Abstract
Background
The accumulation of visceral and ectopic fat comprise a major cause of cardiometabolic diseases. However, novel drug targets for reducing unnecessary visceral and ectopic fat are still limited. Our study aims to provide a comprehensive investigation of the causal effects of the plasma proteome on visceral and ectopic fat using Mendelian randomization (MR) approach.
Methods
We performed two-sample MR analyses based on five large genome-wide association study (GWAS) summary statistics of 2656 plasma proteins, to screen for causal associations of these proteins with traits of visceral and ectopic fat in over 30,000 participants of European ancestry, as well as to assess mediation effects by risk factors of outcomes. The colocalization analysis was conducted to examine whether the identified proteins and outcomes shared casual variants.
Results
Genetically predicted levels of 14 circulating proteins were associated with visceral and ectopic fat (P < 4.99 × 10− 5, at a Bonferroni-corrected threshold). Colocalization analysis prioritized ten protein targets that showed effect on outcomes, including FST, SIRT2, DNAJB9, IL6R, CTSA, RGMB, PNLIPRP1, FLT4, PPY and IL6ST. MR analyses revealed seven risk factors for visceral and ectopic fat (P < 0.0024). Furthermore, the associations of CTSA, DNAJB9 and IGFBP1 with primary outcomes were mediated by HDL-C and SHBG. Sensitivity analyses showed little evidence of pleiotropy.
Conclusions
Our study identified candidate proteins showing putative causal effects as potential therapeutic targets for visceral and ectopic fat accumulation and outlined causal pathways for further prevention of downstream cardiometabolic diseases.
Similar content being viewed by others
Introduction
Obesity is the most prevalent chronic disease worldwide. It is estimated that more than 1 billion people will be living with obesity by 2030 [1]. The fat deposited in people with obesity can be classified into subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), ectopic fat (including epicardial, liver, pancreatic and skeletal muscle fat) and others, according to their patterns of distribution [2]. The accumulation of VAT and ectopic fat is a key driver of cardiometabolic risk [3,4,5,6]. Therefore, therapeutic interventions aimed at reducing unnecessary visceral and ectopic fat may benefit cardiometabolic health.
Plasma proteins play a central role in many biological processes and represent a major source of therapeutic targets for many indications [7, 8]. Observational evidence in search of predictive circulating proteins for risk of VAT and ectopic fat has been limited due to small sample sizes [9, 10]. Moreover, the causal relevance of associations from these observational studies remains largely undetermined. Mendelian randomization (MR) is an established genetic epidemiology method that uses human genetics to ascertain whether a given biomarker is implicated in disease etiology [11]. Due to the rapidly increasing number of genome-wide association studies (GWAS) for plasma proteins [12,13,14,15,16], it is possible to implement an MR analysis to assess the causal association between plasma proteins and disease outcomes based on GWAS summary statistics. Several MR studies investigated the causal association between proteins and obesity traits. Zaghlool et al. [17] performed a bidirectional MR analysis to evaluate the causal association between over 1000 plasma proteins and body mass index (BMI) in ∼ 4600 participants, and identified a bi-directional causal relationship of BMI with leptin receptor (LEPR), insulin like growth factor binding protein 1 (IGFBP1) and netrin domain containing 2 (WFIKKN2), a protein-to-BMI relationship for advanced glycosylation end-product specific receptor (AGER), dermatopontin (DPT) and cathepsin A (CTSA), and a BMI-to-protein relationship for another 21 proteins. In addition, an MR study based on the Chinese Kadoorie Biobank (CKB) cohort explored the causal relationships between BMI and proteins, showing that the genetically predicted BMI was associated with six proteins, such as Interleukin-6 (IL-6), Interleukin-18 (IL-18) and C-C motif chemokine ligand 3 (CCL3) [18]. Goudswaard et al. [19] also performed an MR analysis between BMI and plasma proteins, and they found that BMI was causally associated with eight proteins, such as LEP, fatty acid binding protein 4 (FABP4), C5 (complement C5) and sex hormone binding globulin (SHBG). Besides BMI, the associations between proteome and other obesity-related phenotypes were also detected. A recent MR study based on GWAS summary statistics of 23 body composition traits and 2656 plasma proteins revealed 430 putatively causal effects of 96 plasma proteins on 22 body composition traits, including follistatin (FST) and IGFBP1 on trunk fat-free mass, and R-spondin 3 (RSPO3) on WHR [20]. Moreover, Zheng et al. [21] estimated the effects of 1,002 proteins on 225 phenotypes (including BMI and body fat) using MR, and identified three proteins - regulator of microtubule dynamics 1 (RMDN1), lactase (LCT) and programed cell death 1 ligand 2 (PDCD1LG2), which were causally associated with BMI. However, the MR evidence for proteome on adiposity depots is still scarce.
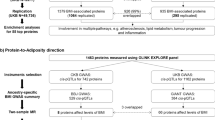
In this study, we systematically linked protein biomarkers to the outcomes of fat depots by taking a four-step approach. First, we applied a two-sample MR to estimate the causal effects of plasma proteins on visceral and ectopic fat, using genetic instruments (pQTLs) for up to 1002 proteins as exposure [12,13,14,15,16] and the summary statistics from a large European GWAS as the outcome [22]. Second, we performed colocalization analyses to verify the robustness of the instrumental variables. Third, we evaluated the causal relationship of proteins on the risk factors associated with visceral and ectopic fat and estimated the mediation effect. Last, we conducted phenotype scanning and characterized the druggability properties of these proteins as potential therapeutic targets for visceral and ectopic fat. Figure 1 summarizes the overall analysis pipeline applied in this study.
