Abstract
Background
Histological transformation (HT) of follicular lymphoma to a more aggressive lymphoma is a serious event affecting patients’ outcomes. To date, no strong clinical HT predictors present at diagnosis have yet been identified. The fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) is highlighted as a non-invasive diagnostic tool for the detection of HT, but its ability to predict HT at early stage of disease has not been clear. Therefore, this study investigated the predictive values of the pre-transformation standardized uptake value (SUVmax) for the risk of transformation in FL.
Methods
This retrospective study involved 219 patients with FL between June 2008 and October 2019 who had undergone 18F-FDG PET/CT scan. One hundred and thirty-two, 64, and 78 patients underwent PET at baseline (PETbaseline), interim (PETinterim) and end-of-induction therapy (PETend), respectively. Qualitative assessment was performed using the 5-point Deauville scale. Statistical analysis was done using Cox regression models, receiver operating characteristic (ROC) analysis, and Kaplan–Meir survival curves.
Results
Of the 219 patients included, 128 had low-grade FL (grade 1–2) and 91 had high-grade FL (grade 3a). HT eventually occurred in 30 patients. The median time to HT was 13.6 months. Among clinical indicators, advance pathological grade was shown as the most significant predictor of HT (HR = 4.561, 95% CI 1.604–12.965). We further assessed the relationship between PET and HT risk in FL. Univariate Cox regression determined that SUVbaseline and SUVend were significant predictors for HT, while neither SUVinterim nor qualitative assessment of Deauville score has predictive value for HT. Due to the noticeable impact of high pathological grade on the HT risk, we conducted the subgroup analysis in patients with low/high pathological grade, and found SUVbaseline could still predict HT risk in both low-grade and high-grade subgroups. Multivariate analysis adjusted by FLIPI2 score showed the SUVbaseline (HR 1.065, 95% CI 1.020–1.111) and SUVend (HR 1.261, 95% CI 1.076–1.478) remained as significant predictors independently of the FLIPI2 score. According to the cut-off determined from the ROC analysis, increased SUVbaseline with a cutoff value of 14.3 and higher SUVend with a cutoff value of 7.3 were highly predictive of a shorter time to HT.
Conclusions
In follicular lymphoma, quantitative assessment used SUVmax at the pre-treatment and end-of-treatment PET/CT scan may be helpful for early screen out patients at high risk of transformation and guide treatment decisions.
Similar content being viewed by others
Background
Follicular lymphoma (FL) is the most common form of inert non-Hodgkin's lymphoma (NHL), accounting for 7–15% of all lymphomas worldwide [1]. The clinical course of FL is highly heterogeneous. Although many patients exhibit the characteristics of indolent lymphoma and can survive for a long time, others show rapid progression and often transformation to a more aggressive form of lymphoma, usually diffuse large B-cell lymphoma (DLBCL), with an annual incidence of 2–3% [2]. Transformation is associated with increased morbidity and mortality. Although the development of rituximab has greatly improved the prognosis of such patients, the survival of patients with HT is still not optimistic, with 5-year OS and PFS reported to be 68% and 58% for patients with HT at primary diagnosis, and even worse with 5-year OS and PFS rates of only 68% and 18% for patients with sequentially HT [3]. Therefore, there is a need to identify predictive markers of HT risk to guide in clinical decision-making so that high-risk patients can benefit from more aggressive treatment. Currently, the prognostic assessment for FL is usually based on the FL International Prognostic Index 2 (FLIPI2), which has been the strongest predictor of FL prognosis but weaker in predictive ability for HT risk [3]. So far, there are no strong clinical HT predictors present at the time of first diagnosis identified [3,4,5,6]. The initial treatment strategy watch-and-wait approach and higher pathological grades appear to more significantly affect FL transformation [7].
Over the past decade, 18F-FDG PET/CT has been widely used in the clinic of lymphoma, including disease diagnosis, initial staging, and follow-up after treatment [8, 9]. The maximum standardized uptake value (SUVmax) derived from PET/CT is the most commonly used quantitative index as a standard of malignancy, and a higher value is generally considered a poor prognostic factor [10]. The First International Workshop held in Deauville, France in 2009 proposed new PET/CT assessment standard for lymphoma named the Deauville standard comprising a 5-point scale (D5PS), which defined a simple visual response criteria at interim and end-of-treatment [11]. In recent years, the quantitative and qualitative indicators of PET/CT have been studied for predicting the prognosis of FL, and the results were inconclusive and contradictory. Most studies suggested higher values of quantitative SUVmax and total metabolic tumor volume (TMTV) parameters, and qualitative Deauville index are considered to be associated with inferior survival [12,13,14], while the other study unexpectedly reported that a low SUVmax correlated with a worse PFS [15]. In term of HT in FL, PET/CT is commonly used as a non-invasive diagnostic tool for the detection of HT and to determine the best suitable re-biopsy site in the context of relapse [16]. There are very few studies evaluating the ability of PET/CT metrics to predict the risk of HT at early diagnosis stage. Mir et al. assessed the relationship between baseline SUVmax and the risk for HT in FL from the GALLIUM study, and found no temporal relationship between baseline SUVmax and HT; neither baseline SUVmax nor baseline SUVrange could predict HT [17]. However, in the study all included patients had high tumor burden, and only 15 of them were patients with HT. The limited sample size and single patient population may lead to bias in the results. More studies are needed to determine the predictive value PET/CT-based measurements for HT risk both in low-grade/tumor burden or high-grade/tumor burden patients. Moreover, the usefulness of interim and terminal PET/CT assessments in predicting HT has not been reported to date, so the significance of PET/CT parameters at different treatment time points remains to be further studied.
