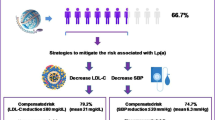
Abstract
Background
Previous studies have investigated the association between the ratio of triglycerides (TG) to high-density lipoprotein cholesterol (HDL-C) and the incidence of diabetes in adults and discovered that a high TG/HDL-C ratio was linked to an elevated risk of new-onset diabetes. However, the comparison of predicting diabetes development among lipid profiles including the TG/HDL-C ratio, and the ratio of TG/HDL-C cut-off value has received limited attention. We examined the relationship between diabetes onset and the TG/HDL-C ratio in addition to the applicable cut-off value for predicting diabetes onset.
Methods
This study included 120,613 participants from the health examination database at Panasonic Corporation from 2008 to 2017. Cox regression analysis employing multivariable models was used to investigate the association between lipid profiles, particularly the ratio of TG/HDL-C and the development of type 2 diabetes (T2D). The multivariable model was adjusted for age, sex, BMI, systolic blood pressure, plasma glucose levels after fasting, smoking status, and exercise habits. Areas under time-dependent receiver operating characteristic (ROC) curves (AUCs) were employed to assess the prediction performance and cut-off values of each indicator. A fasting plasma glucose level of 126 mg/dL, a self-reported history of diabetes, or usage of antidiabetic medicines were used to identify T2D.
Results
During the course of the study, 6,080 people developed T2D. The median follow-up duration was 6.0 (3–10) years. Multivariable analysis revealed that the ratio of TG/HDL-C (per unit, HR; 1.03 [95% CI 1.02–1.03]) was substantially linked to the risk of incident T2D. AUC and cut-off points for the ratio of TG/HDL-C for T2D development after 10 years were 0.679 and 2.1, respectively. Furthermore, the AUC of the ratio of TG/HDL-C was considerably larger compared to that of LDL-C, HDL-C, and TG alone (all P < 0.001). We discovered an interaction effect between sex, BMI, and lipid profiles in subgroup analysis. Females and participants having a BMI of < 25 kg/m2 showed a higher correlation between lipid profile levels and T2D onset.
Conclusions
The ratio of TG/HDL-C was found to be a stronger predictor of T2D development within 10 years than LDL-C, HDL-C, or TG, indicating that it may be useful in future medical treatment support.
Similar content being viewed by others
Background
Diabetes prevalence among individuals aged 20–79 was predicted to be 10.5% (536.6 million) in 2021, escalating to 12.2% (783.2 million) in 2045 worldwide [1]. Diabetes is a risk factor for atherosclerosis and has been related to an increased risk of death from several cancers, infectious diseases, and cardiovascular diseases [2]. Diabetes-related healthcare costs worldwide are expected to reach US$ 966 billion in 2021 and US$ 1,054 billion by 2045 [1]. Thus, the development of diabetes and related complications, as well as the associated increase in healthcare expenses, are worldwide issues, making it critical to manage the onset of diabetes to avoid progression of complications.
Lipid metabolic abnormalities, on the other hand, have been related to an increased likelihood of developing cardiovascular disease [3,4]. Diabetes dyslipidemia is defined by high triglyceride (TG) levels, low high-density lipoprotein cholesterol (HDL-C) levels, and high low-density lipoprotein cholesterol (LDL-C) values, especially small dense LDL-C. Moreover, although abnormalities in lipid metabolism affect diabetes development [5,6], the evidence regarding the association between dyslipidemia and type 2 diabetes (T2D), including cut-off values, is still lacking.
Previous research has revealed that the ratio of TG/HDL-C is an accurate predictor of insulin resistance [7,8,9]. The ratio of TG/HDL-C has also been researched in relation to other events; it raises the chance of developing fatty liver that is independent of other factors [10], and correlates positively with arterial stiffness [11,12]. Previous studies have investigated the association between the ratio of TG/HDL-C and the incidence of diabetes in adults, and discovered that a higher ratio of TG/HDL-C was linked to an elevated risk of new-onset diabetes [13,14,15,16,17,18]. Zhou et al. [19] reported the cut-off values of TG/HDL-C ratio for predicting the development of T2D; however, theirs was a cross-sectional study, leaving the causal relationship unclear. One study reported the cut-off values of the TG/HDL-C ratio for predicting the incidence of T2D [18], but the sample size was small, the observation period was not taken into account, and the performance among lipid profiles for predicting T2D incidence was not compared [18]. To the best of our knowledge, the comparison of predicting diabetes development among lipid profiles, including the ratio of TG/HDL-C and the ratio of TG/HDL-C cut-off value, has received little attention.