Overview of the study design. We identified circulating proteins and potential risk factors causally associated with visceral and ectopic fat using MR. We performed mediation analyses to estimate the risk factors mediating the effect of identified proteins on the outcomes using a two-step MR approach. We also screened the potentially targetable proteins through drug database searches
Methods
Proteomic data sources
MR instruments for circulating proteins were obtained from five proteomic GWAS [12,13,14,15,16]. All participants in the five studies were healthy Europeans. Circulating proteins in the GWAS by Sun et al. [12], Yao et al. [13], Suhre et al. [14] and Emilsson et al. [16] were measured using the SomaLogic platform, while in the GWAS by Folkersen et al. [15], the O-link platform was used. A total of 3606 pQTLs associated with 2656 plasma proteins have been identified.
For each plasma protein, pQTLs from its corresponding GWAS were used as genetic instruments. Consistent with Zheng et al. [21], IVs were selected using the following procedure: [1] SNPs associated with any protein (P ≤ 5 × 10− 8) in at least 1 of the 5 GWAS were included; [2] SNPs encoded by genes within the MHC region (chr6: from 26 Mb to 34 Mb) were excluded; [3] SNPs for each protein were clumped to retain independent SNPs only. The linkage disequilibrium threshold for clumping was set to r2 < 0.001; [4] SNPs associated with 5 or more proteins were excluded to avoid pleiotropy effect. Finally, a total of 1126 pQTLs were selected as potential IVs. We estimated the variance of each protein explained by its IVs by calculating the R2 [23] and the strength of each IV by the F-statistic [24]. IVs with an absolute F-statistic < 10 were considered as weak IVs and excluded from subsequent analyses. For pQTL that were not present in the outcome GWAS, we used proxy SNPs in high LD (R2 > 0.8) with the requested SNP. All palindromic SNPs as well as ambiguous SNPs were also excluded.
Outcome data sources
To assess the association of pQTL with the risk of outcomes, we retrieved the effects of these pQTLs from a large GWAS in over 30,000 participants in the UK Biobank imaging cohort [21]. The estimates for the associations of IVs with outcomes were all of European ancestry will reduce the confounding by population stratification. The primary outcomes were visceral fat (visceral adipose tissue (VAT) volume, N = 32,860) and ectopic fat including: liver fat (N = 32,858), liver volume (N = 32,860), pancreas fat (N = 25,617) and pancreas volume (N = 31,758).
The secondary outcomes were the risk factors for visceral and ectopic fat, which were selected based on literature reviews [2, 25]. We sought well-powered publicly available GWAS summary statistics for these risk factors and removed risk factors that did not have GWAS data. The remaining 18 risk factors were considered for the two-sample MR analyses, including glycemic traits (homeostasis model assessment of insulin resistance [HOMA-IR] [26] and homeostasis model assessment of beta-cell function [HOMA-B] [26]), lipids [27] (high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], total cholesterol [TC] and triglycerides [TG]), blood pressure [28](systolic blood pressure [SBP] and diastolic blood pressure [DBP]), nonalcoholic fatty liver disease [NAFLD] [29], sex hormones [30] (sex hormone binding globulin [SHBG] and total testosterone), lifestyle (moderate to vigorous physical activity [31], time spent watching television [30], coffee intake [30], smoking [32], and alcohol consumption [33]) and stress-related traits (insomnia [30] and major depression [34]). We used the same pQTLs as IVs for the secondary outcomes as for the primary outcomes.
Systematic MR screening for causal proteins of outcome and risk factors
We performed two-sample MR analyses to estimate the effect of genetically predicted protein levels and on outcomes. The MR approach was based on the following assumptions: (i) the genetic variants used as an instrumental variable (IV) are associated with target exposure; (ii) there are no unmeasured confounders of the associations between genetic variants and outcome; (iii) the genetic variants are associated with the outcome only through changes in the exposure. We used the Wald’s ratio method to estimate the causal effects when there was only one IV available for target exposure, if more than one IV was available, we applied the inverse-variance weighting (IVW) method [35]. Additionally, various pleiotropy-robust MR methods (MR-Egger, weighted-median and weighted-mode) were performed in multi-instrument MR analyses [36]. We also performed two sensitivity analyses to evaluate the robustness of the identified causal associations for MR analyses: (i) MR-Egger regression analysis, which estimates an intercept term of the inverse variance weighted test, whose deviation from zero suggests the possibility of horizontal pleiotropy [36]; (ii) Cochran’s Q test, which calculates a statistic that measures heterogeneity across IVs [35].
We employed a two-sample MR framework incorporating the sensitivity analyses for both primary MR (proteins → outcomes of visceral & ectopic fat) and two-step MR (Step-1 MR: risk factors → outcomes of visceral & ectopic fat; Step-2 MR: proteins → risk factors). The MR methods applied in each of the MR settings depend on the number of IVs for each exposure. The results were presented as odds ratio (OR) with their 95% confidence intervals (CIs) scaled to a one standard deviation (SD) higher circulating protein level for the effects on binary outcomes, and presented as the effect size (95% CI) scaled to a 1-SD higher plasma protein levels for the effects on quantitative outcomes. Bonferroni correction was used to control for the total number of distinct proteins tested in our MR experiments (P < 4.99 × 10− 5 = 0.05/1002 proteins). The MR analyses were conducted using TwoSampleMR (version: 0.5.6) [37] package in R v4.0.3.