To this end, in this study, we investigated the correlation between clinical indicators and HT risk, and further analyzed the quantitative SUVmax parameter and qualitative D5PS index of PET/CT evaluation at different treatment stages in predicting HT risk. Subgroup analysis was conducted in patients with low and high pathological grade, and the best cutoff values of PET/CT parameters were calculated as the basis for early screening and judgment of high-risk HT patients.
Patients and methods
Patients
Two hundred and twenty-eight patients with histologically proven FL underwent PET/CT from June 2008 to October 2019. Among them, 219 patients who met the following inclusion criteria were included: patients having histologically confirmed FL with grading, including subsequent histologically confirmed transformation to other invasive lymphomas; patients having no evidence of biopsy-confirm transformation when underwent PET/CT scan; patients aged ≥ 18 years; and patients whose PETbaseline, PETinterim (after 3 or 4 cycles of chemotherapy), or PETend (after 5 or 6 cycles of chemotherapy) data were available. The treatment of low-grade (1–2) FL was decided by the hematooncologist depending on the patient’s condition. High-grade FL (3a) was treated with the following regimens: R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) and R-CVP (rituximab, cyclophosphamide, vincristine and prednisone). Nine individuals were excluded: two for whom pathologic grading data were lacking, two who showed lower FDG uptake in the tumor than in the liver at PET/CTbaseline, four who received radiotherapy due to a massive mass but did not receive chemotherapy, and one for whom time data for transformation were lacking. Clinical parameters and hematological data (Ann Arbor stage, NCCN-FLIPI1, NCCN-FLIPI2, pathological grade, lactate dehydrogenase [LDH] level, bone marrow involvement, area of enlarged lymph nodes, hemoglobin (HGB) level, fibrinogen, and subsequent treatment planned) were obtained from the patients’ medical records. The study was conducted per the ethical principles of the Declaration of Helsinki. All patients provided written informed consent to participate in the study (Additional file 1).
PET/CT technique and evaluation
The patients received an intravenous injection of 18F-FDG at 4.44–5.55 MBq/kg of body weight after at least 4–6 h of fasting. Adequate hydration was ensured by giving the patients 1000 ml of water 1 h before image acquisition. Blood glucose levels were checked in all patients before FDG injection. The patients’ blood glucose levels were lower than 11 mmol/L. Whole-body PET/CT scans were acquired using a combined PET/CT scanner (Siemens Biograph Sensation 16, Germany). The whole-body CT and PET covered a region ranging from the meatus of the ear to the mid-thigh. PET/CT was performed approximately 1 h after FDG injection. The procedure for data acquisition was as follows: 16-section multi-detection row CT scanning was performed first, from the head to the mid-thigh with 120 kV and 100 mA. The tube rotation time and section thickness were 0.5 s and 5 mm, respectively, which matched the PET section thickness. Immediately after the CT scan, a whole-body PET scan was performed with 1.5- to 2-min acquisition per bed position by using a 3-dimensional acquisition mode. Attenuation-corrected PET images were reconstructed with an ordered-subset expectation maximization iterative reconstruction algorithm (8 subsets, 3 iterations). PET, CT, and fused PET/CT images were generated and reviewed on a Syngo workstation. The scans were independently interpreted by two staff members of the PET center. Disagreements between respective independent interpretations were resolved by consensus.
The SUV refers to the ratio of the radioactivity of the imaging agent taken from local tissues to the average injection activity of the whole body. SUVmax was obtained by a software program from Siemens. The PETInterim and PETend scans were assessed using the D5PS as follows: 1, no intake; 2, intake less than or equal to that in the mediastinum; 3, intake higher than that in the mediastinum but less than that in the liver; 4, intake moderately higher than that in the liver; and 5, intake significantly higher than that in the liver or at a new disease site.
