Abstract
Background
Lung cancer bone metastasis causes poor prognosis. Basigin-2, a novel cancer-associated biomarker, is upregulated in lung cancer and has been linked with tumor progression. But little is known about the role of basigin-2 in lung cancer bone metastasis and osteolytic lesion.
Methods
Basigin-2 expression was evaluated in biopsy tissue specimens of 20 lung cancer patients with bone metastases via immunohistochemistry. Invasion assay and MTT proliferation assay were performed to test the invasion and proliferation of lung cancer cell after modulated basigin-2 expression. The osteoclastic activity of basigin-2 was detected in tibia cancer model by injected of lung cancer cells. The regulation role of receptor activator of NF-κB ligand (RANKL) on basigin-2 and its downstream molecules were measured by real-time quantitative RT-PCR, gelatin zymography and western blot analysis.
Results
We found that basigin-2 was highly expressed in lung cancer bone metastases. Then, we demonstrated that basigin-2 could promote lung cancer cells invasion, metastasis and proliferation through upregulating metalloproteinases-2 (MMP-2), MMP-9 and vascular endothelial growth factor (VEGF) expression. The lung cancer cells overexpressing basigin-2 strongly induced the osteolytic lesions in immunodeficient mice, which were reduced by treatment with basigin-2 blocking antibody. Furthermore, we explored the enhanced basigin-2 molecular mechanism in lung cancer bone metastasis. Our results indicated the RANKL, pivotal for the control of bone resorption, could increase basigin-2 and its downstream molecules MMP-2, MMP-9 and VEGF expression in vitro.
Conclusions
Basigin-2 upregulated by RANKL induces MMPs and VEGF, which may increase lung cancer cell metastasis ability and support osteoclastic activity. Thus, our data suggest important roles for basigin-2 in lung cancer-induced osteolytic lesion and implicate this protein potential application as a target for lung cancer bone metastasis therapy.
Similar content being viewed by others
Background
Lung cancer is the most frequent cause of cancer-related death in the world, with about 15 % 5-year survival rate of patients with non-small cell lung cancer (NSCLC) [1] and 6 % of small cell lung cancer (SCLC) [2]. In the United States, it is expected that 224,210 people will receive diagnoses of lung and bronchial cancer and that 159,260 will die of the disease in 2014 [3, 4]. One of the characteristics of lung cancer is its high potential for invasion and metastasis. Bone metastasis is one of the most frequent sites of secondary lesions in lung cancer [5]. When the bone metastases established, the chance of survival as well as the quality of life dramatically drop, with intractable pain, nerve compression syndromes, increased risk of fractures, and hypercalcaemia [6]. Lung cancer bone metastasis is usually osteolytic in nature and relies on the ability of tumor cells to activate osteoclast bone resorption, which is a pivotal event in the disruption of bone and in the creation of physical space into which the tumor intrudes [7–9]. Therefore, it is significant to study the molecular mechanism of bone metastasis in lung cancer.
Basigin-2, also known as CD147, is a transmembrane glycoprotein present on the cancer cell surface belonging to the immunoglobulin superfamily, which was found to induce expression of matrix metalloproteinases (MMPs), particular MMP-2 and MMP-9 in fibroblasts or tumor cells. Several laboratories have subsequently identified the basigin-2 molecule in different origins of human cells and tissues, designating it the names of extracellular matrix metalloproteinase inducer (EMMPRIN) [10], HAb18G [11], and M6 antigen [12]. Several data have clearly shown a key role for basigin-2 in tumor progression and metastasis [13, 14]. Basigin-2 is also able to induce MMPs production in tumor cells in an autocrine fashion [15] and to enhance in vivo tumor angiogenesis by upregulating VEGF [16].
Since, our previous work have showed the up-regulation of basigin-2 in lung cancer [17]. The aim of this study was to test the potential involvement of basigin-2 in the ability of lung cancer cells to induce osteolytic lesions in bone. In this study, we found that basigin-2 was highly expressed in the lung cancer bone metastases. We also demonstrated that basigin-2 could upregulate MMP-2, MMP-9 and VEGF expression. In addition, overexpression of basigin-2 promoted the invasion, metastasis and proliferation in lung cancer cell. The immunodeficient mice, which were injected with lung cancer cell overexpressing basigin-2, had a greater ability to induce the development of radiographically detectable osteolytic lesions. But this effect was reduced by treatment with basigin-2 blocking antibody. Furthermore, we explored the enhanced basigin-2 molecular mechanism that involved the activation of the receptor activator of NF-κB ligand (RANKL) pathway, pivotal for the control of bone resorption. Thus, our data suggest important roles for basigin-2 in supporting osteoclastic activity and increased incidence of lung cancer bone metastases.
