Abstract
Background
Cardiovascular diseases (CVDs) remain a major global health concern, necessitating advanced risk assessment beyond traditional factors. Early vascular aging (EVA), characterized by accelerated vascular changes, has gained importance in cardiovascular risk assessment.
Methods
The EVasCu study in Spain examined 390 healthy participants using noninvasive measurements. A construct of four variables (Pulse Pressure, Pulse Wave Velocity, Glycated Hemoglobin, Advanced Glycation End Products) was used for clustering. K-means clustering with principal component analysis revealed two clusters, healthy vascular aging (HVA) and early vascular aging (EVA). External validation variables included sociodemographic, adiposity, glycemic, inflammatory, lipid profile, vascular, and blood pressure factors.
Results
EVA cluster participants were older and exhibited higher adiposity, poorer glycemic control, dyslipidemia, altered vascular properties, and higher blood pressure. Significant differences were observed for age, smoking status, body mass index, waist circumference, fat percentage, glucose, insulin, C-reactive protein, diabetes prevalence, lipid profiles, arterial stiffness, and blood pressure levels. These findings demonstrate the association between traditional cardiovascular risk factors and EVA.
Conclusions
This study validates a clustering model for EVA and highlights its association with established risk factors. EVA assessment can be integrated into clinical practice, allowing early intervention and personalized cardiovascular risk management.
Similar content being viewed by others
Introduction
Currently, cardiovascular diseases (CVDs) continue to be a leading cause of morbidity and mortality on a global scale, presenting an ongoing challenge to healthcare systems and medical research communities [1]. Traditionally, CVD risk has been evaluated by using conventional methods focused on established risk factors, such as hypertension, elevated cholesterol levels, diabetes, or smoking habits [2]. While these traditional parameters have provided valuable insights, they have been shown to be insufficient in accounting for the actual incidence of CVD events [3]. Specifically, the number of individuals who ultimately experience cardiovascular events often exceeds those predicted to be at high risk, particularly among populations over the age of 40 [4]. This incongruence, commonly referred to as the “detection gap,” raises questions about the completeness of current risk assessment models and points out the need for additional predictive variables that go beyond these classical factors [5].
Beyond its role as a detection bridge in cardiovascular risk assessment, Early Vascular Aging (EVA) presents a multifaceted challenge, unraveling the intricate complexity of its underlying mechanisms [6]. The accelerated vascular changes observed in EVA, such as arterial stiffening and endothelial dysfunction, pose a complex puzzle for researchers and clinicians alike [7]. Understanding the nuanced interplay of these structural and functional alterations is crucial not only for accurate risk assessment but also for devising targeted interventions to impede or reverse the progression of vascular aging [8]. Unraveling the molecular and cellular pathways involved in EVA remains an ongoing pursuit, holding the key to unlocking novel therapeutic avenues in the battle against cardiovascular diseases [6].
EVA has increasingly been recognized as a key factor that could fill the detection gap inherent in traditional cardiovascular risk assessment models [9]. EVA manifests through a collection of both structural and functional alterations in the vascular system. Such changes include arterial stiffness, endothelial dysfunction, and variations in the intima-media thickness of the vessel wall [10]. Moreover, these vascular alterations, while associated with normal ageing, occur at an accelerated rate in EVA, culminating in decreased arterial distensibility and increased arterial stiffness. Consequently, accelerated vascular aging contributes to a number of cardiovascular complications, most notably related to coronary artery disease and stroke [11]. Therefore, the quantification of EVA risk has become a key factor in the strategic prevention and management of cardiovascular diseases [12].
As we delve into the realm of EVA assessment, a paradigm shift towards unsupervised learning techniques unfolds, which can reveal hidden patterns and relationships within vascular risk [13]. Unlike traditional classification methods, which predominantly focus on individuals with documented cardiovascular events, unsupervised techniques cover latent risks that may not manifest explicit symptoms [14]. This shift, while promising, is not without its challenges, particularly the absence of a universally accepted gold standard for EVA prediction. Consequently, the validation of unsupervised models becomes paramount, prompting innovative approaches such as comparing emergent risk patterns with external variables like age, body fat percentage, or cholesterol levels. Navigating these uncharted territories in EVA assessment holds the potential to revolutionize risk prediction models and enhance our understanding of vascular aging dynamics [15].