Statistical analysis
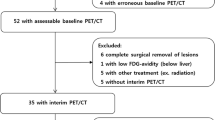
Continuous variables were expressed as mean ± SD values. Estimates of the predictive effect for HT were expressed as hazard ratios (HRs) in the Cox proportional hazard regression analysis with 95% confidence intervals (CI). Receiver operating characteristic (ROC) analysis was performed to determine the optimal cut-off values for PET parameters for predicting HT. Transformation-free survival curves were constructed using the Kaplan–Meier method and were compared with the log-rank test. Differences between the results of comparative tests were considered significant if the 2-sided P-value was < 0.05. All statistical analyses were performed using STATA version 12.0 (StataCorp, College Station, TX, USA). The flowchart of the study protocol and methodology was shown in Fig. 1.
Result
Patient characteristics
Of the 219 patients included (median follow-up: 46 months), 128 had low-grade FL (grade 1–2) and 91 had high-grade (grade 3a) FL. Thirty (13.7%) patients showed biopsy-confirmed HT to diffuse large B-cell lymphoma (DLBCL), with six cases (4.7%) categorized in the low-grade (1–2) group and 24 (26.4%) in the high-grade (3a) group. The median time to transformation was 13.6 months, and the earliest transformation was reported at 186 days (6 months). In the low-grade group, treatment was determined according to the Groupe d’Etude des Lymphomes Folliculaires (GELF) criteria for tumor load (lymph node involvement, large mass, symptom B, splenomegaly, pleural effusion, and blood abnormalities). Fifty-one (39.8%) patients adopted a watch-and-wait approach, 47 (36.7%) received involved site radiation therapy (ISRT) for a local mass, and only 30 (23.4%) received systemic chemotherapy. In the high-grade group, all included patients received chemotherapy, with 61 (67%) receiving R-CHOP and 30 (33%) receiving R-CVP. The median patient age was 51.1 years, and 47% of the patients were male. Table 1 shows baseline characteristics of patients with HT and those that did not show transformation. In comparison with the non-transformed patients, HT patients were older, had higher LDH and fibrinogen levels, and showed a higher proportion of high-grade FL.
Association of baseline characteristics and the risk of HT
Univariate Cox regression was used to determine the baseline characteristics that could serve as predictors for HT. The results showed that older age, higher fibrinogen levels, higher FLIPI2 score, and advanced pathological grade were correlated with transformation (Table 2). Subsequently, multivariate Cox regression was used for the four significant variables identified by univariate analysis. Higher fibrinogen level (hazard ratio [HR] = 1.385, 95% confidence interval [CI] 1.002–1.915; P = 0.048), higher FLIPI2 score (HR = 1.550, 95% CI 1.002–2.398; P = 0.049), and advanced pathological grade (HR = 4.561, 95% CI 1.604–12.965; P = 0.004) were significant independent predictors of HT. Increased fibrinogen level and higher FLIPI2 score showed modest HR values, ranging from 1.3 to 1.6. Advanced pathological grade was highly predictive of HT risk with HR value of > 4.
Predictive value of PET/CT data for HT risk in FL
Among the 219 patients with PET/CT data, 132 underwent pre-treatment PET/CT evaluation (PETbaseline), 64 received post-treatment interim evaluation (PETinterim, after 3 or 4 cycles), and 78 underwent post-treatment terminal evaluation (PETend, after 5 or 6 cycles). The median SUVbaseline was 8.1 (range, 0.7–46.6), median SUVinterim was 2.9 (range, 0–17.3), and median SUVend was 2.6 (range, 0–20.2). SUVinterim and SUVend were assessed according to the Deauville scoring criteria, named as Deauvilleinterim and Deauvilleend, respectively. The PET/CT data for patients with HT and without HT are presented in Table 3. Median SUVbaseline in patients with HT vs. without HT was 16.9 (range 2.12–29.72) vs. 7.9 (range 0.7–46.6), respectively; median SUVinterim was 2.49 (range 0–17.3) vs. 3.0 (range 0–11.4), respectively; and median SUVend was 7.3 (range 0–20.2) vs. 2.5 (range 0–15.5), respectively. The proportion of patients with higher qualitative assessment Deauvilleinterim (scored 5–6 points) was 25% (2/8) and 10.9% (6/55) in the HT and non-HT groups, respectively; the percentage of patients classified as higher at Deauvilleend (scored 5–6 points) was 50% (3/6) and 14.2% (10/70) in the HT and non-HT groups, respectively.Univariate Cox regression analysis showed that the SUVbaseline (HR = 1.073, 95%CI 1.031–1.115, P = 0.000) and SUVend (HR = 1.163, 95%CI 1.023–1.323, P = 0.021) could predict HT. Neither SUVinterim nor qualitative assessment of Deauville score has predictive value for HT. A total of 128 patients with pathological grade 1–2 received multiple mild treatments and 91 patients with pathological grade 3a received rituximab-based systemic chemotherapy. To minimize the confounding effects of pathological classification and treatment strategies on the results, we conducted subgroup analysis in low (grade 1–2) and high (grade 3a) pathological grade patients, respectively. The result showed the baseline SUVmax was still an important predictor for HT in both high-grade (HR = 1.055, 95%CI 1.009–1.103, P = 0.017) and low-grade subgroups (HR = 1.224, 95%CI 1.033–1.449, P = 0.019); SUVend also remained as a prognostic indicator for HT in high-grade subgroup (HR = 1.147, 95%CI 1.022–1.288, P = 0.020). Due to the insufficient sample size, statistical data on predictive value of SUVend in low-grade subgroup could not be obtained. In addition, we conducted a multivariate Cox analysis of adjusted by FLIPI2 score on the significant indicators screened by univariate analysis, and found the SUVbaseline (HR = 1.065, 95%CI 1.020–1.111, P = 0.004) and SUVend (HR = 1.261, 95%CI 1.076–1.478, P = 0.004) remained predictive factors of HT risk independently of FLIPI2 score.