Consequently, we undertook this cohort study conducted in the past to investigate the relationship between the lipid profile, including the ratio of TG/HDL-C, alongside the development of diabetes over 10 years, and to estimate the ratio of TG/HDL-C cut-off value for predicting T2D development.
Methods
Study population and study design
This long-term retrospective cohort study used data from the Panasonic Cohort Study, which were collected between 2008 and 2018. This database contains information on annual medical health checks, medical costs, medical history, and mortality among Panasonic Corporation employees in Osaka, Japan. This examination program was designed to promote the health of employees by detecting chronic diseases early, such as metabolic abnormalities, and evaluating the underlying risk factors. All employees received yearly medical health checks.
Blood samples were taken following > 10 h of fasting. A weight and height meter that is automatic was used to record the subjects' weight and height. A previously established and verified self-administered questionnaire was employed to assess baseline features, including smoking habits, exercise habits, history of medical treatment, and history of diabetes. Three groups of participants were formed: nonsmokers, former smokers, and current smokers. Regular exercisers were those who engaged in at least 30 min of exercise at least 2 days per week for at least a year. A fasting plasma glucose level of 126 mg/dL, a self-reported history of diabetes, or usage of antidiabetic medicines were used to identify T2D.
This study was approved by the Panasonic Health Insurance Organization's local ethics committee (permission number: 2021–001) and was carried out in conformity with the principles of the Helsinki Declaration.
Inclusion and exclusion criteria
Figure 1 depicts the participant registration flow diagram for the study. Employees who had medical health checks between 2008 and 2017 were all registered. Between 2008 and 2017, a total of 236,603 employees had medical health checks. The observation period for the development of T2D was set until 2018. Participants who did not have a blood test at baseline, those with incomplete data, those taking antidyslipidemic medicines at baseline, those with diabetes at baseline, and those who had undergone a medical health checkup at baseline only because they were retiring were excluded from the study.
Statistical analyses
The potential confounding variables' means and frequencies were computed. All potential confounding variables were baseline values. The primary outcome measure was the relationship between lipid profiles, especially the ratio of TG/HDL-C, and T2D incidence. The T2D incidence and lipid profiles were evaluated in the annual medical health checks. Cox regression analysis with multivariable models was utilized to investigate the connection between LDL-C, HDL-C, TG, and the ratio of TG/HDL-C and T2D incidence. The multivariable model was adjusted for age, sex, BMI, systolic blood pressure, plasma glucose levels after fasting, smoking status, and exercise habits. We also conducted a likelihood ratio test to examine whether lipid profiles have a significant prognostic impact. We used time-dependent ROC curves for censored survival data and AUCs as criteria to assess the predictive performance of LDL-C, HDL-C, TG, and the ratio of TG/HDL-C. The best cut-off values for LDL-C, HDL-C, TG, and the ratio of TG/HDL-C for incident T2D were investigated. Moreover, we compared the AUCs of LDL-C, HDL-C, TG, and ratios of TG/HDL-C using 1,000 bootstrap samples with the Bonferroni method.
The impact of sex and BMI was evaluated using a subgroup analysis. The subgroups were categorized as male and female, and individuals with BMI < 25 kg/m2 and ≥ 25 kg/m2, according to the Japan Society for the Study of Obesity's definition. Except for sex or BMI, the multivariable model was adjusted for variables in subgroup analyses as appropriate. Hazard ratios (HRs), AUCs, and cut-off values were evaluated according to sex and BMI category. We also tested the potential interaction effects of sex, BMI category, and lipid profile.
The mean, standard deviation, or absolute numbers are used to represent all continuous variables. At P < 0.05, differences were judged to be statistically significant. When the Bonferroni method was applied, differences were considered statistically significant at P < 0.008. HRs with 95% confidence intervals (CIs) were used to represent associations. JMP software version 17 (SAS Institute, Cary, NC, USA) was used for statistical analyses.
Results
Main analyses
The analysis included 120,613 participants in total (Fig. 1). The baseline characteristics of the individuals are depicted in Table 1. During the study period, 6,080 participants developed T2D. Additional file 1: Table S1 depicts the unadjusted HRs for the incidence of T2D. Figure 2 presents the adjusted HRs of lipid profiles for the incidence of T2D. Multivariable analysis demonstrated that LDL-C (per 10 mg/dl, HR: 1.02 [95% CI 1.02–1.03]), HDL-C (per 10 mg/dl, HR: 0.88 [95% CI 0.86–0.90]), TG (per 10 mg/dl, HR: 1.008 [95% CI 1.006–1.010]), and the ratio of TG/HDL-C (HR: 1.03 [95% CI 1.02–1.03]) were linked to an increased risk of developing T2D. The multivariable analysis showed the c-index of 0.88.