Traditionally, vascular risk assessment using EVA has been approached through classification techniques [16]. While these methods have provided valuable insights, their primary focus has been on patients who have either manifested a cardiovascular event or for whom such an event has been documented. However, this methodology might inadvertently omit individuals exhibiting latent risk who have yet to display explicit symptoms or have an event recorded [12]. For this reason, a shift towards unsupervised learning techniques in EVA assessment could uncover underlying risk patterns and identify novel and potentially overlooked relationships between risk factors. However, the absence of a recognized gold standard for EVA prediction presents a significant challenge, emphasizing the need for validating the risk models developed through unsupervised techniques [9]. Given this context, one promising approach for validation involves employing a set of external variables not initially included in the unsupervised model [17]. Specifically, comparing the unsupervised groups against external features, recognized as indicators of premature vascular risk, such as age, body fat percentage or cholesterol, could serve as valuable benchmarks to assess and corroborate the reliability and robustness of these emergent risk models.
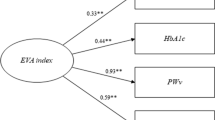
In recent research, a construct composed of four variables consisting of pulse pressure (PP), pulse wave velocity (PWv), glycated hemoglobin (HbA1c), and advanced glycation end products (AGEs) measured by skin autofluorescence (SAF) was introduced by our research group as a potential tool for assessing vascular risk [9]. Preliminary analyses of this construct indicated that optimal differentiation into two clusters provided the most coherent grouping of individuals regarding the risk of suffering from EVA. The selection of these variables is based on an understanding of the physiological changes associated with EVA that goes beyond classical risk factors. This comprehensive approach addresses both the structure and function of the vascular system, providing a more complete picture of the mechanisms underlying cardiovascular risk [18]. PP serves as an indicator of the force exerted by the blood on the arterial walls during each heartbeat [19], PWv reflects arterial stiffness [20], HbA1c serves as an indicator of long-term glycaemic control that impacts vascular health [21], and SAF indicate accumulated tissue damage [22].
While this construct offers a novel perspective, it remains essential to further validate its effectiveness. By doing so, we can not only potentially highlight the inherent relationships and patterns within the data but also confirm the robustness and validity of both the construct and the clustering model. Therefore, the objective of this work is to validate the proposed clustering model by comparing its outcomes with external vascular risk indicators, ensuring a comprehensive and practical understanding of EVA. Successful validation of this approach could facilitate its integration into clinical settings, providing healthcare professionals with a robust tool for early and accurate risk assessment.
Materials and methods
Study design
The EVasCu study was a cross-sectional investigation conducted in the province of Cuenca, Spain, to evaluate the validity of an early vascular aging model as an index of cardiovascular risk in healthy adults [9]. The study adhered to the principles outlined in the Declaration of Helsinki and received prior approval from the Clinical Research Ethics Committee of the Cuenca Health Area (REG: 2022/PI2022). The study design followed the guidelines provided by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [23].
Study sample
A total of 390 participants from Cuenca (Spain) were recruited for the EVasCu study between June 2022 and December 2022. Individuals with preexisting conditions that could significantly influence arterial stiffness, including diabetes mellitus, dyslipidemia, and arterial hypertension, were excluded from the study. Participants were provided with detailed information about the purpose and procedures of the study, and written informed consent was obtained. After the data collection, participants received validated reports from a physician, along with appropriate recommendations if needed.
EVA construct variables
PWv was measured using oscillometric techniques with Mobil-O-Graph® (IEM GmbH). Mobil-O-Graph® measures aortic pulse wave velocity (PWv) calculated as the mean of two repeated measurements, separated by 5 min each. These parameters were measured in a quiet place and after a 5-min rest period using a cuff size according to the participant’s arm/s and/or lower limb circumference.
Pulse pressure (PP) was obtained from the difference between mean systolic blood pressure (SBP) and diastolic blood pressure (DBP). Blood pressure was measured in a quiet place and after a 5-min rest period using the Omron® M5-I monitor (Omron Healthcare UK Ltd.) with a cuff size according to the participant’s arm circumference.
Glycated hemoglobin A1c (HbA1c) was determined by high-performance liquid chromatography using the ADAMS A1c HA-8180 V analyser from A. Menarini Diagnostics®. Samples were collected between 8 a.m. and 9 a.m. and after 12 h of fasting.
AGEs were measured by SAF with the AGE Reader® device. SAF were calculated as the mean of the measurements from both arms. The mean for each arm was calculated as the mean of three repeated measurements.
External validation variables
Sociodemographic and lifestyle factors
Age, gender, and smoking status were collected through direct questioning. Smoking status was classified into five groups: smoker, ex-smoker < 1 year, ex-smoker 1–5 years, ex-smoker > 5 years, and nonsmoker.