Kaplan–Meier survival analysis
We used ROC curve analysis to determine the accuracy and optimal cut-off values of SUV for predicting HT. The Area Under the Curve (AUC) for SUVbaseline and SUVend was 0.761 and 0.669, respectively; their cut-off values were 14.3 and 7.3, respectively. A Kaplan–Meier survival analysis was performed according to the cut-off values. The median time to HT were 96 months and not reached between patients with SUVbaseline above and below the 14.3 cut-off, respectively. The higher SUVbaseline was significantly associated with a shorter time to HT (log-rank test, P = 0.000, Fig. 2). In addition, a significant difference in time to HT was also observed between patients with SUVend above and below the 7.3 cut-off (logrank test, P = 0.000) (Fig. 3).
Discussion
Follicular lymphoma shows a heterogeneous clinical course ranging from long-term responses to initial therapy to frequent recurrences or transformation to more aggressive subtypes. Histological transformation is a serious clinical event with a marked effect on the disease outcomes. In the past few decades, the incidence of HT has been reported to vary from 10 to 70% [2, 18,19,20,21,22]. This variability has been attributed to several factors, including the lack of a unique histological definition for transformation, the heterogeneity in study populations, differences in treatment strategies, and differences in the follow-up period. In our study, the rate of HT of FL was 13.6% over a median follow-up period of 46 months, which was slightly higher than the recently reported values. The HT incidence in high-grade patients was 26.4%, which was significantly higher than that in low-grade patients (4.7%). We speculate that a higher proportion of patients with advanced 3a pathological grade (more than 50%) may be a non-negligible reason for the increase in the HT rate in our study.
Even though in rituximab era, HT is still a serious event affecting the prognosis and survival of follicular lymphoma, especially in patients with subsequent transformation, with a 5-year PFS rate of less than 20% and 5-year OS rate of less than 70% [3]. The transformation of follicular lymphoma is a multi-step event, from an indolent lymphoma to a more aggressive lymphoma subtype, which is accompanied by an increase in tumor burden. Early screening to identify patients with high HT risk, and giving more aggressive treatment strategies may help reduce the occurrence of transformation, thereby reduce serious complications caused by high tumor burden [23] and improve patient survival. In 2018, Federico et al. conducted a retrospective pooled analysis to explore the risk factors influencing FL transformation [7]. In their study, 8116 patients from 11 cooperative groups or institutions across Europe were eligible for analysis, and the 10-year cumulative hazard of HT was 7.7%, with a high FLIPI score, grade 3a follicular lymphoma, and a previous watch-and-wait approach were associated with increased cumulative hazards of HT. In addition, in the PRIMA study, the risk factors for HT were high LDH levels, an altered performance status, anemia and systemic symptoms [24]. This has been well documented for most of the individual clinical to confirm the adverse factors, such as elevated serum LDH, high values of ECOG-PS, IPI and FLIPI2, but these indicators have no strong power to predict HT risk with weaker HR values ranging from 1 to 2 [3,4,5,6]. We speculate most transformations in FL are from the divergent evolution of small progenitor clones that is usually not detectable at diagnosis, which makes it difficult to predict it reliably with available clinical indicators. In this study, we also identified higher FLIPI2 score and 3a pathological grading were independent predictors of HT. Advanced pathological grade was shown to be most significantly related to HT risk with HR value > 4, while FLIPI2 showed a weaker HR value of only 1.55. Notably, the coagulation factor fibrinogen was a newly identified possible important factor predicting transformation. At present, research on the clinical significance of fibrinogen in FL is limited. Elevated pretreatment levels of fibrinogen are reported to be unfavorable predictors in clinical response and prognosis in DLBCL [25] and nasal-type natural/killer T cell lymphoma [26]. For FL, one study reported that elevated CRP levels showed a significant association with elevated fibrinogen levels, and patients with higher CRP levels had a significantly shorter PFS, implicating the potential poor prognosis of fibrinogen in FL [27]. Treatment strategies on the risk of HT in FL were also investigated. The largest study to date has shown that patients who are managed by watch and waiting are at increased HT risk [5]. A retrospective international study involving nearly 10,000 patients also stated that patients who did not receive rituximab as the initial treatment had an increased risk of HT [28].