Methods
Tissue specimens and immunohistochemical analysis
Twenty biopsy tissue specimens of lung cancer bone metastases were collected from Tangdu Hospital of Fourth Military Medical University (FMMU, Xi’an, China) and were histologically confirmed by staining with hematoxylin and eosin (H & E). All individuals provided written informed consent, and the study was approved by the Hospital Ethics Committee.
Immunohistochemistry was performed using the Histostain-SP kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s manual. Anti-basigin-2 antibody was previously prepared using hybridoma technique in our laboratory, which was a murine monoclonal antibody with a clone number as HAb18, targeting Ig-C2 domain of human basigin-2 [18–20]. Immunohistochemistry was performed using a basigin-2 diagnostic kit (Jiangsu Pacific Meinuoke Biopharmaceutical Company) according to the manufacturer’s manual [21]. Immunopositivity was independently evaluated by two pathologists. In case of discrepancy, the two pathologists will re-review the slides together to reach a consensus. Detailed evaluation of IHC staining score was according to our previous work [13, 17, 22].
Cell lines and culture conditions
The human lung cancer cell line A549 (lung adenocarcinoma cells) was obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The A549 cell was routinely cultured in RPMI-1640 medium (Hyclone Laboratories, Logan, UT, USA) supplemented with 10 % fetal calf serum (Gibco, Rockville, MD, USA) at 37 °C in a humidified atmosphere of 5 % CO2. The cell line authentication was assessed using short tandem repeat (STR) DNA profiling method every year in our laboratory and the latest verification was done in March 2014.
To obtain the conditioned medium from osteoblasts, primary cultures of calvarial osteoblasts were prepared by a modification of the sequential collagenase/trypsin digestion method [23, 24]. In brief, calvaria were removed from 7 to 9-day-old CD1 mice, cleaned free from soft tissue, and digested with 1 mg/mL Clostridium histolyticum type IV collagenase and 0.025 % trypsin for 20 min at 37 °C in HBSS with gentle agitation. The procedure was repeated three times and cells from the second and third digestions were plated in petri dishes and grown to confluence in DME supplemented with antibiotics and 10 % FCS. At confluence, cells were trypsinized by the standard procedure and plated in wells for experiments. The cells obtained with this method were positive for alkaline phosphatase (ALP) activity and expression of the osteoblast markers [24]. Then, cells were grown in DMEM plus 10 % FBS until 80 % confluence. The media were then replaced with serum-free media, and after 48 h, supernatants were collected, centrifuged, and stored at −80 °C until use. For the experiments of RANKL inhibition, primary osteoblasts were treated with 100 ng/mL osteoprotegerin (OPG), then after 48 h, supernatants were collected, centrifuged, and stored at −80 °C until use.
Vector construction, stable transfection and siRNA
The coding regions of basigin-2 was inserted into pcDNA3.1 (Invitrogen, Carlsbad, CA, USA) [20]. Stable transfectant was screened with G418 (Calbiochem, San Diego, CA) after transfection. siRNAs targeting basigin-2 and scrambled negative control siRNA (SNC) were purchased from Invitrogen [13]. Next, we constructed the shBasigin-2 vevtor containing small hairpin RNA (shRNA) targeting basigin-2 mRNA. The stable shRNA transfection A549 cells were screened with purine (Calbiochem) [25].
Real-time quantitative RT-PCR
Real-time quantitative RT-PCR was performed as described previously [26]. Expression data were uniformly normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control, and the relative expression levels were evaluated using the ΔΔCt method [20, 27]. Primers were used as described previously [13, 14, 20].
Western blot analysis
Cells were lysed with RIPA buffer (Beyotime, NanTong, China). Equal amounts (10 μg) of total protein were loaded, and then subsequently immunoblotted with the primary antibodies, including anti-basigin-2, VEGF and tubulin monoclonal antibodies (Santa Cruz, CA, USA). Proteins were detected using the Amersham enhanced chemiluminescence system (Pierce, Rockford, IL, USA) according to the manufacturer’s instructions.