Phenotype scanning
To further eliminate potential bias due to horizontal pleiotropy, we used PhenoScanner v2 [38] to assess any reported associations of the pQTL of the MR-prioritized proteins with potential confounders (P ≤ 1 × 10− 8), and with other circulating protein levels.
Bayesian colocalization analysis
To assess potential confounding by LD, we checked whether the loci harboring the pQTL of the MR-prioritized proteins are also associated with outcomes of visceral & ectopic fat using colocalization as implemented in the coloc R package [39]. We conducted colocalization analysis for each of the 14 proteins associated with one or more of the outcomes to investigate whether the protein level and outcome genetic associations are due to the same causal variants. To estimate the posterior probability of each genomic locus containing a single variant affecting both the protein and outcomes, we analyzed all SNPs with minor allele frequency > 0.01 within 1 Mb of the pQTLs. In this study, we tested the posterior probability of hypothesis 3 (PPH3), in which both the protein and outcomes were associated with the region by different variants, and hypothesis 4 (PPH4), in which both the protein and outcomes were associated with the region by shared variants. We defined a gene as having evidence of colocalization based on gene-based PPH4 > 80% [40].
Mediation analysis
For proteins that were causally associated with both visceral & ectopic fat outcomes and risk factors, we conducted a mediation analysis to quantify the effects of proteins on the outcomes via risk factors. Reverse MR between the mediator and identified proteins was conducted to determine whether there was bidirectionality that might affect the validity of the mediation model. The ‘total’ effect of exposure on outcome includes both ‘direct’ effect and any ‘indirect’ effect via one or more mediators. In this study, the total effect was captured by a standard univariable MR analysis - the primary MR. To decompose direct and indirect effects, we used results from two-step MR and chose the Product method to estimate the beta of indirect effect and the Delta method to estimate the standard error (SE) and confidence interval (CI) [41].
Evaluation of druggability
To assess the druggability of identified proteins, we searched identified proteins in DrugBank and in a list of druggable genes from a previous study [42]. For proteins identified in drug database, information on drug molecule types, approved indications, and target outcomes in clinical trials was documented.
Ethics approval
All the included studies had been approved by corresponding ethical review committees.
Results
Causal association between plasma proteins and outcomes of visceral and ectopic fat
We obtained 1126 pQTL from five proteomic GWAS and used them as instruments to evaluate the causal role of circulating proteins on outcomes. At the Bonferroni-corrected significance level (P < 4.99 × 10− 5), MR analyses revealed 14 putatively causal proteins with at least one outcome (Fig. 2, Supplementary Table 1), including: IL6R, GSTA1, RGMB, RSPO3, IL12RB1, CTSA, DNAJB9, FLT4, FST, IGFBP1, IL6ST, PNLIPRP1, PPY and SIRT2. Notably, IL12RB1 was associated with a lower risk of VAT volume (β[95% CI] = -0.04[-0.06, -0.02], P = 1.97 × 10− 5). Among the ectopic fat subtypes, FST, SIRT2, DNAJB9 and IGFBP1 were associated with a higher risk of liver fat; FST, IL6R and IGFBP1 were associated with a higher risk of liver volume, while CTSA and GSTA1 were associated with a lower risk of liver volume; RGMB was associated with a higher risk of pancreas fat (β[95% CI] = 0.18[0.10, 0.26], P = 4.31 × 10− 6); RSPO3, FLT4 and IL6ST were associated with a higher risk of pancreas volume, while PNLIPRP1 and PPY were associated with a lower risk of pancreas volume.
All pQTLs for the candidate proteins had an F-statistic > 10. Results of sensitivity analyses showed little evidence for heterogeneity based on Cochran Q statistics (Supplementary Table 1). The MR-Egger regression requires at least 3 IVs for analysis. Since we had at most 2 IVs for causal associations with strong MR evidence, we were not able to perform the MR-Egger analysis.
We also assessed whether the pQTL of proteins were associated with other phenotypes in PhenoScanner (Supplementary Table 2). We found that pQTL associated with GSTA1 (rs2290758) was not associated with any other traits, and was strongly associated with plasma GSTA1 levels, as well as two other proteins: GSTA2 and GSTA6P, suggesting potential pleiotropic effects. The pQTL for IL6R (rs4129267) was strongly associated with circulating IL6R, but was also associated with other proteins and traits. The pQTL for IL12RB1 (rs376008) was not associated with any other traits, but was associated with other proteins. Querying the pQTL for RGMB (rs1563317) in database, we noticed that it was strongly associated with RGMB levels and was not associated with any other traits or proteins, which reduces the probability that our MR estimate for RGMB was driven by horizontal pleiotropy. The pQTL for RSPO3 (rs2489623) was strongly associated with circulating IL6R and the other protein and trait (waist-to-hip ratio).