Recently, some studies have investigated the potential predictive value of quantitative parameters of PET/CT for FL prognosis. As the most commonly used semi-quantitative indicator in PET/CT, SUVmax reflects the glucose metabolism of tumors, and has been proved to be closely related to tumor invasion [14, 29, 30]. Rossi et al. reported that baseline SUVmax > 14.5 was associated with poorer PFS than baseline SUVmax ≤ 14.5 [12]. With the development of software programs, TMTV may provide additional valuable information to assess response to treatment and patient prognosis. MTV is an indicator of the tumor score in vivo and may be a better estimator of the tumor burden than anatomical imaging. TMTV measured on the basis of baseline PET examinations can also predict PFS and overall survival (OS) better than the FLIPI score, thereby indicating progression within 2 years [13]. Cottereau et al. established a model including TMTV and PETend and found that a high TMTV (> 510 cm3) and positive PETend results were independent important risk factors for PFS [30]. In addition, for the qualitative evaluation of PET/CT, one study in a pooled analysis of three prospective trials demonstrated the prognostic value of Deauville score in FL. They found the positive results in post-induction PET scan (D5PS of 4, 5) could identify a high-risk group with shorter PFS, and indicated a significantly higher risk of death [14]. Boo et al. also retrospectively reviewed 33 FL patients who had three sets of initial staging, interim and end-of-therapy to evaluate the predictive value of Deauville score. The results confirmed that patients with interim and end-of –therapy D5PS of 4 or 5 showed shorter PFS than patients with scores 1, 2, or 3, and terminal Deauville score is an independent factor in FL progress [30].
In term of relationship between PET/CT and HT in FL, retrospective studies showed that the nodal sites of HT may have a higher SUVmax than non-transformed sites on PET/CT scans. Numerous studies have confirmed that higher SUVmax values increase the odds of higher grade or more aggressive lymphoma, and PET/CT is often used as an important non-invasive diagnostic tool to find the best biopsy site when transformation is suspected [16]. To date, only a few studies have assessed the ability of pre-transformation PET/CT parameters for predicting the risk of transformation in early stage of disease. A brief report published on Blood in 2020 assessed the relationship between baseline SUVmax and the risk for HT in FL from the GALLIUM study [17]. They found no temporal relationship between baseline SUVmax and HT. Neither baseline SUVmax nor baseline SUVrange could predict HT, suggesting that repeat biopsy of lesions may offer limited benefit in excluding HT based on SUVmax alone before initiating therapy in patients with FL with a high tumor burden. This study highlighted for the first time the insignificance of baseline SUVmax in predicting transformation risk, but the limited sample size (only 15 patients with HT) and a single population (patients with high tumor burden) may lead bias in results. Moreover, the usefulness of mid-term and terminal PET/CT assessments in predicting HT has not been reported to date. Therefore, we retrospectively collected a decade data to analyze the predictive power of pre-transformation PET/CT for the risk of HT in FL. All patients had no evidence of histological transformation when underwent PET/CT scans.
Our result showed that in quantitative assessments, higher levels of initial and post-treatment SUVmax both indicated a shorter time to HT. While in visual analysis, the results of the interim or post-treatment Deauville 5-point scale assessment, which uses liver activity as the reference, were not associated with the time to HT in FL. In our study, grade 3a disease was found to be the most important factor affecting transformation, and treatments containing rituximab have been reported to be closely related to a reduced incidence of transformation. Here, the treatment protocols for patients with grade 3a disease (R-CHOP or R-CVP) were more uniform than those for low-grade patients. To minimize the confounding effects of pathological classification and treatment strategy, we analyzed the predictive ability of PET/CT parameters for HT in low-grade and high-grade subgroups, respectively. The results showed the SUV baseline was still an important predictor for HT in both high-grade and low-grade subgroups. SUVend also remained as a prognostic indicator for HT in high-grade subgroup. These findings imply that a higher baseline SUVmax represents higher tumor invasiveness, leading to a higher probability of subsequent transformation, and poor efficacy of induction therapy is also an important factor influencing transformation. The NCCN-FLIPI2 was proposed recently and proved more accurate for identifying high-risk patients in the era of immune-chemotherapy. In our study, the NCCN-FLIPI2 score was also shown to be highly predictive for patients at risk of transformation. Therefore, we used multivariate analysis to evaluate the prognostic values adjusted by NCCN- FLIPI2 score, and concluded that SUVbaseline and SUVend were significant HT predictors independent of NCCN- FLIPI2 score.