Gelatin zymography
Gelatin zymography was performed using 10 % SDS-PAGE containing 1 mg/mL gelatin. Transfected A549 cells (1.0 × 105 per well) were cultured in 96-well plates. After attachment, the cells were washed and incubated in serum-free medium for 12 h. Then, the supernatants were collected and prepared in non-reducing loading buffer. After electrophoresis, SDS was removed using 2.5 % Triton X-100 to renature gelatinases. Gels were then incubated at 37 °C for 16 h in developing buffer (50 mM Tris–HCl (pH 7.8), 200 mM NaCl, 5 mM CaCl2, and 0.02 % Brij-35) and then stained with Coomassie Blue R-250. MMPs activity was visualized as clear bands against the blue-stained gelatin background.
In vitro invasion assay and migration assay
MilliCell (12 mm diameter with 8 µm pores) chambers (Millipore Corporation, Billerica, MA, USA) were pre-coated with Matrigel (BD, Bedford, MA, USA) on the upper side. A total of 1 × 105 serum-starved A549 cells were added to the upper compartment in medium supplemented with 0.1 % serum, and the chambers were placed into 24-well plates with medium containing 10 % serum. After 24 h at 37 °C, invaded cells on the lower membrane surface were fixed and stained with 0.1 % crystal violet. Invasive activity was quantified by counting nine high-power fields (HPFs, 400×) per chamber. Mean values were obtained from at least three individual chambers for each experimental point per assay. The migration assay was the same as the invasion assay, except that no Matrigel was used and the cell permeating time was 12 h.
Wound-healing assay
1 × 106 cells were seeded in six-well plates, cultured overnight, and transfected with basigin-2 siRNA, basigin-2/pcDNA3.1 or scrambled negative control (SNC), respectively. When the culture had reached nearly 90 % confluency, the cell layer was scratched with a sterile plastic tip and then washed with culture medium twice and cultured again for up to 24 h with serum-reduced medium containing 1 % FBS. At different time points, photographic images of the plates were acquired under a microscope and the data were summarized based on sextuple assays for each experiment.
Cell proliferation assay
Cells were plated in sextuplicate in 96-well plates (2 × 103 per well) in 100 μL complete medium and allowed to attach overnight. 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) (20 μL at 5 mg/mL; Sigma, St. Louis, MO, USA) was added at different time points (24 h interval) for the different groups and then incubated for 4 h. The supernatant was discarded, the precipitate was dissolved in 200 μL dimethyl sulfoxide (DMSO), and plates were read with a microplate reader at 570 nm [28].
Tibia cancer model by injected of lung cancer cells
Female BALB/c nude mice at 4–6 weeks of age were provided by the Laboratory Animal Research Center of FMMU, and the animal study was reviewed and approved by the Animal Care and Use Committee of FMMU. Mice were anaesthetized with i.p. injection of pentobarbital. A syringe with a 26 1/2 G needle was subsequently inserted in the proximal end of the tibia, and tumor cells (5 × 104/10 μL Matrigel) were injected into the intramedullary space [29]. Radiographs were taken every week after injection using Carestream MS FX Pro in vivo imaging system (Carestream Health, Cheektowaga, NY, United States) [14]. Osteolytic lesions were identified on radiographs as radio-opaque regions. For the antibody blocking treatment, 5 μg of basigin-2 antibody [18], or negative control IgG were injected twice weekly until the end of the experiment.
Statistical analysis
All statistical analyses were performed using the SPSS statistical software package (version 16.0, Chicago, IL, United States). Each in vitro quantitative test was independently replicated, and all data are presented as mean ± SEM. All the statistical tests were two sided, and P < 0.05 was considered with statistical significance.
Results
High expression level of basigin-2 in lung cancer bone metastases
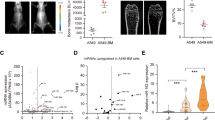
In the first set of experiments, basigin-2 expression was evaluated in biopsy tissue specimens of 20 lung cancer patients with bone metastases via immunohistochemistry. As showed in Fig. 1, basigin-2 protein was predominantly localized in tumor epithelial cells, whereas little was detected in stroma. We also found that basigin-2 displayed positive membranous and cytoplasmic staining. Basigin-2 positive expression found in lung cancer bone metastases was 65 % (13/20). However, basigin-2 expression was not detected in the bone tissues of lung cancer patients without bone metastasis. So our results indicated that basigin-2 was highly expressed in lung cancer bone metastases, which may participate in the cancer distant metastasis.