Colocalization analysis
We conducted colocalization analyses of 14 causal proteins with visceral and ectopic fat. The high support of colocalization evidence was observed between three proteins (FST, SIRT2 and DNAJB9) and liver fat (PPH4 > 80%) (Supplementary Table 3), suggesting the associations in these regions were likely due to the same underlying causal variants. Similarly, FST, IL6R and CTSA were colocalized with liver volume, RGMB was colocalized with pancreas fat, and four proteins including PNLIPRP1, FLT4, PPY and IL6ST were colocalized with pancreas volume. The colocalization evidence at RSPO3, was less strong than with the other proteins, with colocalization PPH4 > 60% for pancreas volume, and there was no colocalization evidence for IGFBP1, GSTA1 and IL12RB1 with outcomes, implying a possible bias due to LD.
Identification of likely causal risk factors for visceral and ectopic fat
To understand potential causal mechanisms between plasma proteins and outcomes of visceral and ectopic fat, we conducted two-step mediation MR analyses. First, we performed two-sample MR analyses to characterize the causal relationship of the potential risk factors with all the outcomes. Second, we assessed the causal effects of the proteins on the significant risk factors.
Of the 18 risk factors we considered, seven risk factors were causally associated with at least one of the outcomes (P < 0.05/18 = 0.0028, Bonferroni-adjusted for 18 risk factors; Table 1, Supplementary Table 4). The higher NAFLD risk was associated with a increased risk of liver fat (β[95% CI] = 0.40[0.33, 0.46]), while associated with a decreased risk of pancreas fat (β[95% CI] = -0.04[-0.06, -0.03]). Genetically predicted HDL-C was associated with a decreased risk of liver volume (β[95% CI] = -0.13[-0.21, -0.05]). Genetically predicted HOMA-B was associated with a decreased risk of liver fat (β[95% CI] = -0.06[-0.07, -0.04]). Genetically predicted SHBG was associated with a decreased risk of both liver fat (β[95% CI] = -0.21[-0.32, -0.09]) and liver volume (β[95% CI] = -0.14[-0.22, -0.06]). Moreover, the unhealthy lifestyle including longer time spent watching television, smoking initiation and alcohol consumption was associated with a higher risk of the outcomes. No association was observed between HOMA-IR, LDL, TC, TG, blood pressure, total testosterone, MVPA, coffee intake and stress-related traits (insomnia and major depression) with any of the outcomes.
Identification of plasma proteins associated with risk factors for outcomes
We performed MR analyses of all 1126 pQTLs with the seven likely causal risk factors for the outcomes, and found 30 proteins were associated with at least one risk factor after multiple testing correction (P < 4.99 × 10− 5), of which 18 with HDL-C, 21 with SHBG, two with alcohol consumption, three with time spent watching television and five with smoking initiation (Supplementary Table 5). The instrument validity test presented sufficient instrument strength of all the pQTLs for MR analyses (F-statistic > 10), but sensitivity analyses showed potential heterogeneity for some of the tested associations.
Among the 14 proteins causally associated with outcomes of visceral and ectopic fat based on the primary MR analyses, seven proteins were found to be associated with one or more of the potential risk factors (Table 2). Of note, we found that genetically predicted higher CTSA was associated with higher HDL-C (β[95% CI] = 0.11 [0.07, 0.15], P = 1.16 × 10− 8) and SHBG (β[95% CI] = 0.51 [0.47, 0.54], P = 6.72 × 10− 188). Genetically determined higher DNAJB9 levels were associated with lower risk of SHBG (β[95% CI] = -0.12 [-0.15, -0.1], P = 2.09 × 10− 21). Genetically higher FST and IGFBP1 levels were both associated with lower risk of alcohol consumption and SHBG. Genetically higher IL12RB1 levels were associated with higher risk of SHBG. Genetically higher IL6R levels were associated with higher risk of SHBG. Genetically higher RSPO3 levels were associated with lower risk of HDL-C. The effect direction of the association of IL6R with SHBG was consistent with its association with liver volume, indicating that SHBG might be the potential mediator of the association between IL6R and liver volume (Table 2, Supplementary Table 1). In bidirectional MR analyses, there was little evidence that mediators decreased or increased these proteins significantly, except the associations between HOMA-B and CTSA, SHBG and FST, as well as NAFLD and RSPO3 (Supplementary Table 6).
Among the 30 proteins that were associated with at least one stroke risk factor, 23 were found to be associated with the risk factors but not the primary outcomes of visceral and ectopic fat (Supplementary Table 5).
Mediation effect of plasma proteins on outcomes via risk factors
Two-step MR analyses were conducted to estimate the mediation effect of each risk factor in the association between proteins and outcomes of visceral and ectopic fat. The proportion of mediation effect of DNAJB9 on liver fat via SHBG was 14.5%. The causal mediators from CTSA to liver volume was SHBG (24.4%) and HDL-C (4.9%) respectively. The proportion of mediation effect of IGFBP1 on liver fat via SHBG is 31.5%, and its effect on liver volume via SHBG was 14.3% (Fig. 3 and Supplementary Table 7).