Recently, cytogenetics and tumor microenvironment to predict HT have also been studied. No single gene lesion could explain all cases of HT. TP53 mutation, loss of CKDN2A, and gains of MYC were reported to be participated in development of HT [31]. The microenvironment of patients with HT seems to be different but HT cannot be predicted based on microenvironment gene expression profile (GEP) [32, 33]. In addition, some emerging technologies such as the detection of blood ctDNA [16] and the application of Raman-Enhanced Spectroscopy (RESpect) probe to identify molecular chemical composition in tissue [34] may be used as potential diagnostic or predictive tools for the HT of FL.
Our study has several limitations. First, the study design was retrospective. Second, although the study included 219 patients, the PET/CT dataset provided was insufficient. The PET/CT data for the pre-treatment phase, interim period, and the end of treatment were obtained from scattered patients, and data for all three stages were available for very few individual patients. The lack of parallel data of patients and insufficient data for analysis reduced the reliability of the conclusion. In addition, although the initial biopsy-confirmed HT did not occur, sites with a high SUVmax may not be suitable for biopsy, and the possibility of de novo transformation cannot be completely ruled out.
Conclusions
Although HT occurs in a minority of patients with follicular lymphoma, it significantly worsens the survival, prompting the clinical significance of early screening of high-risk HT patients. The biology of HT is complex, no single genetic lesion or mechanism could explain all cases of HT, and no clinical indicators or biomarks to strongly predict HT have been identified to date. PET/CT is used as a non-invasive diagnosis tool to help determine the best biopsy site when suspecting HT, but its role in predicting the HT risk in the early stage of disease is still unclear. The present study provides a decade of retrospective data to analyze the relationship between HT risk and PET/CT parameters at the initial, interim and end-treatment stages. SUVbaseline and SUVend were validated to show predictive value on HT risk independently of FLIPI2 score, and may help to identify high risk patients in clinical. Given the bias caused by the analysis of scattered data in this study, more prospective studies with larger samples are needed to confirm the predictive ability of pre-trasformation PET/CT and whether patients could benefit from a change in treatment strategy based on PET/CT-based risk stratification of HT.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HT:
-
Histological transformation
- SUVmax:
-
Maximum standardized uptake value;
- PET:
-
Positron emission tomography
- 18F-FDG PET/CT:
-
18F-fluorodeoxyglucose positron emission tomography/computed tomography
- PETinterim:
-
Interim PET
- PETend:
-
End-of-induction therapy
- FLIPI2:
-
FL International Prognostic Index 2
- PETbaseline:
-
PET at baseline
- FL:
-
Follicular lymphoma
- NHL:
-
Non-Hodgkin's lymphoma
- DLBCL:
-
Diffuse large B-cell lymphoma
- D5PS:
-
Deauville standard comprising a 5-point scale
- HGB:
-
Hemoglobin
- HRs:
-
Hazard ratios
- CI:
-
Confidence intervals
- ROC:
-
Receiver operating characteristic
- GELF:
-
Groupe d’Etude des Lymphomes Folliculaires
- ISRT:
-
Involved site radiation therapy
- AUC:
-
Area Under the Curve
- TMTV:
-
The total metabolic tumor volume
- OS:
-
Overall survival
- bSUVmax:
-
Baseline SUVmax
References
Izumo T, Maseki N, Mori S, Tsuchiya E. Practical utility of the revised European-American classification of lymphoid neoplasms for Japanese non-Hodgkin’s lymphomas. Jpn J Cancer Res. 2000;91:351–60. https://doi.org/10.1111/j.1349-7006.2000.tb00952.x.
Link BK, Maurer MJ, Nowakowski GS, Ansell SM, Macon WR, Syrbu SI, et al. Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: a report from the University of Iowa/MayoClinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J Clin Oncol. 2013;31:3272–8. https://doi.org/10.1200/JCO.2012.48.3990.
Madsen C, Plesner TL, Bentzen HH, Jørgensen J, Sillesen IB, Himmelstrup BM, et al. Real world data on histological transformation in patients with follicular lymphoma: Incidence, clinico-pathological risk factors and outcome in a nationwide Danish cohort. Leuk Lymphoma. 2020;61:2584–94. https://doi.org/10.1080/10428194.2020.1779254.