High expression of basigin-2 in lung cancer bone metastases. Representative immunohistochemistry analysis of basigin-2 expression in lung cancer tissues and the bone metastases. The left panel, magnification, ×200. The right panel is the amplification with magnification ×400. Top, the basigin-2 was high expressed in the lung adenocarcinoma. The middle and bottom were bone metastases of the lung cancer patient detected by H & E and IHC staining
Characterization of lung cancer cells with modulated basigin-2 expression
To explore the effect of modulated basigin-2 expression in lung cancer cell, we transfected the A549 cell with basigin-2 overexpression vector or siRNA, respectively. As showed in Fig. 2a, we exhibited that the ectopic expression or siRNA knockdown, respectively, increased or reduced basigin-2 mRNA and protein expression. Modulated basigin-2 expression were accompanied by a significant increase or decrease of MMP-2 and MMP-9 mRNA expression and proteinase activity (Fig. 2b). In addition, the expression of both VEGF mRNA and protein were significantly upregulated or downregulated in A549 cells (Fig. 2c).
The regulation effect of basigin-2 on MMP-2, MMP-9 and VEGF expression in lung cancer cell. a The mRNA and protein expression of basigin-2 in A549 cells transfected with overexpression vector or siRNA detected by realtime RT-PCR and western blot, respectively. SNC means scrambled negative control of siRNA. After modulating basigin-2 expression, the downstream molecular MMP-2, MMP-9 (b) and VEGF (c) mRNA and protein expression level (proteinase activity for MMPs) were detected using realtime RT-PCR, gelatin zymography and western blot in A549 cells. *P < 0.05, compared to negative control (pcDNA3.1 or SNC), by Student’s t test
Based on above results, we detected whether basigin-2 could change the capacities of lung cancer cells for migration, invasion and proliferation. As expected, transfection of basigin-2 expression plasmid into A549 cells resulted in increased migration rate and invasion rate compared with the control (Fig. 3a). In contrast, transfected with siRNA displayed the opposite results (Fig. 3b). Next, the wound-healing assay showed that A549 cells with overexpression of basigin-2 presented a quicker closing of scratch wound, compared with the controls (Fig. 3c). Transfection with siRNA showed the opposite result (Fig. 3d). The lung cancer cell proliferation was also promoted by modulated basigin-2 expression (Fig. 3e). Our results indicated that basigin-2 could regulate the downstream molecules MMP-2, MMP-9 and VEGF expression and contributed to a promotion in migration, invasion and proliferation of lung cancer cells.
The furtherance role of basigin-2 on lung cancer cell invasion, metastasis and proliferation. a Effect of basigin-2 on the invasion and migration of A549 cells transfected with basigin-2 expression plasmid. b The invasion and migration of A549 cells transfected with basigin-2 siRNA were tested. SNC means scrambled negative control of siRNA. Morphologic comparison of cells penetrating the artificial basement membrane was shown. c and d Wound-healing assay of A549 cells transfected with basigin-2 expression plasmid or siRNA, respectively. *P < 0.05, compared to negative control (pcDNA3.1 or SNC), by one-way ANOVA followed by the Dunnett test. Scale bars 200 μm. e Cell proliferation of lung cancer cells transfected as above (a) and (b) was measured in the indicated time periods using MTT assay. *P < 0.05, compared to negative control (pcDNA3.1 or SNC), by 2-way repeated measures ANOVA followed by the Bonferroni test
The promotion effect of basigin-2 on the osteolytic lesions
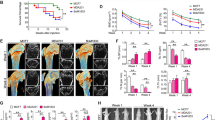
The lung cancer A549 cells used in our experiments were highly osteotropic, and when injected into the bone of immunodeficient mice, they grew causing osteolytic lesions that increased over time in a progressive fashion (Fig. 4a middle panel). Thus we compared the effects of A549 cells with overexpression of basigin-2, downexpression of basigin-2 and A549 empty vector cells after their inoculation into the tibia of nude mice. When injected with basigin-2 stable overexpression A549 cells, the incidence and extension area of osteolytic lesions were significantly higher in nude mice than the empty vector control (Fig. 4a, b). Downregulation of basigin-2 showed the opposite results (Fig. 4a right panel). So our results indicated the basigin-2 could significantly promote the lung cancer osteolytic lesions.