The likelihood ratio test showed that lipid profiles, including TG/HDL cholesterol ratio, had a significant prognostic impact (p < 0.001) (Table 2).
Table 3 displays the AUC and optimal cut-off values after ten years of time-dependent ROC curve analysis. The AUC and optimal cut-off values for LDL-C, HDL-C, TG, and the ratio of TG/HDL-C for incident T2D were 0.609 and 124 mg/dL, 0.638 and 54 mg/dL, 0.672 and 106 mg/dl, and 0.679 and 2.1, respectively, at 10 years. Table 3 also compares the AUCs of LDL-C, HDL-C, TG, and the ratio of TG/HDL-C. The AUC of the ratio of TG/HDL-C was greater than that of LDL-C (difference value, 0.069; 95% CI 0.061–0.078; P < 0.001), HDL-C (difference value, 0.041, 95% CI 0.035–0.046, P < 0.001), or TG (difference value, 0.007; 95% CI 0.004–0.009; P < 0.001).
Subgroup analyses
The adjusted HRs of lipid profiles for the occurrence of T2D in subgroup analysis according to sex and BMI category are shown in Fig. 2. Additional file 2: Table S2 shows the AUC and cut-off values according to sex and BMI category. We discovered an interaction effect between sex and LDL-C and TG. The HR of LDL-C (p for interaction < 0.001) and TG (p for interaction = 0.002) was higher in females than in males. In males, LDL-C (HR: 1.02 [95% CI 1.01–1.03]) and TG (HR: 1.008 [95% CI 1.006–1.009]) were substantially related to the probability of developing T2D. In females, LDL-C (HR: 1.09 [95% CI 1.06–1.13]) and TG (HR: 1.02 [95% CI 1.01–1.03]) were substantially linked with the probability of developing T2D. The optimal ratio of TG/HDL-C cut-off values for T2D development at 10 years were 2.1 and 1.2 in males and females, respectively.
Furthermore, we discovered an interaction impact between the BMI category and TG, as well as the ratio of TG/HDL-C. The HR of TG (p for interaction = 0.0001) and TG/HDL-C (p for interaction = 0.0001) was higher in participants with a BMI of < 25 kg/m2 than in those with a BMI of ≥ 25 kg/m2. In participants with a BMI < 25 kg/m2, TG (HR: 1.01 [95% CI 1.010–1.014]) and the ratio of TG/HDL-C (HR: 1.04 [95% CI 1.03–1.05]) were substantially linked with the development of T2D. In participants with a BMI ≥ 25 kg/m2, TG (HR: 1.006 [95% CI 1.003–1.008]) and the ratio of TG/HDL-C (HR: 1.02 [95% CI 1.02–1.03]) were substantially linked with the probability of developing T2D. The optimized cut-off values for the ratio of TG/HDL-C for T2D development at 10 years were 1.7 and 2.5 in participants with BMI < 25 kg/m2 and BMI ≥ 25 kg/m2, respectively.
Discussion
The following are the study's three key findings. First, the ratio of TG/HDL-C cut-off value for T2D development within 10 years was 2.1. Second, the ratio of TG/HDL-C outperformed LDL-C, HDL-C, and TG levels in predicting the development of diabetes within 10 years. Third, females and those with a BMI of < 25 kg/m2 may be more sensitive to lipid profile levels in terms of the risk of developing T2D.
Though the precise mechanism through which a high ratio of TG/HDL-C causes insulin resistance is unknown, several reports suggest a theory. In cellular experiments, LDL-C was shown to decrease the expression of cyclin B1 in pancreatic β-cells, resulting in increased insulin resistance, and HDL-C is thought to improve insulin resistance by suppressing the effects of LDL-C [20,21]. HDL has also been shown to possibly regulate glucose homeostasis through mechanisms such as insulin secretion, direct glucose uptake by muscle, and increased insulin sensitivity [22]. In contrast, hypertriglyceridemia increases free fatty acids, which accumulate in skeletal muscle and cause insulin resistance, while accumulation in pancreatic islets has been reported to cause β-cell dysfunction and apoptosis [23]. Moreover, hypertriglyceridemia increases the activity of cholesteryl ester transfer protein (CETP) [24,25]. While CETP enhances TG enrichment of HDL and LDL by causing cholesteryl ester to be converted to TG, it leads to lower HDL-C concentrations [24,25]. These findings may be related to the fact that the ratio of TG/HDL-C was more beneficial than LDL-C, HDL-C, and TG levels in predicting diabetes development. In addition, insulin resistance increases TG levels and reduces HDL-C through compensatory hyperinsulinemia and activation of fatty acid degradation [26], and in this regard, the ratio of TG/HDL-C is an important finding in conducting diabetes care.