Sorigue M, Sancho JM. Current prognostic and predictive factors in follicular lymphoma. Ann Hematol. 2018;97:209–27. https://doi.org/10.1007/s00277-017-3154-z.
Wagner-Johnston ND, Link BK, Byrtek M, Dawson KL, Hainsworth J, Flowers CR, et al. Outcomes of transformed follicular lymphoma in the modern era: A report from the National LymphoCare Study (NLCS). Blood. 2015;126:851–7. https://doi.org/10.1182/blood-2015-01-621375.
Janikova A, Bortlicek Z, Campr V, Kopalova N, Benesova K, Hamouzova M, et al. The incidence of biopsy-proven transformation in follicular lymphoma in the rituximab era. A retrospective analysis from the Czech Lymphoma Study Group (CLSG) database. Ann Hematol. 2018;97:669–78. https://doi.org/10.1007/s00277-017-3218-0.
Federico M, Caballero BM, Marcheselli L, Tarantino V, Manni M, Sarkozy C, et al. Rituximab and the risk of transformation of follicular lymphoma: a retrospective pooled analysis. Lancet Haematol. 2018;5:e359–67. https://doi.org/10.1016/S2352-3026(18)30090-5.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. https://doi.org/10.1200/JCO.2006.09.2403.
Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25:571–8. https://doi.org/10.1200/JCO.2006.08.2305.
Zhu SH, Zhang Y, Yu YH, Fu Z, Kong L, Han DL, et al. FDG PET-CT in non-small cell lung cancer: relationship between primary tumor FDG uptake and extensional or metastatic potential. Asian Pac J Cancer Prev. 2013;14:2925–9. https://doi.org/10.7314/apjcp.2013.14.5.2925.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68. https://doi.org/10.1200/JCO.2013.54.8800.
Rossi C, Tosolini M, Gravelle P, Pericart S, Kanoun S, Evrard S, et al. Baseline SUVmax is related to tumor cell proliferation and patient outcome in follicular lymphoma. Haematologica. 2020. https://doi.org/10.3324/haematol.2020.263194.
Meignan M, Cottereau AS, Versari A, Chartier L, Dupuis J, Boussetta S, et al. Baseline metabolic tumor volume predicts outcome in high-tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J Clin Oncol. 2016;34:3618–26. https://doi.org/10.1200/JCO.2016.66.9440.
Trotman J, Luminari S, Boussetta S, Versari A, Dupuis J, Tychyj C, et al. Prognostic value of PET-CT after first-line therapy in patients with follicular lymphoma: a pooled analysis of central scan review in three multicentre studies. Lancet Haematol. 2014;1:e17-27. https://doi.org/10.1016/S2352-3026(14)70008-0.
Cottereau AS, Versari A, Chartier L, Dupuis J, Tarantino V, et al. Low suvmax measured on baseline FDG-PET/CT and elevated beta 2 microglobulin are negative predictors of outcome in high tumor burden follicular lymphoma treated by immunochemotherapy: a pooled analysis of three prospective studies. Blood. 2016;128:22. https://doi.org/10.1182/blood.V128.22.1101.1101.
Ohmoto A, Fuji S. Histological transformation in malignant lymphoma: a possible role of PET/CT and circulating tumor DNA as noninvasive diagnostic tools. Expert Rev Hematol. 2020;13:23–30. https://doi.org/10.1080/17474086.2020.1690987.
Mir F, Barrington SF, Brown H, Nielsen T, Sahin D, Meignan M, et al. Baseline SUVmax did not predict histological transformation in follicular lymphoma in the phase 3 GALLIUM study. Blood. 2020;135:1214–8. https://doi.org/10.1182/blood.2019001091.
Casulo C, Burack WR, Friedberg JW. Transformed follicular non-Hodgkin lymphoma. Blood. 2015;125:40–7. https://doi.org/10.1182/blood-2014-04-516815.
Acker B, Hoppe RT, Colby TV, Cox RS, Kaplan HS, Rosenberg SA. Histologic conversion in the non-Hodgkin’s lymphomas. J Clin Oncol. 1983;1:11–6. https://doi.org/10.1200/JCO.1983.1.1.11.
Garvin AJ, Simon RM, Osborne CK, Merrill J, Young RC, Berard CW. An autopsy study of histologic progression in non-Hodgkin’s lymphomas. 192 cases from the National Cancer Institute. Cancer. 1983;52:393–8. https://doi.org/10.1002/1097-0142(19830801)52:3.