The promotion effect of basigin-2 on the osteolytic lesions. a The X-rays of the hind limb of mice injected with A549, basigin-2/A549 or shbasigin-2/A549 cells compared with normal mouse. b Evaluation of the incidence of tumor take (left two panel) and bone densitometric analysis of the radiographs assessing the osteolytic areas taken at the end point of the experiment (right panel). Inset numbers, number of tibia developing osteolytic lesions mice/number of tibia injected mice. *P < 0.05, by Student’s t test. c The X-rays of the tibia of mice injected with basigin-2/A549 cells treated using basigin-2 blocking antibody compared with IgG control. d Evaluation of the incidence of tumor take (left two panel) and bone densitometric analysis of the radiographs assessing the osteolytic areas taken at the end point of the experiment (right panel). Inset numbers, number of tibia developing osteolytic lesions mice/number of tibia injected mice. *P < 0.05, by Student’s t test
To validate the notion, mice intratibially inoculated with A549 cells were treated with a basigin-2 blocking antibody [30]. Our results exhibited that the ability of the blocking antibody to reduce the incidence and extension area of osteolytic lesions (Fig. 4c, d). There was no significant difference in mice body weight among the four groups of different treatment (Additional file 1: Figure S1). Our above results have indicated that basigin-2 could promote the lung cancer cells migration and invasion. Hence, these results suggest that basigin-2 play important roles in lung cancer bone metastasis induced osteolytic lesions.
The regulation role of RANKL on basigin-2 expression
To unravel the regulatory mechanisms of basigin-2 promoting osteolytic lesion, the A549 cells were exposed to conditioned medium from osteoblasts (OBsCM). The medium contains the osteoblast markers alkaline phosphatase, runt-related transcription factor 2, parathyroid hormone/parathyroid hormone–related peptide receptor, and type I collagen and osteocalcin, which could produce paracrine effectors of lung cancer growth into the bone [14]. Notably, the expression levels of basigin-2, MMP-2, MMP-9 and VEGF were increased by the OBsCM than the control (Fig. 5a). Previous works have suggested that RANKL is a key inducer of osteolytic bone metastasis through its pro-osteoclastogenic activity. There was RANK expression in A549 cells [31]. So we detected the effect of RANKL on the basigin-2 expression regulation. Interestingly, the upregulated expression of basigin-2, MMP-2, MMP-9 and VEGF induced by OBsCM were abolished through treating with OPG, a decoy receptor that physiologically inhibited RANKL activity (Fig. 5a).
Effect of RANKL on the expression of basigin-2 and its downstream genes. a A549 cells were treated with DMEM (negative control, NC) or with conditioned medium from primary mouse osteoblast cultures treated with vehicle (OBsCM) or OPG (OBsCM + OPG) for 48 h. The mRNA expression of basigin-2, MMP-2, MMP-9 and VEGF were detected by realtime RT-PCR. *P < 0.05, compared to negative control (NC), by Student’s t test. b–d A549 cells were treated with 30 ng/mL RANKL for 4, 8, and 16 h. Then, the mRNA and protein expression level were measured for b basigin-2, d VEGF and proteinase activities for c MMP-2 and MMP-9. *P < 0.05, compared to 0 h, by Student’s t test
To further prove the role of RANKL in modulating basigin-2 and its downstream genes, the A549 cells were treated with recombinant RANKL. As showed, the basigin-2 mRNA and protein expression level were increased in a time-dependent pattern (Fig. 5b). The downstream genes, MMP-2, MMP-9 and VEGF were also increased by RANKL (Fig. 5c, d). A parallel increase of MMP-2, MMP-9 activity (Fig. 5c, inset) and VEGF protein expression (Fig. 5d, inset) was seen after treatment with RANKL. Hence, our results suggest that RANKL may through basigin-2 pathway enhance osteoclast activity.
Discussion
In our study, we showed that basigin-2 was highly expressed in the lung cancer bone metastases and could regulate the downstream molecules MMP-2, MMP-9 and VEGF expression, which promoted migration, invasion and proliferation of lung cancer cells. When injected with basigin-2 stable overexpression A549 cells in the tibia of nude mice, the incidence and extension area of osteolytic lesions were significantly higher than those of empty vector control, which was reduced by treatment with basigin-2 blocking antibody. Furthermore, our results revealed that RANKL could increase basigin-2 and its downstream molecules MMP-2, MMP-9 and VEGF expression. So RANKL may through basigin-2 pathway enhance osteoclast activity.