Subgroup analysis revealed that an elevated lipid profile is more likely to affect diabetes development in females than in males and in those with a BMI of < 25 kg/m2 than in those with a BMI of ≥ 25 kg/m2. Generally, females have lower LDL-C and higher HDL-C levels than males. Due to the effect of female hormones, females have lower LDL-C levels and higher HDL-C levels than males of the same age [27]. Even though estrogen is known to increase TG levels [28], females still have lower TG levels than males. Females have been reported to have greater TG uptake by muscle cells and higher TG clearance than males [29]. Given that estrogen deficiency causes dysregulation of lipid metabolism and accumulation of visceral adipose tissue [30], it is possible that estrogen suppresses TG elevation indirectly by inhibiting the accumulation of visceral adipose tissue. In addition, females are known to be more sensitive to TG levels with respect to cardiovascular disease risk than males [31], suggesting that these results might be consistent with our findings. Furthermore, BMI is strongly connected to the risk of acquiring T2D [32], and several studies have linked increased visceral fat to insulin resistance [33]. Compared to participants with low BMI, the effect of BMI on insulin resistance may have been greater than the effect of lipid profiles in participants with high BMI. Females and individuals with a BMI < 25 kg/m2 may be more sensitive to levels of lipid profiles based on these findings.
Previous studies have evaluated the association between categorical or continuous TG/HDL-C ratio values and the incidence of T2D [13,14,15,16,17,18]. Kim et al. [13] and Lim et al. [14] conducted a study in a Korean population, Liu et al. [15] conducted a study in Chinese individuals (aged 75 years and older), Tohidi et al. [16] have studied the relationship in Iranians, and Wang et al. [17] investigated the relationship in Japanese people. While these studies have discovered a significant association between higher ratios of TG/HDL-C and an increased risk of new-onset diabetes, cut-off values and comparisons among lipid profiles were not presented [13,14,15,16,17], unlike in our study, which estimated cut-off values and compared ratio of TG/HDL-C, LDL-C, HDL-C, and TG parameters. Additionally, the sample size of some studies was not large enough [14,15,16]. Although Hadaegh et al. [18] reported the cut-off values of the ratio of TG/HDL-C, their sample size was small and they did not compare the performance among lipid profiles for predicting T2D incidence. Furthermore, the cut-off values of the TG/HDL-C ratio reported in their study were higher than the values reported by us [18]. This may be because their study was conducted in a Middle Eastern population, in which the pathophysiology of diabetes may be different from that in Japanese people.
It is also noteworthy that our study's sample size was large, and the follow-up period was lengthy, due to the use of a large corporate health checkup database. Furthermore, several dyslipidemia medications, such as statins, are known to increase the likelihood of developing diabetes [34,35], and by excluding participants taking these drugs, we avoided this confounding effect on our risk assessment.
This study had several limitations. First, only reasonably young Japanese were included. It is unknown whether our findings apply to other age and ethnic groups. Most of the participants in our study were males. Therefore, further research will be needed on sex differences in the relationship between lipid profiles and the development of T2D. Additionally, our study did not take into account other factors, such as genetic predisposition, diet, and medication other than antidiabetic drugs, that might influence diabetes risk. Furthermore, neither HbA1c nor a 75-g oral glucose tolerance test was employed in the diagnosis of T2D. The data of a self-reported history of diabetes or usage of antidiabetic medicines were collected using the self-administered questionnaire. Therefore, some participants with T2D might not have been identified. Finally, the lipid data was only available at baseline and was not tracked over time.
Conclusions
In conclusion, it was demonstrated that the ratio of TG/HDL-C was a stronger predictor of T2D development within 10 years than LDL-C, HDL-C, or TG. These results indicated the importance of providing future medical care support.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- HDL-C:
-
High-density lipoprotein cholesterol
- HR:
-
Hazard ratio
- LDL-C:
-
Low-density lipoprotein cholesterol
- ROC:
-
Receiver operating characteristic
- T2D:
-
Type 2 diabetes
- TG:
-
Triglyceride
References
Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. https://doi.org/10.1016/j.diabres.2021.109119.
Seshasai SRK, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–41. https://doi.org/10.1056/NEJMoa1008862.
Costa J, Borges M, David C, et al. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: Meta-analysis of randomised controlled trials. BMJ. 2006;332(7550):1115–24. https://doi.org/10.1136/bmj.38793.468449.AE.