Gallagher CJ, Gregory WM, Jones AE, Stansfeld AG, Richards MA, Dhaliwal HS, et al. Follicular lymphoma: prognostic factors for response and survival. J Clin Oncol. 1986;4:1470–80. https://doi.org/10.1200/JCO.1986.4.10.1470.
Al-Tourah AJ, Gill KK, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:5165–9. https://doi.org/10.1200/JCO.2008.16.0283.
Ghosh S, Somasundaram V, Deepti M. The domino effect-treatment of superior vena cava obstruction triggering tumor lysis syndrome: a case report. Sci Med J. 2021;3(1):44–50. https://doi.org/10.28991/SciMedJ-2021-0301-6.
Sarkozy C, Trneny M, Xerri L, Wickham N, Feugier P, Leppa S, et al. Risk factors and outcomes for patients with follicular lymphoma who had histologic transformation after response to first-line immunochemotherapy in the PRIMA trial. J Clin Oncol. 2016;34:2575–82. https://doi.org/10.1200/JCO.2015.65.7163.
Wang X, Wu CY, Zeng PY, Li LL, Ma CC, Xiang X, et al. Value of red blood cell distribution width and fibrinogen level for evaluation of the therapeutic efficacy and prognosis in patients wi. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28:153–9. https://doi.org/10.19746/j.cnki.issn.1009-2137.2020.01.026.
Chai Y, Qi F, Chen B, Gui L, Yang J, Qi S, et al. Abnormal pretreatment coagulation factor levels correlate with poor prognosis in patients with early-stage extranodal nasal-type natural/killer T cell lymphoma. Ann Hematol. 2020;99:1303–9. https://doi.org/10.1007/s00277-020-04035-0.
Kawaguchi Y, Saito B, Nakata A, Matsui T, Sasaki Y, Shimada S, et al. Elevated C-reactive protein level is associated with poor prognosis in follicular lymphoma patients undergoing rituximab-containing chemotherapy. Int J Hematol. 2020;112:341–8. https://doi.org/10.1007/s12185-020-02910-0.
Federico M, Caballero D, Marcheselli L, et al. The risk of transformation of follicular lymphoma “transformed” by rituximab: the Aristotle study promoted by the european lymphoma institute. Hematol Oncol. 2017;35(S2):115–6. https://doi.org/10.1002/hon.2437_104.
Cottereau AS, Versari A, Luminari S, Dupuis J, Chartier L, Casasnovas RO, et al. Prognostic model for high-tumor-burden follicular lymphoma integrating baseline and end-induction PET: a LYSA/FIL study. Blood. 2018;131:2449–53. https://doi.org/10.1182/blood-2017-11-816298.
Boo SH, O JH, Kwon SJ, Yoo IR, Kim SH, Park GS, et al. Predictive value of interim and end-of-therapy 18F-FDG PET/CT in patients with follicular lymphoma. Nucl Med Mol Imaging. 2019;53:263–9. Doi: https://doi.org/10.1007/s13139-019-00602-0
Pasqualucci L, Khiabanian H, Fangazio M, Vasishtha M, Messina M, Holmes AB, et al. Genetics of follicular lymphoma transformation. Cell Rep. 2014;6:130–40. https://doi.org/10.1016/j.celrep.2013.12.027.
Montoto S, Fitzgibbon J. Transformation of indolent B-cell lymphomas. J Clin Oncol. 2011;29:1827–34. https://doi.org/10.1200/JCO.2010.32.7577.
Glas AM, Knoops L, Delahaye L, Kersten MJ, Kibbelaar RE, Wessels LA, et al. Gene-expression and immunohistochemical study of specific T-cell subsets and accessory cell types in the transformation and prognosis of follicular lymphoma. J Clin Oncol. 2007;25:390–8. https://doi.org/10.1200/JCO.2006.06.1648.
Agsalda-Garcia M, Shieh T, Souza R, Kamada N, Loi N, Oda R, et al. Raman-Enhanced spectroscopy (RESpect) probe for childhood Non-Hodgkin lymphoma. Sci Med J. 2020;2:1–7. https://doi.org/10.28991/scimedj-2020-0201-1.
Acknowledgements
Not applicable.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
MX and LW: conception and design of the study, protocol development, analysis and interpretation of data, drafting the article; QJ, XL and XZ: screening patients, collection of data, analysis and interpretation of data; JJ, XY and KZ: conception and design of the study, protocol development, revision of the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All patients provided written informed consent to participate in the study. The Ethics Committee of the First Affiliated Hospital, Zhejiang University approved the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. The original data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xie, M., Wang, L., Jiang, Q. et al. Significance of initial, interim and end-of-therapy 18F-FDG PET/CT for predicting transformation risk in follicular lymphoma. Cancer Cell Int 21, 394 (2021). https://doi.org/10.1186/s12935-021-02094-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-021-02094-5