Lung cancer has a high potential for metastasis and is normally diagnosed when the disease has already progressed locally or systemically because there are few symptoms in the early stages of the disease [32]. A recent study reported that 41 % of patients had distant disease at the time of the diagnosis and of these 38 % suffered bone metastasis during the first year following their cancer diagnosis [33]. Our previous work showed that basigin-2 was more strongly upregulated in lung cancer tissues than in the adjacent tissues [17]. Our results indicated that basigin-2 could regulate its downstream molecules MMP-2, MMP-9 and VEGF expression and promote migration, invasion and proliferation of lung cancer cells. One hallmark of metastasis and invasive growth is the transition of tumor cells from an epithelial to a mesenchymal morphology, known as the epithelial–mesenchymal transition (EMT). MMPs help cancer cells spread by breaking down the extracellular matrix (ECM) and other barriers MMP-2 and MMP-9 were previously reported to be involved in human tumorigenesis and cancer metastasis [34]. VEGF is a key regulatory factor in angiogenesis and vascular permeability in both physiological and pathological states [35]. Several studies have reported the association between the VEGF polymorphisms with lung cancer risk [36]. Our current data are in keeping with the known functions of basigin-2 in human malignancies and strengthen the key role played by this molecule in lung cancer progression.
Bone metastasis is a frequent complication of cancer, occurring in up to 70 percent of patients with advanced breast or prostate cancer and in approximately 15–30 percent of patients with carcinoma of the lung, colon, stomach, bladder, uterus, rectum, thyroid and kidney [37]. Our findings indicated that basigin-2 could significantly promote the lung cancer osteolytic lesions in vivo. Thus, basigin-2 plays important roles in lung cancer bone metastasis induced osteolytic lesions. The bone microenvironment plays a critical role in the formation of osteoclasts through the production of macrophage colony-stimulating factor and RANKL [38] by stromal cells or osteoblasts. RANKL binds to the RANK receptor on osteoclast precursors and induces the formation of osteoclasts by signaling through the nuclear factor-κB and Jun N-terminal kinase pathways [39]. In this study, RANKL could increase basigin-2 and regulate its downstream molecules. Herein, we have shown a link between tumor basigin-2 and the RANKL pathway. Increased RANKL expression by osteoblasts is a hallmark of bone metastases and is deemed to contribute to enhanced osteoclast activity at sites of osteolytic lesions [39]. Our work is consistent with that conditioned medium from primary osteoblasts enhanced basigin-2 expression in lung cancer cells. Therefore, our data suggest that the mechanism whereby basigin-2 induces osteolysis relies to a direct basigin-2 activity as well as to the induction of the release of downstream effectors MMPs and VEGF.
Conclusion
To the best of our knowledge, this is the first study to examine the role of basigin-2 in lung cancer-induced osteolytic lesion and to reveal that a RANKL-basigin-2-MMPs/VEGF pathway plays an important role in this process. These findings will contribute to our understanding of the molecular mechanism of lung cancer-induced osteolytic lesion and might aid in an alternative target for bone metastasis therapy.
References
Araujo A, Ribeiro R, Azevedo I, Coelho A, Soares M, Sousa B, Pinto D, Lopes C, Medeiros R, Scagliotti GV. Genetic polymorphisms of the epidermal growth factor and related receptor in non-small cell lung cancer—a review of the literature. Oncologist. 2007;12(2):201–10.
Wu K, House L, Liu W, Cho WC. Personalized targeted therapy for lung cancer. Int J Mol Sci. 2012;13(9):11471–96.
Groome PA, Bolejack V, Crowley JJ, Kennedy C, Krasnik M, Sobin LH, Goldstraw P, Committee IIS, Cancer R. Biostatistics, observers to the C, participating I: the IASLC lung cancer staging project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thoracic Oncol Off Publ Int Assoc Study Lung Cancer. 2007;2(8):694–705.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Peters S, Meylan E. Targeting receptor activator of nuclear factor-kappa B as a new therapy for bone metastasis in non-small cell lung cancer. Curr Opin Oncol. 2013;25(2):137–44.
Shi L, Wang L, Hou J, Zhu B, Min Z, Zhang M, Song D, Cheng Y, Wang X. Targeting roles of inflammatory microenvironment in lung cancer and metastasis. Cancer Metastasis Rev. 2015;34(2):319–31.