Adiposity factors
Weight and height were measured twice using appropriate equipment and averaged for analysis. Body mass index (BMI) was calculated as weight in kilograms divided by the squared height in meters (kg/m2), and participants were classified as underweight, normal weight, overweight, or obese using cut-off points of 18.5, 25.0, and 30.0, respectively [24]. Fat percentage was measured by calculating the average of two measurements using Tanita® BC-418 MA 8-electrode electrical bioimpedance.
Glycemic and inflammatory factors
Glucose and ultrasensitive C-reactive protein (CRP) determinations were measured on a Roche Diagnostics® Cobas 8000 system, and insulin determinations were measured on the Abbott ® Architect platform. Samples were collected between 8 a.m. and 9 a.m. and after 12 h of fasting. Diabetic status was established according to the HbA1c criteria for the diagnosis of diabetes of the American Diabetes Association (ADA) [25]: nondiabetic (HbA1c < 5.7%), prediabetic (HbA1c 5.7–6.4%) and diabetic (HbA1c ≥6.5%).
Lipid profile factors
Total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL) and triglyceride determinations were measured on a Roche Diagnostics® Cobas 8000 system, and insulin determinations were obtained on the Abbott ® Architect platform. Samples were collected between 8 a.m. and 9 a.m. and after 12 h of fasting. Hypercholesterolemia and hypertriglyceridaemia status were established according to the total cholesterol and triglyceride criteria of the American Heart Association (AHA) and the American College of Cardiology (ACC) [26], considering total cholesterol > 200 mg/dL for hypercholesterolemia and triglycerides > 150 mg/dL for hypertriglyceridaemia.
Vascular factors
The augmentation index (AIx75) was measured using Mobil-O-Graph®, which was calculated as the mean of two repeated measurements, separated by 5 min each. Cardio-ankle vascular index (CAVI) and ankle-brachial index (ABI) were measured with the VaSera system VS-1500 N (Fukuda Denshi UK Ltd.). Finally, intima-media thickness (IMT) was measured by ultrasound with the Sonosite SII device (Sonosite Inc., Bothell, Washington, USA). IMT was calculated as the mean measurement of the right and left carotid arteries.
Blood pressure factors
Blood pressure was measured in a quiet place and after a 5-min rest period using the Omron® M5-I monitor (Omron Healthcare UK Ltd. with a cuff size according to the participant’s arm circumference. SBP and DBP were calculated as the mean of two repeated measurements, separated by 5 min each. Hypertension status was established according to the SBP and DBP criteria of the AHA and ACC [26]: Optima blood pressure (SBP < 120 mmHg and/or DBP < 80 mmHg), normal blood pressure (SBP 120–129 mmHg and/or DBP 80–84 mmHg), normal-high blood pressure (SBP 130–139 mmHg and/or DBP 85–89 mmHg), hypertension grade I (SBP 140–159 mmHg and/or DBP 90–99 mmHg), hypertension grade II (SBP ≥160 mmHg and/or DBP ≥100 mmHg) and hypertension grade III (SBP ≥180 mmHg and/or DBP ≥110 mmHg).
It should be clarified that all subjects included in the EVasCu study were healthy subjects, so diabetic, hypercholesterolemia, hypertriglyceridaemia and hypertension status were established on the basis of the guidelines mentioned above and would not be subjects diagnosed with these diseases at the time of data collection, so they were not treated for these diseases and were informed with a report to be diagnosed by a physician.
Statistical analysis
Data description and preprocessing
Before any analyses, a preprocessing stage was conducted on the dataset. First, when features presented missing values, they were imputed using their respective medians for each feature. Second, to ensure a consistent scale across variables, all features were standardized using z score normalization.
Cluster formation and data representation
Initially, the optimal number of risk groups, denoted as K, was determined based on the inherent characteristics of the four-variable construct. Different values of K (ranging from 2 to 5) were tested using the Calinski‒Harabasz, Davies‒Bouldin, and Silhouette indices to evaluate the quality and coherence of the formed clusters [27, 28]. These indices were also crucial to determine the cohesion and separation within and between the clusters once they were computed, thus measuring the quality of the grouping [29].
Later, patients were assigned to these groups using the K-means algorithm [30]. Mathematically, this algorithm minimizes the sum of squared Euclidean distances between each point and its assigned centroid. In this process, each patient was allocated to the nearest group or centroid based on the shortest Euclidean distance. This assignment process was rigorously iterated until the centroid positions experienced negligible changes or until a maximum of 100 iterations was reached. To further elucidate the structure of the data and to visualize the delineation between clusters, principal component analysis (PCA) was utilized, especially emphasizing the distinction between clusters derived from the original four-variable construct.