Mediating role of risk factors in the causal association between identified proteins and outcomes. A), Two-step MR analysis framework. β1 effect of exposure on mediator, β2 effect of mediator on outcome, β3 effect of exposure on outcome. B), MR estimates for the mediation effect of each mediator and the proportion mediated by each mediator. The squares and bars represent the mediated proportions (95% CIs)
Druggability of identified proteins
We searched 14 circulating proteins identified through MR analysis as possible drug targets in drug database, and no direct evidence showed them as the drug targets for visceral fat or ectopic fat (Supplementary Table 8). However, the drug Mecasermin targeting IGFBP1 has been approved to treat growth failure in pediatric patients with primary IGF-1 deficiency or with growth hormone gene deletion and is likely to be used for treating obesity. Moreover, one drug targeting GSTA1 named Curcumin has been approved to treat type 2 diabetes. Drugs targeting SIRT2 and FLT4 were mainly designed to treat cancers. Two drugs targeting IL6R have been approved to treat rheumatoid arthritis. Several drugs targeting CTSA have been approved for the treatment of chronic hepatitis B virus infection, hepatitis C virus infection and RNA virus infections including COVID-19. No information was available for DNAJB9, RGMB, PNLIPRP1, RSPO3, PPY, IL6ST and IL12RB1 in the drug database.
Discussion
We conducted two-sample MR and colocalization analyses to explore the causal roles of 1002 circulating proteins in visceral and ectopic fat to provide preclinical clues for the drug development. MR analysis identified 14 proteins causally associated with primary outcomes, and ten of them showed strong colocalization evidence. We found that seven risk factors includingHDL-C, HOMA-B, NAFLD, SHBG, alcohol consumption, smoking and time spent watching television were causally associated with primary outcomes, demonstrating a key role of these risk factors in the development of visceral and ectopic fat accumulation. We showed the associations of CTSA, DNAJB9 and IGFBP1 with primary outcomes were likely to be mediated by SHBG and HDL-C. Finally, we searched drug database for the selected proteins that may represent future intervention and treatment targets.
In the current study, we identified IL6R associated with increased risk of liver volume with robust evidence. Interleukin-6 (IL6), a proinflammatory cytokine, is shown to have strong associations with obesity, insulin resistance [43], type 2 diabetes [44] and liver disease [45, 46]. Several studies point to a role of IL-6 in the regulation of energy homeostasis and body weight through the classical IL6-IL6R signaling pathway [47,48,49,50,51]. Enhanced inflammation in liver and skeletal muscles and insulin resistance was observed in hepatocyte-specific IL6R deficient animals [52, 53]. Notably, a MR study reported inhibiting the IL6 signaling pathway via IL6R blockade might increase the risk of NAFLD, suggesting IL6R should play a protective role in NAFLD [54]. The above evidence supports the potential effect of IL6R on the risk of ectopic fat and is worthy of further studies.
In humans, circulating IGF1 is mostly produced by hepatocytes and over 99% bound to six different insulin growth factor-binding proteins (IGFBPs) [55]. IGFBP1 was involved in glucose metabolism [56] and molecular regulation of obesity [57]. A global IGFBP1 deletion in mice showed a significant increase in body weight and body fat mass [58]. In addition, IGFBP1 was identified as potential target in the previous MR analyses of plasma proteome in obesity [17, 20]. NAFLD patients with advanced fibrosis had higher levels of IGFBP1 [59]. In alcoholic cirrhosis, serum IGFBP1 levels was elevated when compared with controls [60, 61]. With our finding that IGFBP1 is associated with liver fat and volume, further investigation of IGFBP1 on ectopic fat is justified.
Follistatin (FST) was originally discovered as an endogenous inhibitor of follicle-stimulating hormone [62]. Liver is a key contributor to the circulating FST in humans [63]. FST can promote the differentiation and browning of adipocytes and mitigate systemic metabolic inflammation [64, 65]. Clinical studies showed that NAFLD patients had elevated circulating levels of FST [66]. The previous evidence supported that FST is associated with insulin resistance and energy metabolism [67], which is consistent with our results of the significant associations between FST and liver fat/volume, suggesting that FST might represents a potentially promising therapeutic target for ectopic fat.
Lysosomal protective protein (CTSA) is a lysosomal serine carboxypeptidase [68]. A recent MR study revealed that CTSA was potentially causal for the development of obesity [17]. Repulsive Guidance Molecule b (RGMB) is a member of the RGM family, which consists of RGMA, RGMB, and RGMC [69]. While RGMB neural expression in adult mice is limited to the basal ganglia and pituitary, the protein is expressed more broadly in the gut, bone, heart, lung, liver, kidney, testis, ovary, uterus, epididymis and pancreas of adult mice [70, 71], and humans [72, 73]. RGMB deficiency significantly altered the diversity of gut microbiota and also induced dysbiosis [74]. However, literature on the associations of both CTSA and RGMB with ectopic fat was scarce. Further cohort and functional studies are warranted.
