over-expressing breast cancer. Mol Cancer. 2014;13:71.
Huan LC, Wu JC, Chiou BH, Chen CH, Ma N, Chang CY, et al. MicroRNA regulation of DNA repair gene expression in 4-aminobiphenyl-treated HepG2 cells. Toxicology. 2014;322:69–77.
Cao JX, Lu Y, Qi JJ, An GS, Mao ZB, Jia HT, et al. MiR-630 inhibits proliferation by targeting CDC7 kinase, but maintains the apoptotic balance by targeting multiple modulators in human lung cancer A549 cells. Cell Death Dis. 2014;5(9):e1426.
Sakurai MA, Ozaki Y, Okuzaki D, Naito Y, Sasakura T, Okamoto A, et al. Gefitinib and luteolin cause growth arrest of human prostate cancer PC-3 cells via inhibition of cyclin G-associated kinase and induction of miR-630. PLoS ONE. 2014;9(6):e100124.
Farhana L, Dawson MI, Murshed F, Das JK, Rishi AK, Fontana JA. Upregulation of miR-150* and miR-630 induces apoptosis in pancreatic cancer cells by targeting IGF-1R. PLoS ONE. 2013;8(5):e61015.
Zhang L, Wang C, Xue ZX. Inhibition of miR-630 enhances the cell resistance to radiation by directly targeting CDC14A in human glioma. Am J Transl Res. 2017;9(3):1255–65.
Rupaimoole R, Ivan C, Yang D, Gharpure KM, Wu SY, Pecot CV, et al. Hypoxia-upregulated microRNA-630 targets Dicer, leading to increased tumor progression. Oncogene. 2016;35(33):4312–20.
Zahoor MA, Yao XD, Henrick BM, Verschoor CP, Abimiku A, Osawe S, et al. Expression profiling of human milk derived exosomal microRNAs and their targets in HIV-1 infected mothers. Sci Rep. 2020;10(1):12931.
Liao Q, Wang J, Pei Z, Xu J, Zhang X. Identification of miRNA-mRNA crosstalk in CD4(+) T cells during HIV-1 infection by integrating transcriptome analyses. J Transl Med. 2017;15(1):41.
Liu C, Ye MY, Yan WZ, Peng XF, He LY, Peng YM. microRNA-630 regulates underglycosylated IgA1 production in the tonsils by targeting TLR4 in IgA nephropathy. Front Immunol. 2020;11:563699.
Wang S, Guo C, Yu M, Ning X, Yan B, Zhao J, et al. Identification of H(2)O(2) induced oxidative stress associated microRNAs in HLE-B3 cells and their clinical relevance to the progression of age-related nuclear cataract. BMC Ophthalmol. 2018;18(1):93.
Khalyfa A, Kheirandish-Gozal L, Khalyfa AA, Philby MF, Alonso-Álvarez ML, Mohammadi M, et al. Circulating plasma extracellular microvesicle MicroRNA cargo and endothelial dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194(9):1116–26.
Gao W, Zhou X, Lin R. miR-378a-5p and miR-630 induce lens epithelial cell apoptosis in cataract via suppression of E2F3. Braz J Med Biol Res. 2020;53(5):e9608.
Lin S-J, Chou F-J, Li L, Lin C-Y, Yeh S, Chang C. Natural killer cells suppress enzalutamide resistance and cell invasion in the castration resistant prostate cancer via targeting the androgen receptor splicing variant 7 (ARv7). Cancer Lett. 2017;398:62–9.
Sun Y, Cai J, Yu S, Chen S, Li F, Fan C. MiR-630 inhibits endothelial–mesenchymal transition by targeting slug in traumatic heterotopic ossification. Sci Rep. 2016;6:22729.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SGF wrote the draft and revised it. SK collected the data and designed the tables and figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent forms were obtained from all study participants. The study protocol was approved by the ethical committee of Shahid Beheshti University of Medical Sciences.
Consent of publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kadkhoda, S., Ghafouri-Fard, S. The importance of miRNA-630 in human diseases with an especial focus on cancers. Cancer Cell Int 22, 105 (2022). https://doi.org/10.1186/s12935-022-02531-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02531-z