External validation analysis
To assess the distribution of external variables within each cluster, Shapiro-Wilks and Levene’s tests were employed, which determine the normality and homoscedasticity of the data, respectively. Based on the outcomes of these preliminary tests, the appropriate statistical analysis to discern differences between clusters was chosen. Thus, when a quantitative variable exhibited a normal distribution and homogenous variances across both clusters, Student’s t test was applied. Conversely, when the assumptions of normality or homoscedasticity were not met, the nonparametric Mann‒Whitney U test was chosen. For categorical variables, the chi-square test was used. These tests ensure robust identification of significant differences between clusters based on the data characteristics.
Clustering modelling was conducted using Python version 3.10 and the scikit-learn package [31], whereas statistical analyses were performed with SPSS version 28.
Results
Characteristics of study participants
The EVasCu study sample included a total of 390 participants, of whom 246 (63.1%) were women. The mean age of the participants was 42.0 ± 13.1 years. Table 1 shows the baseline characteristics of the enrolled population.
Cluster analysis
This study is constructed upon a clustering model previously established in preceding research [6]. In that study, the robustness and coherence of the clustering groups were rigorously demonstrated, showcasing the model’s reliability [6]. Building on this solid foundation, the following analyses seek to further validate the clustering, with the objective of affirming its applicability and consistency across practical applications. Figure 1 shows a 2-D visual representation of clusters using the first two principal components with normalized data, which explains more than 75% of the total data variability of the construct variables. Notably, the most robust model configuration was identified when utilizing two distinct groups.
Identifying health and risk groups in the cluster model
Table 2 presents the definitions for the two clusters identified within the EVasCu study, referred to as Cluster 1 and Cluster 2 (see Fig. 1), based on the selected construct variables. The median values and interquartile ranges (IQRs) for each variable are presented, and p values are provided to highlight any statistically significant differences between the two clusters. The data in Table 2, complemented by the visual representation in Fig. 2, reveal marked disparities between the two clusters across all the construct variables. Indeed, Cluster 2 exhibited higher values of pulse pressure, HbA1c, PWv, and SAF than Cluster 1, with these differences achieving statistical significance (p < 0.001). Consequently, Cluster 1 can be confidently associated with the HVA, while Cluster 2 can be associated with the EVA. In subsequent analyses, this grouping aims to be further validated, employing external variables not used in the initial construct of the model, thus ensuring the robustness and applicability of this assignment.
Quantitative and categorical risk indicators
Tables 3 and 4 provide a comprehensive analysis of quantitative and categorical risk indicators, categorized into distinct factors, enabling an in-depth exploration of the multifaceted risk factors present within the EVasCu study.
Differences between the cluster categories (HVA and EVA) for sociodemographic and lifestyle factors
For A, B, C and D, p values were estimated using the Mann‒Whitney U test. HVA, healthy vascular aging; EVA, early vascular aging; HbA1c: glycated hemoglobin A1c; PWv, pulse wave velocity; SAF: advanced glycation products measured by skin autofluorescence
In terms of sociodemographic and lifestyle factors, age emerged as a substantial differentiator, with individuals in the EVA cluster showing a marked increase in age compared to their HVA counterparts (p < 0.001). In contrast, the gender distribution remained statistically homogeneous between the two clusters. However, a notable trend emerged in smoking status, where although similar prevalences were observed in current and ex-smoker (0–1 year) categories, there were more nonsmokers in the HVA cluster than in the EVA cluster, resulting in a significant distinction (p < 0.001) (Fig. 3).
Within the adiposity factors, our analysis revealed that individuals in the EVA cluster had significantly higher BMI values than those in the HVA cluster (p < 0.001). Similarly, waist circumference showed a substantial increase in the EVA cluster, emphasizing the role of adiposity in vascular aging (p < 0.001). Furthermore, a discernible elevation in fat percentage was observed in the EVA cluster, suggesting the importance of adipose tissue in the early vascular aging process (p < 0.001). In terms of weight status, the EVA cluster exhibited a higher prevalence of individuals classified as overweight and obese in comparison to the HVA cluster (p < 0.001) (Fig. 4).
Regarding glycemic and inflammatory factors, our examination showed a significant increase in glucose levels in the EVA cluster compared to the HVA cluster (p < 0.001). While insulin levels and CRP showed slight increases in the EVA cluster, these differences were statistically significant (p = 0.034 and p = 0.031, respectively). Notably, although the majority of individuals in both clusters were nondiabetic, the prevalence of prediabetes and diabetes was slightly higher in the EVA cluster (p = 0.012) (Fig. 5).