Another noteworthy finding of this study is the identification and quantification of the mediating roles of risk factors in the association between proteome and adiposity depots. In the current study, we selected 18 candidate mediators covering lifestyle, metabolic and stress related traits, SHBG and HDL-C were finally identified as the causal mediators. Interestingly, SHBG plays a significant role in mediating the pathways between proteins and adiposity depots, with the proportion of mediation effect of IGFBP1 on liver fat 31.5%, DNAJB9 on liver fat 14.5%, IGFBP1 on liver volume 14.3%, and CTSA on liver volume 24.4%. These results are consistent with previous epidemiological and MR evidence that SHBG was associated with lower liver fat [75,76,77], implicating the protective role of SHBG against liver fat accumulation. Cai et al. found that genetically determined circulating SHBG were inversely associated with liver fat content in women but not men [76], but we could not conduct subgroup analysis by sex in the current study as the lack of sex-specific data. Although the mechanism underlying the association between SHBG and liver fat regulation remains unclear, animal experiments implied that increased SHBG level can downregulate the expression of the crucial enzymes involved in the hepatic lipogenesis, such as the adenosine triphosphate (ATP) citrate lyase (production of precursor for fatty acid), Acetyl-CoA-carboxylase and fatty acid synthase (further restriction of fatty acid synthesis) in the liver [78, 79], which could consequently reduce liver fat content. In addition, in vitro experiments showed that SHBG can repress inflammatory cytokines, including interleukin-6 and tumor necrosis factor-alpha in adipocytes and macrophages, modulating inflammatory processes [79].
The main strength of our study is that it allowed for assessment of the causal role of biomarkers using MR, an approach that is robust to unmeasured error due to confounding. Colocalization analysis has been proven a powerful tool in revealing the pleiotropic effects of certain loci on multiple traits. The large sample sizes of both the GWAS ensure adequate statistical power to show clinically relevant associations between protein levels and risk of visceral and ectopic fat. In addition, we confined our analysis to individuals of European ancestry, which minimized the population stratification bias.
Some limitations of our analysis are worth noting. Firstly, our approach tested effects of circulating protein levels and could not capture potential tissue-specific effects. Secondly, the causal associations should be interpreted with caution, as several assumptions of the methods are untestable, and potential sample overlap, heterogeneity of the IVs and residual horizontal pleiotropy might bias the results. Finally, our findings were based on GWAS of European ancestry and cannot be generalized to other ancestries. This underscores the importance of large-scale biobanks including individuals of non-European ancestry.
In conclusion, our results provided evidence that genetically altered levels of ten circulating proteins were associated with the risk of visceral and ectopic fat, which might be the potential drug targets for future therapies. Future studies are needed to validate these candidate proteins in independent longitudinal case-control cohorts and non-European ancestry populations.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- BMI:
-
Body mass index
- DBP:
-
Diastolic blood pressure
- GWAS:
-
Genome-wide association study
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-B:
-
Homeostasis model assessment of beta-cell function
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- IV:
-
Instrumental variable
- IVW:
-
Inverse-variance weighting
- LDL-C:
-
Low-density lipoprotein cholesterol
- MR:
-
Mendelian randomization
- NAFLD:
-
Nonalcoholic fatty liver disease
- SAT:
-
Subcutaneous adipose tissue
- SBP:
-
Systolic blood pressure
- SHBG:
-
Sex hormone binding globulin
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- WHR:
-
Waist-to-hip ratio
- VAT:
-
Visceral adipose tissue
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Neeland IJ, Ross R, Despres JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–25.
Hiuge-Shimizu A, Kishida K, Funahashi T, Ishizaka Y, Oka R, Okada M, et al. Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). Ann Med. 2012;44(1):82–92.
Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–13.
Martin S, Sorokin EP, Thomas EL, Sattar N, Cule M, Bell JD, et al. Estimating the effect of liver and pancreas volume and Fat Content on risk of diabetes: a mendelian randomization study. Diabetes Care. 2022;45(2):460–8.
Zhao L, Zhou X, Chen Y, Dong Q, Zheng Q, Wang Y et al. Association of visceral fat area or BMI with arterial stiffness in ideal cardiovascular health metrics among T2DM patients. J Diabetes. 2023.
Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, et al. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. 2017;16(1):19–34.
Imming P, Sinning C, Meyer A. Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006;5(10):821–34.
Hruska P, Kucera J, Pekar M, Holeczy P, Mazur M, Buzga M, et al. Proteomic Signatures of Human Visceral and subcutaneous adipocytes. J Clin Endocrinol Metab. 2022;107(3):755–75.
Alfadda AA, Masood A, Al-Naami MY, Chaurand P, Benabdelkamel H. A Proteomics Based Approach reveals Differential Regulation of visceral adipose tissue proteins between metabolically healthy and unhealthy obese patients. Mol Cells. 2017;40(9):685–95.
Smith GD, Ebrahim S. Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32(1):1–22.
Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, et al. Genomic atlas of the human plasma proteome. Nature. 2018;558(7708):73–9.
Yao C, Chen G, Song C, Keefe J, Mendelson M, Huan T, et al. Genome-wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat Commun. 2018;9(1):3268.
Suhre K, Arnold M, Bhagwat AM, Cotton RJ, Engelke R, Raffler J, et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat Commun. 2017;8:14357.
Folkersen L, Fauman E, Sabater-Lleal M, Strawbridge RJ, Franberg M, Sennblad B, et al. Mapping of 79 loci for 83 plasma protein biomarkers in cardiovascular disease. PLoS Genet. 2017;13(4):e1006706.
Emilsson V, Ilkov M, Lamb JR, Finkel N, Gudmundsson EF, Pitts R, et al. Co-regulatory networks of human serum proteins link genetics to disease. Science. 2018;361(6404):769–73.
Zaghlool SB, Sharma S, Molnar M, Matias-Garcia PR, Elhadad MA, Waldenberger M, et al. Revealing the role of the human blood plasma proteome in obesity using genetic drivers. Nat Commun. 2021;12(1):1279.