Clezardin P, Teti A. Bone metastasis: pathogenesis and therapeutic implications. Clin Exp Metastasis. 2007;24(8):599–608.
Sone S, Yano S. Molecular pathogenesis and its therapeutic modalities of lung cancer metastasis to bone. Cancer Metastasis Rev. 2007;26(3–4):685–9.
Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6(10):2609–17.
Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55(2):434–9.
Xu HY, Qian AR, Shang P, Xu J, Kong LM, Bian HJ, Chen ZN. siRNA targeted against HAb18G/CD147 inhibits MMP-2 secretion, actin and FAK expression in hepatocellular carcinoma cell line via ERK1/2 pathway. Cancer Lett. 2007;247(2):336–44.
Kasinrerk W, Fiebiger E, Stefanova I, Baumruker T, Knapp W, Stockinger H. Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J Immunol. 1992;149(3):847–54.
Kong LM, Liao CG, Zhang Y, Xu J, Li Y, Huang W, Zhang Y, Bian H, Chen ZN. A regulatory loop involving miR-22, Sp1, and c-Myc modulates CD147 expression in breast cancer invasion and metastasis. Cancer Res. 2014;74(14):3764–78.
Rucci N, Millimaggi D, Mari M, Del Fattore A, Bologna M, Teti A, Angelucci A, Dolo V. Receptor activator of NF-kappaB ligand enhances breast cancer-induced osteolytic lesions through upregulation of extracellular matrix metalloproteinase inducer/CD147. Cancer Res. 2010;70(15):6150–60.
Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metalloproteinase inducer interactions. Cancer Res. 2001;61(5):2276–81.
Zhu D, Wang Z, Zhao JJ, Calimeri T, Meng J, Hideshima T, Fulciniti M, Kang Y, Ficarro SB, Tai YT, Hunter Z, McMilin D, Tong H, Mitsiades CS, Wu CJ, Treon SP, Dorfman DM, Pinkus G, Munshi NC, Tassone P, Marto JA, Anderson KC, Carrasco RD. The Cyclophilin A-CD147 complex promotes the proliferation and homing of multiple myeloma cells. Nat Med. 2015;21(6):572–80.
Kong LM, Liao CG, Fei F, Guo X, Xing JL, Chen ZN. Transcription factor Sp1 regulates expression of cancer-associated molecule CD147 in human lung cancer. Cancer Sci. 2010;101(6):1463–70.
Chen ZN. Significance and application of anti-malignant hepatoma MAb HAb18 in radioimmunal diagnosis of human hepatocellular carcinoma. Zhonghua zhong liu za zhi [Chinese J Oncol]. 1992;14(1):9–12.
Ku XM, Liao CG, Li Y, Yang XM, Yang B, Yao XY, Wang L, Kong LM, Zhao P, Chen ZN. Epitope mapping of series of monoclonal antibodies against the hepatocellular carcinoma-associated antigen HAb18G/CD147. Scand J Immunol. 2007;65(5):435–43.
Liao CG, Kong LM, Song F, Xing JL, Wang LX, Sun ZJ, Tang H, Yao H, Zhang Y, Wang L, Wang Y, Yang XM, Li Y, Chen ZN. Characterization of basigin isoforms and the inhibitory function of basigin-3 in human hepatocellular carcinoma proliferation and invasion. Mol Cell Biol. 2011;31(13):2591–604.
Bian H, Zheng JS, Nan G, Li R, Chen C, Hu CX, Zhang Y, Sun B, Wang XL, Cui SC, Wu J, Xu J, Wei D, Zhang X, Liu H, Yang W, Ding Y, Li J, Chen ZN. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J Natl Cancer Inst. 2014;106(9):dju239.
Fei F, Li X, Xu L, Li D, Zhang Z, Guo X, Yang H, Chen Z, Xing J. CD147–CD98hc complex contributes to poor prognosis of non-small cell lung cancer patients through promoting cell proliferation via the PI3K/Akt signaling pathway. Ann Surg Oncol. 2014;21(13):4359–68.
Robey PG, Termine JD. Human bone cells in vitro. Calcif Tissue Int. 1985;37(5):453–60.