Differences between the cluster categories (HVA and EVA) for glycemic and inflammatory factors
For A, C and D, p values were estimated using the Mann‒Whitney U test, and for B, p values were estimated using the chi-squared test. HVA, healthy vascular aging; EVA, early vascular aging; CRP, c-reactive protein
In terms of lipid profile factors, our findings highlighted substantial elevations in the levels of total cholesterol, LDL, and triglycerides in the EVA cluster compared to the HVA cluster (p < 0.001). In particular, no statistically significant variations in HDL levels were observed between the two clusters. The EVA cluster also had a higher proportion of individuals with hypercholesterolemia than the HVA cluster (p < 0.001). Additionally, a modest inclination in hypertriglyceridaemia was observed in the EVA cluster, although it did not reach statistical significance (p = 0.051) (Fig. 6).
Differences between the cluster categories (HVA and EVA) for lipid profile factors
For A, B, C and E, p values were estimated using the Mann‒Whitney U test, and for D and F, p values were estimated using the chi-squared test. HVA, healthy vascular aging; EVA, early vascular aging; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol
Among the vascular factors, AIx-75 showed a significant increase in the EVA cluster compared to the HVA cluster, suggesting altered arterial stiffness and wave reflection properties in early vascular aging (p < 0.001). However, no statistically significant differences in ABI were observed between the two clusters. CAVI showed a significant elevation in the EVA cluster, signifying greater vascular stiffness (p < 0.001). Additionally, our analysis revealed that IMT was slightly greater in the EVA cluster, with statistically significant differences (p = 0.015) (Fig. 7).
Differences between the cluster categories (HVA and EVA) for vascular factors
For A, B, C and D, p values were estimated using the Mann‒Whitney U test. HVA, healthy vascular aging; EVA, early vascular aging; AIx@75, augmentation index; ABI, ankle-brachial index; CAVI, cardio-ankle vascular index; IMT: intima media thickness
Finally, in terms of blood pressure factors, both SBP and DBP were substantially higher in the EVA cluster than in the HVA cluster (p < 0.001). Importantly, our analysis revealed a higher proportion of hypertension, especially in the categories of hypertension grade I and grade II, in the EVA cluster (p < 0.001) (Fig. 8).
Differences between the cluster categories (HVA and EVA) for blood pressure factors
For A and B, p values were estimated using the Mann‒Whitney U test, and for C, p values were estimated using the chi-squared test. HVA, healthy vascular aging; EVA, early vascular aging; SBP, systolic blood pressure; DBP, diastolic blood pressure
Discussion
In summary, our findings provide valuable insights into the potential utility of the clustering model for the assessment of EVA and its associated cardiovascular risk. The results indicate that the clustering model effectively stratifies individuals into two distinct groups, HVA and EVA, based on a set of construct variables. These findings align with the growing recognition of EVA as a key factor in cardiovascular risk assessment. EVA manifests through structural and functional alterations in the vascular system, including arterial stiffness, endothelial dysfunction, and variations in intima-media thickness. Our results confirm that individuals with EVA exhibit significant differences in various risk factors, including age, adiposity, glycemic control, lipid profiles, vascular health, and blood pressure, compared to those in the HVA group.
Age is a well-established risk factor for cardiovascular disease, but its specific role in EVA has been a subject of interest in recent research [4]. In this study, individuals in the EVA cluster were significantly older than those in the HVA group. This finding aligns with the concept that EVA is a process characterized by accelerated vascular aging [32]. As individuals age, a number of structural and functional changes occur in the vascular system, including increased arterial stiffness and endothelial dysfunction [7]. EVA may represent an extreme manifestation of these age-related changes. Early detection and intervention in older populations is crucial because these individuals may already be in an advanced stage of vascular aging, which may result in an increased risk of cardiovascular events [33]. This finding underscores the need for age-specific interventions and risk assessments in clinical practice [34].
The significantly higher levels of adiposity factors, including BMI, waist circumference, and fat percentage, in the EVA group suggest a strong association between adipose tissue and early vascular aging. Adipose tissue is known to produce various bioactive substances, such as cytokines and adipokines, which can contribute to inflammation and endothelial dysfunction [35]. Moreover, adiposity is often associated with other risk factors, such as insulin resistance, dyslipidemia, and hypertension, all of which are known to accelerate vascular aging [36]. Therefore, addressing weight management and reducing adiposity may be crucial in EVA prevention. Lifestyle modifications, including dietary changes and regular physical activity, may play a significant role in mitigating the adverse effects of adiposity on vascular health [37].