Pang Y, Kartsonaki C, Lv J, Fairhurst-Hunter Z, Millwood IY, Yu C, et al. Associations of Adiposity, circulating protein biomarkers, and risk of Major Vascular diseases. JAMA Cardiol. 2021;6(3):276–86.
Goudswaard LJ, Bell JA, Hughes DA, Corbin LJ, Walter K, Davey Smith G, et al. Effects of adiposity on the human plasma proteome: observational and mendelian randomisation estimates. Int J Obes (Lond). 2021;45(10):2221–9.
Han BX, Yan SS, Xu Q, Ni JJ, Wei XT, Feng GJ, et al. Mendelian randomization analysis reveals Causal effects of plasma proteome on body composition traits. J Clin Endocrinol Metab. 2022;107(5):e2133–40.
Zheng J, Haberland V, Baird D, Walker V, Haycock PC, Hurle MR, et al. Phenome-wide mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat Genet. 2020;52(10):1122–31.
Liu Y, Basty N, Whitcher B, Bell JD, Sorokin EP, van Bruggen N et al. Genetic architecture of 11 organ traits derived from abdominal MRI using deep learning. Elife. 2021;10.
Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740–52.
Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. 2012;21(3):223–42.
Wagner R, Eckstein SS, Yamazaki H, Gerst F, Machann J, Jaghutriz BA, et al. Metabolic implications of pancreatic fat accumulation. Nat Rev Endocrinol. 2022;18(1):43–54.
Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–16.
Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–83.
Evangelou E, Warren HR, Mosen-Ansorena D, Mifsud B, Pazoki R, Gao H, et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018;50(10):1412–25.
Kurki MI, Karjalainen J, Palta P, Sipila TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508–18.
Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–9.
Klimentidis YC, Raichlen DA, Bea J, Garcia DO, Wineinger NE, Mandarino LJ, et al. Genome-wide association study of habitual physical activity in over 377,000 UK Biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes (Lond). 2018;42(6):1161–76.
Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237–44.
Clarke TK, Adams MJ, Davies G, Howard DM, Hall LS, Padmanabhan S, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N = 112 117). Mol Psychiatry. 2017;22(10):1376–84.
Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22(3):343–52.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7.
Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–3.
Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10(5):e1004383.
Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 2019;4:186.
Carter AR, Sanderson E, Hammerton G, Richmond RC, Davey Smith G, Heron J, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36(5):465–78.
Finan C, Gaulton A, Kruger FA, Lumbers RT, Shah T, Engmann J et al. The druggable genome and support for target identification and validation in drug development. Sci Transl Med. 2017;9(383).
Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280(5):E745–51.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–34.
Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18(8):727–35.
Carbonaro M, Wang K, Huang H, Frleta D, Patel A, Pennington A, et al. IL-6-GP130 signaling protects human hepatocytes against lipid droplet accumulation in humanized liver models. Sci Adv. 2023;9(15):eadf4490.
Rothaug M, Becker-Pauly C, Rose-John S. The role of interleukin-6 signaling in nervous tissue. Biochim Biophys Acta. 2016;1863(6 Pt A):1218–27.
Michalopoulou M, Nikolaou C, Tavernarakis A, Alexandri NM, Rentzos M, Chatzipanagiotou S, et al. Soluble interleukin-6 receptor (sIL-6R) in cerebrospinal fluid of patients with inflammatory and non inflammatory neurological diseases. Immunol Lett. 2004;94(3):183–9.
Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20.
Schele E, Fekete C, Egri P, Fuzesi T, Palkovits M, Keller E, et al. Interleukin-6 receptor alpha is co-localised with melanin-concentrating hormone in human and mouse hypothalamus. J Neuroendocrinol. 2012;24(6):930–43.
Schele E, Benrick A, Grahnemo L, Egecioglu E, Anesten F, Palsdottir V, et al. Inter-relation between interleukin (IL)-1, IL-6 and body fat regulating circuits of the hypothalamic arcuate nucleus. J Neuroendocrinol. 2013;25(6):580–9.
Wunderlich FT, Strohle P, Konner AC, Gruber S, Tovar S, Bronneke HS, et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 2010;12(3):237–49.
Skuratovskaia D, Komar A, Vulf M, Quang HV, Shunkin E, Volkova L et al. IL-6 reduces mitochondrial replication, and IL-6 receptors reduce chronic inflammation in NAFLD and type 2 diabetes. Int J Mol Sci. 2021;22(4).
Li S, Chen L, Lv G. Interleukin-6 receptor blockade can increase the risk of nonalcoholic fatty liver disease: indications from mendelian randomization. Front Pharmacol. 2022;13:905936.
Livingstone C. Insulin-like growth factor-I (IGF-I) and clinical nutrition. Clin Sci (Lond). 2013;125(6):265–80.
Clemmons DR. Role of IGF binding proteins in regulating metabolism. Trends Endocrinol Metab. 2016;27(6):375–91.
Haywood NJ, Slater TA, Matthews CJ, Wheatcroft SB. The insulin like growth factor and binding protein family: novel therapeutic targets in obesity & diabetes. Mol Metab. 2019;19:86–96.
Gray A, Aronson WJ, Barnard RJ, Mehta H, Wan J, Said J, et al. Global Igfbp1 deletion does not affect prostate cancer development in a c-Myc transgenic mouse model. J Endocrinol. 2011;211(3):297–304.