Marzia M, Sims NA, Voit S, Migliaccio S, Taranta A, Bernardini S, Faraggiana T, Yoneda T, Mundy GR, Boyce BF, Baron R, Teti A. Decreased c-Src expression enhances osteoblast differentiation and bone formation. J Cell Biol. 2000;151(2):311–20.
Zhao P, Zhang W, Wang SJ, Yu XL, Tang J, Huang W, Li Y, Cui HY, Guo YS, Tavernier J, Zhang SH, Jiang JL, Chen ZN. HAb18G/CD147 promotes cell motility by regulating annexin II-activated RhoA and Rac1 signaling pathways in hepatocellular carcinoma cells. Hepatology. 2011;54(6):2012–24.
Kong LM, Liao CG, Chen L, Yang HS, Zhang SH, Zhang Z, Bian HJ, Xing JL, Chen ZN. Promoter hypomethylation up-regulates CD147 expression through increasing Sp1 binding and associates with poor prognosis in human hepatocellular carcinoma. J Cell Mol Med. 2011;15(6):1415–28.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–8.
Zhang J, Yang Y, Yang T, Liu Y, Li A, Fu S, Wu M, Pan Z, Zhou W. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br J Cancer. 2010;103(8):1215–20.
Angelucci A, Gravina GL, Rucci N, Festuccia C, Muzi P, Vicentini C, Teti A, Bologna M. Evaluation of metastatic potential in prostate carcinoma: an in vivo model. Int J Oncol. 2004;25(6):1713–20.
Damsker JM, Okwumabua I, Pushkarsky T, Arora K, Bukrinsky MI, Constant SL. Targeting the chemotactic function of CD147 reduces collagen-induced arthritis. Immunology. 2009;126(1):55–62.
Chen LM, Kuo CH, Lai TY, Lin YM, Su CC, Hsu HH, Tsai FJ, Tsai CH, Huang CY, Tang CH. RANKL increases migration of human lung cancer cells through intercellular adhesion molecule-1 up-regulation. J Cell Biochem. 2011;112(3):933–41.
da Silva GT, Bergmann A, Thuler LC. Skeletal related events in patients with bone metastasis arising from non-small cell lung cancer. Supportive Care Cancer Off J Multinatl Assoc Support Care Cancer. 2015.
Sathiakumar N, Delzell E, Morrisey MA, Falkson C, Yong M, Chia V, Blackburn J, Arora T, Kilgore ML. Mortality following bone metastasis and skeletal-related events among patients 65 years and above with lung cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999–2006. Lung India Off Organ Indian Chest Soc. 2013;30(1):20–6.
Chung TW, Moon SK, Chang YC, Ko JH, Lee YC, Cho G, Kim SH, Kim JG, Kim CH. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004;18(14):1670–81.
Bhattacharya R, Sinha S, Yang SP, Patra C, Dutta S, Wang E, Mukhopadhyay D. The neurotransmitter dopamine modulates vascular permeability in the endothelium. J Mol Signaling. 2008;3:14.
Zhai R, Liu G, Zhou W, Su L, Heist RS, Lynch TJ, Wain JC, Asomaning K, Lin X, Christiani DC. Vascular endothelial growth factor genotypes, haplotypes, gender, and the risk of non-small cell lung cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14(2):612–7.
Roodman GD. Mechanisms of bone metastasis. New England J Med. 2004;350(16):1655–64.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76.
Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4(8):638–49.
Authors’ contributions
CGL and LMK carried out the molecular genetic studies, animal studies and drafted the manuscript. LY participated in the histologic study. JGH, LLL, SDW and WX participated in cell biology experiments. NL performed the statistical analysis. LMK and HLZ conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the Project funded by China Postdoctoral Science Foundation (2013M542504), National Natural Science Foundation of China (81472632, 81101614 and 81171995) and Natural Science Foundation of Shaanxi (2013JQ4039).
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Cheng-Gong Liao, Li Yao and Wei Xie contributed equally to this work
Additional file
12935_2016_302_MOESM1_ESM.docx
Additional file 1: Figure S1. The body weight of mice among the four groups of different treatment. There was no significant difference in the four groups analyzed by 2-way repeated measures ANOVA followed by the Bonferroni test.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liao, CG., Yao, L., Xie, W. et al. Basigin-2 upregulated by receptor activator of NF-κB ligand enhances lung cancer-induced osteolytic lesions. Cancer Cell Int 16, 28 (2016). https://doi.org/10.1186/s12935-016-0302-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-016-0302-9