The elevated glucose levels and the observed higher prevalence of prediabetes and diabetes in the EVA group suggest a strong link between glycemic control and EVA. Chronic hyperglycemia is known to induce oxidative stress and inflammation, which can contribute to endothelial dysfunction and vascular damage [38]. Moreover, the inclusion of HbA1c in the construct is primarily based on the analysis of the EVA construct previously analysed [9]. HbA1c in apparently healthy individuals is crucial because of its ability to provide an integrative measure of long-term glycaemic control [39]. Since chronic hyperglycaemia is a known risk factor for the development and progression of cardiovascular disease [40], the inclusion of HbA1c in the EVA construct may more accurately identify those individuals who, despite having apparently normal fasting glucose values, may experience detrimental fluctuations in their glucose levels over time. Consequently, integrating HbA1c in apparently healthy subjects may improve the predictive ability of cardiovascular risk by more effectively capturing chronic glycaemic load, thus contributing to more personalised and effective preventive strategies [41].
Similarly, the elevated lipid profiles (total cholesterol, LDL, and triglycerides) in the EVA group highlight the significance of lipid management in EVA prevention. Dyslipidemia can lead to atherosclerosis and increased vascular stiffness, contributing to early vascular aging [42]. The findings related to vascular factors (arterial stiffness and IMT) reinforce the importance of assessing vascular health for the early detection and management of EVA. The higher prevalence of hypertension in the EVA cluster underlines the role of blood pressure management in EVA risk reduction. Elevated blood pressure can lead to structural changes in blood vessels and increased vascular resistance, further accelerating vascular aging [43].
The external validation of the clustering model is crucial for several reasons. First, it confirms that the model’s clusters align with established risk factors. This strengthens the model’s clinical utility and suggests that it can identify individuals at risk of cardiovascular events based on readily available parameters. Second, the external validation underscores the importance of these traditional risk factors in EVA. This suggests that EVA is not an isolated phenomenon but rather a manifestation of cardiovascular risk factors that can be identified and managed using established guidelines. This, in turn, highlights the potential for early intervention in individuals showing signs of EVA.
However, important limitations should be acknowledged for this study. First, the size of the study population, deemed too small, may impact the statistical power and generalizability of the findings. Additionally, the age composition, primarily comprising young to middle-age subjects with a low rate of cardiovascular events in the general population, introduces a potential limitation in extrapolating the results to broader age groups with different cardiovascular risk profiles. The cross-sectional design of the research restricts our ability to test the proposed hypothesis thoroughly. Longitudinal studies are imperative to elucidate causal relationships and comprehensively assess the impact of identified risk factors on the development of early vascular aging (EVA) over time. Additionally, the study’s findings are based on a specific population, and their generalizability to other demographic groups should be investigated. Furthermore, the clustering model’s performance may vary in different clinical and research settings, and its robustness needs further validation in diverse populations.
In conclusion, the results of this study support the validity and utility of the clustering model for assessing EVA and its association with cardiovascular risk. The differentiation between the HVA and EVA groups based on various risk factors provides a comprehensive understanding of EVA’s distinct characteristics. These findings have implications for clinical practice, as they suggest that interventions targeting age, adiposity, glycemic control, lipid profile, vascular health, and blood pressure may be particularly relevant for individuals at risk of EVA. Further research and longitudinal studies are needed to confirm these associations and to assess the long-term impact of EVA on cardiovascular outcomes. Successful validation of this clustering model could confirm its utility for model integration into clinical settings, offering healthcare professionals a robust tool for early and accurate cardiovascular risk assessment and management.
Data Availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- ABI:
-
Ankle-brachial index
- ACC:
-
American College of Cardiology
- ADA:
-
American Diabetes Association
- AGEs:
-
Advanced glycation end products
- AHA:
-
American Heart Association
- Aix-75:
-
Augmentation index
- BMI:
-
Body mass index
- CAVI:
-
Cardio ankle vascular index
- CRP:
-
Ultrasensitive C-reactive protein
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- EVA:
-
Early vascular aging
- HDL:
-
High-density lipoprotein cholesterol
- HbA1c:
-
Glycated hemoglobin A1c
- HVA:
-
Healthy vascular aging
- IMT:
-
Intima media thickness
- IQR:
-
Interquartile range
- LDL:
-
Low-density lipoprotein cholesterol
- PCA:
-
Principal component analysis
- PCR:
-
Protein c-reactive
- PP:
-
Pulse pressure
- PWv:
-
Pulse wave velocity
- SAF:
-
Skin autofluorescence
- SBP:
-
Systolic blood pressure
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021.
deGoma EM, Knowles JW, Angeli F, Budoff MJ, Rader DJ. The evolution and refinement of traditional risk factors for Cardiovascular Disease. Cardiol Rev. 2012 May-Jun;20(3):118–29.