Hagstrom H, Stal P, Hultcrantz R, Brismar K, Ansurudeen I. IGFBP-1 and IGF-I as markers for advanced fibrosis in NAFLD - a pilot study. Scand J Gastroenterol. 2017;52(12):1427–34.
Jeyaratnaganthan N, Gronbaek H, Holland-Fischer P, Espelund U, Chen JW, Flyvbjerg A, et al. Ascites from patients with alcoholic liver cirrhosis contains higher IGF-I bioactivity than serum. Clin Endocrinol (Oxf). 2010;72(5):625–32.
Nedic O, Malenkovic V, Dukanovic B, Baricevic I. Association of elevated IGFBP-1 with increased IGF-II concentration in patients with carcinoma of the liver. Int J Biol Markers. 2008;23(4):225–30.
Welt C, Sidis Y, Keutmann H, Schneyer A. Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med (Maywood). 2002;227(9):724–52.
Hansen JS, Rutti S, Arous C, Clemmesen JO, Secher NH, Drescher A, et al. Circulating follistatin is liver-derived and regulated by the glucagon-to-insulin ratio. J Clin Endocrinol Metab. 2016;101(2):550–60.
Braga M, Reddy ST, Vergnes L, Pervin S, Grijalva V, Stout D, et al. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J Lipid Res. 2014;55(3):375–84.
Tang R, Harasymowicz NS, Wu CL, Collins KH, Choi YR, Oswald SJ, et al. Gene therapy for follistatin mitigates systemic metabolic inflammation and post-traumatic arthritis in high-fat diet-induced obesity. Sci Adv. 2020;6(19):eaaz7492.
Polyzos SA, Kountouras J, Anastasilakis AD, Triantafyllou G, Mantzoros CS. Activin A and follistatin in patients with nonalcoholic fatty liver disease. Metabolism. 2016;65(10):1550–8.
Hansen JS, Plomgaard P. Circulating follistatin in relation to energy metabolism. Mol Cell Endocrinol. 2016;433:87–93.
Zhou XY, van der Spoel A, Rottier R, Hale G, Willemsen R, Berry GT, et al. Molecular and biochemical analysis of protective protein/cathepsin A mutations: correlation with clinical severity in galactosialidosis. Hum Mol Genet. 1996;5(12):1977–87.
Xia Y, Cortez-Retamozo V, Niederkofler V, Salie R, Chen S, Samad TA, et al. Dragon (repulsive guidance molecule b) inhibits IL-6 expression in macrophages. J Immunol. 2011;186(3):1369–76.
Corradini E, Babitt JL, Lin HY. The RGM/DRAGON family of BMP co-receptors. Cytokine Growth Factor Rev. 2009;20(5–6):389–98.
Xia Y, Sidis Y, Mukherjee A, Samad TA, Brenner G, Woolf CJ, et al. Localization and action of Dragon (repulsive guidance molecule b), a novel bone morphogenetic protein coreceptor, throughout the reproductive axis. Endocrinology. 2005;146(8):3614–21.
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419.
Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteom. 2014;13(2):397–406.
Shi Y, Zhong L, Li Y, Chen Y, Feng S, Wang M, et al. Repulsive Guidance Molecule b Deficiency induces gut microbiota dysbiosis and increases the susceptibility to intestinal inflammation in mice. Front Microbiol. 2021;12:648915.
Lazo M, Zeb I, Nasir K, Tracy RP, Budoff MJ, Ouyang P, et al. Association between Endogenous Sex Hormones and Liver Fat in a multiethnic study of atherosclerosis. Clin Gastroenterol Hepatol. 2015;9:1686–e932.
Cai X, Thorand B, Hohenester S, Prehn C, Cecil A, Adamski J, et al. Association of sex hormones and sex hormone-binding globulin with liver fat in men and women: an observational and mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1223162.
Zhang X, Mou Y, Aribas E, Amiri M, Nano J, Bramer WM, et al. Associations of sex steroids and sex hormone-binding globulin with non-alcoholic fatty liver disease: a population-based study and meta-analysis. Genes (Basel). 2022;6:966.
Saez-Lopez C, Barbosa-Desongles A, Hernandez C, Dyer RA, Innis SM, Simo R, et al. Sex hormone-binding globulin reduction in metabolic disorders may play a role in NAFLD development. Endocrinology. 2017;3:545–59.
Yamazaki H, Kushiyama A, Sakoda H, Fujishiro M, Yamamotoya T, Nakatsu Y et al. Protective effect of sex hormone-binding globulin against metabolic syndrome: in vitro evidence showing anti-inflammatory and lipolytic effects on adipocytes and macrophages. Mediators Inflammation. 2018; 2018:3062319.
Acknowledgements
The authors would like to thank the investigators and participants who contributed to and shared the data sources incorporated to our work.
Funding
This study was funded by the National Key R&D Program of China [grant number: 2018YFC1314802].
Author information
Authors and Affiliations
Contributions
M.C. and B.C. designed the study. M.C. analyzed the data and drafted the manuscript. B.C. revised the manuscript. All authors reviewed the manuscript and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cao, M., Cui, B. Clinically relevant plasma proteome for adiposity depots: evidence from systematic mendelian randomization and colocalization analyses. Cardiovasc Diabetol 23, 126 (2024). https://doi.org/10.1186/s12933-024-02222-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-024-02222-1