Javaid A, Zghyer F, Kim C, Spaulding EM, Isakadze N, Ding J, et al. Medicine 2032: the future of Cardiovascular Disease prevention with machine learning and digital health technology. Am J Prev Cardiol. 2022;12:100379.
Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, et al. Cardiovascular risks Associated with gender and aging. J Cardiovasc Dev Dis. 2019;6(2):19.
Knoflach M, Kiechl S, Penz D, Zangerle A, Schmidauer C, Rossmann A, et al. Cardiovascular risk factors and Atherosclerosis in young women: Atherosclerosis risk factors in female youngsters (ARFY study). Stroke. 2009;40(4):1063–9.
Papakatsika S, Stabouli S, Antza C, Kotsis V. Early vascular aging: a New Target for Hypertension Treatment. Curr Pharm Des. 2016;22(1):122–6.
Laurent S. Defining vascular aging and cardiovascular risk. J Hypertens. 2012;30 Suppl:S3-8.
Matz RL, Andriantsitohaina R. Age-related endothelial dysfunction: potential implications for pharmacotherapy. Drugs Aging. 2003;20(7):527–50.
Saz-Lara A, Cavero-Redondo I, Pascual-Morena C, Martínez-García I, Rodríguez-Gutiérrez E, Lucerón-Lucas-Torres M, et al. Early vascular aging as an index of cardiovascular risk in healthy adults: confirmatory factor analysis from the EVasCu study. Cardiovasc Diabetol. 2023;22(1):209.
Cunha PG, Boutouyrie P, Nilsson PM, Laurent S. Early vascular ageing (EVA): definitions and clinical applicability. Curr Hypertens Rev. 2017;13(1):8–15.
Nilsson PM. Hemodynamic aging as the Consequence of Structural Changes Associated with early vascular aging (EVA). Aging Dis. 2014;5(2):109–13.
Climie RE, Alastruey J, Mayer CC, Schwarz A, Laucyte-Cibulskiene A, Voicehovska J, et al. Vascular ageing: moving from bench towards bedside. Eur J Prev Cardiol. 2023;30(11):1101–17.
Suri JS, Bhagawati M, Paul S, Protogerou AD, Sfikakis PP, Kitas GD, et al. A powerful paradigm for Cardiovascular Risk Stratification using Multiclass, Multi-label, and ensemble-based machine learning paradigms: a narrative review. Diagnostics (Basel). 2022;12(3):722.
Šmuc T, Gamberger D, Krstačić G. Combining unsupervised and supervised machine learning in analysis of the CHD patient database. Lect Notes Comput Sci. 2001; 109–12.
Bednarski B, Williams M, Pieszko K, Miller R, Huang C, Kwiecinski J et al. Unsupervised machine learning improves risk stratification of patients with visual normal SPECT myocardial perfusion imaging assessments. Eur Heart J. 2022.
Bikia V, Fong T, Climie RE, Bruno RM, Hametner B, Mayer C, et al. Leveraging the potential of machine learning for assessing vascular ageing: state-of-the-art and future research. Eur Heart J Digit Health. 2021;2(4):676–90.
Deo RC. Machine learning in Medicine. Circulation. 2015;132(20):1920–30.
El Assar M, Angulo J, Vallejo S, Peiró C, Sánchez-Ferrer CF. Rodríguez-Mañas L. mechanisms involved in the aging-induced vascular dysfunction. Front Physiol. 2012;3:132.
Mitchell GF, Lacourcière Y, Ouellet JP, Izzo JL Jr, Neutel J, Kerwin LJ, et al. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic Hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation. 2003;108(13):1592–8.
Milan A, Tosello F, Fabbri A, Vairo A, Leone D, Chiarlo M, et al. Arterial stiffness: from physiology to clinical implications. High Blood Press Cardiovasc Prev. 2011;18(1):1–12.
Guo K, Zhao Q, Wang M, Lu Y, Wo M, Zhou X, et al. The scope of HbA1c variability and risk of vascular Complications among patients with type 2 Diabetes: a systematic review and Meta-analysis of prospective studies. Horm Metab Res. 2022;54(2):94–103.
Smit AJ, Gerrits EG. Skin autofluorescence as a measure of advanced glycation endproduct deposition: a novel risk marker in chronic Kidney Disease. Curr Opin Nephrol Hypertens. 2010;19(6):527–33.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet. 2007;370(9596):1453–7.
NHLBI Obesity Education Initiative Expert Panel on the Identification., Evaluation, and Treatment of Obesity in Adults (US). Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda (MD): National Heart, Lung, and Blood Institute; 1998 Sep. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2003/.
American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of Diabetes: standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):17–S38.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, Michos ED, Miedema MD, et al. 2019 ACC/AHA Guideline on the primary Prevention of Cardiovascular Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation. 2019;140(11):e563–95.
Calinski T, Harabasz J. A dendrite method for cluster analysis. Commun Stat Theory Methods. 1974;3(1):1–27.
Davies DL, Bouldin DW. A cluster separation measure. TPAMI. 1979;1(2):224–7.
Vendramin L, Campello RJ, Hruschka ER. Relative clustering validity criteria: a comparative overview. Statistical analysis and data mining: the ASA data science journal. 2010;3(4):209–35.
Arthur D, Vassilvitskii S. K-means++: the advantages of careful seeding. In: ACM‐SIAM. 2007. p. 1027–35.
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–30.
Xu X, Wang B, Ren C, Hu J, Greenberg DA, Chen T, et al. Age-related impairment of vascular structure and functions. Aging Dis. 2017;8(5):590–610.
Laurent S, Boutouyrie P, Cunha PG, Lacolley P, Nilsson PM. Concept of extremes in Vascular Aging. Hypertension. 2019;74(2):218–28.
Nilsson PM. Early vascular aging (EVA): consequences and prevention. Vasc Health Risk Manag. 2008;4(3):547–52.
Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory Diseases. Mediators Inflamm. 2010;2010:802078.
Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation. 2021;143(21):e984–e1010.
Fruh SM, Obesity. Risk factors, Complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017;29(S1):3–S14.
Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in Diabetes Mellitus related Cardiovascular Diseases. Curr Pharm Des. 2013;19(32):5695–703.
Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of Diabetic patients. Biomark Insights. 2016;11:95–104.
Cavero-Redondo I, Peleteiro B, Álvarez-Bueno C, Rodriguez-Artalejo F, Martínez-Vizcaíno V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open. 2017;7(7):e015949.
Ikeda F, Doi Y, Ninomiya T, Hirakawa Y, Mukai N, Hata J, et al. Haemoglobin A1c even within non-diabetic level is a predictor of Cardiovascular Disease in a general Japanese population: the Hisayama Study. Cardiovasc Diabetol. 2013;12:164.
Kılıç A, Baydar O, Elçik D, Apaydın Z, Can MM. Role of dyslipidemia in early vascular aging syndrome. Turk J Med Sci. 2021;51(2):727–34.
Harvey A, Montezano AC, Touyz RM. Vascular biology of ageing-implications in Hypertension. J Mol Cell Cardiol. 2015;83:112–21.
Acknowledgements
Not applicable.
Funding
This study was funded by 42nd edition of the nursing award of the University Pontificia Comillas and Escuela de Enfermeria y Fisioterapia San Juan de Dios, and co-funded by the European Union (ERDF/ESF) and by the Carvascare Research Group from the Universidad de Castilla‐La Mancha (2023‐GRIN‐ 34459). IMG is supported by a grant from the Ministry of Science, Innovation and Universities. (FPU19/06866).
Author information
Authors and Affiliations
Contributions
Conceptualization, IC-R and AS-L; methodology, ICR and AM-R; software, AM‐R; validation, IM‐G and IO‐L; formal analysis, IC‐R and AM‐R; investigation, IC‐R; resources, AL‐S; data curation, IC‐R and AL‐S; writing—original draft preparation, IC‐R and AM‐R; writing—review and editing, AL‐S; visualization, IM‐G and IO‐L; supervision, AS‐L. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research protocol of this study was approved by the Clinical Research Ethics Committee of the Cuenca Health Area (REG: 2022/PI2022). Written informed consent to participate was obtained from all subjects included in the study.
Consent for publication
Written informed consent for publication was obtained from all subjects included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cavero-Redondo, I., Saz-Lara, A., Martínez-García, I. et al. Validation of an early vascular aging construct model for comprehensive cardiovascular risk assessment using external risk indicators for improved clinical utility: data from the EVasCu study. Cardiovasc Diabetol 23, 33 (2024). https://doi.org/10.1186/s12933-023-02104-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-02104-y