Fujihara K, Matsubayashi Y, Yamamoto M, et al. Impact of body mass index and metabolic phenotypes on coronary artery disease according to glucose tolerance status. Diabetes Metab. 2017;43(6):543–6. https://doi.org/10.1016/j.diabet.2017.08.002.
Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014;63(12):1469–79. https://doi.org/10.1016/j.metabol.2014.08.010.
von Eckardstein A, Sibler RA. Possible contributions of lipoproteins and cholesterol to the pathogenesis of diabetes mellitus type 2. Curr Opin Lipidol. 2011;22(1):26–32. https://doi.org/10.1097/MOL.0b013e3283412279.
Lin D, Qi Y, Huang C, et al. Associations of lipid parameters with insulin resistance and diabetes: a population-based study. Clin Nutr. 2018;37(4):1423–9. https://doi.org/10.1016/j.clnu.2017.06.018.
Ren X, Chen ZA, Zheng S, et al. Association between triglyceride to HDL-C ratio (TG/HDL-C) and insulin resistance in Chinese patients with newly diagnosed type 2 diabetes mellitus. PLoS ONE. 2016;11(4):e0154345. https://doi.org/10.1371/journal.pone.0154345.
Zhou M, Zhu L, Cui X, et al. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance but not of β cell function in a Chinese population with different glucose tolerance status. Lipids Health Dis. 2016;15:104. https://doi.org/10.1186/s12944-016-0270-z.
Fukuda Y, Hashimoto Y, Hamaguchi M, et al. Triglycerides to high-density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; a population-based cohort study. Liver Int. 2016;36(5):713–20. https://doi.org/10.1111/liv.12977.
Chen C, Dai JL. Triglyceride to high-density lipoprotein cholesterol (HDL-C) ratio and arterial stiffness in Japanese population: a secondary analysis based on a cross-sectional study. Lipids Health Dis. 2018;17(1):130. https://doi.org/10.1186/s12944-018-0776-7.
Wu Z, Zhou D, Liu Y, et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. 2021;20(1):134. https://doi.org/10.1186/s12933-021-01330-6.
Kim J, Shin SJ, Kim YS, et al. Positive association between the ratio of triglycerides to high-density lipoprotein cholesterol and diabetes incidence in Korean adults. Cardiovasc Diabetol. 2021;20(1):183. https://doi.org/10.1186/s12933-021-01377-5.
Lim TK, Lee HS, Lee YJ, et al. Triglyceride to HDL-cholesterol ratio and the incidence risk of type 2 diabetes in community dwelling adults: a longitudinal 12-year analysis of the Korean genome and epidemiology study. Diabetes Res Clin Pract. 2020;163:108150. https://doi.org/10.1016/j.diabres.2020.108150.
Liu H, Liu J, Liu J, et al. Triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, a simple but effective indicator in predicting type 2 diabetes mellitus in older adult. Front Endocrinol (Lausanne). 2022;13:828581. https://doi.org/10.3389/fendo.2022.828581.
Tohidi M, Asgari S, Chary A, et al. Association of triglycerides to high-density lipoprotein cholesterol ratio to identify future prediabetes and type 2 diabetes mellitus: over one-decade follow-up in the Iranian population. Diabetol Metab Syndr. 2023;15:13. https://doi.org/10.1186/s13098-023-00988-0.
Wang H, Wang C, Xuan X, et al. Association between triglyceride to high-density lipoprotein cholesterol ratio and type 2 diabetes risk in Japanese. Sci Rep. 2023;13(1):3719. https://doi.org/10.1038/s41598-022-25585-5.
Hadaegh F, Hatami M, Tohidi M, et al. Lipid ratios and appropriate cut off values for prediction of diabetes: a cohort of Iranian men and women. Lipids Health Dis. 2010;9:85. https://doi.org/10.1186/1476-511X-9-85.
Zhou Y, Yang G, Qu C, et al. Predictive performance of lipid parameters in identifying undiagnosed diabetes and prediabetes: a cross-sectional study in eastern China. BMC Endocr Disord. 2022;22(1):76. https://doi.org/10.1186/s12902-022-00984-x.
Sokooti S, Flores-Guerrero JL, Heerspink HJL, et al. Triglyceride-rich lipoprotein and LDL particle subfractions and their association with incident type 2 diabetes: the PREVEND study. Cardiovasc Diabetol. 2021;20(1):156. https://doi.org/10.1186/s12933-021-01348-w.
Rütti S, Ehses JA, Sibler RA, et al. Low- and high-density lipoproteins modulate function, apoptosis, and proliferation of primary human and murine pancreatic β-cells. Endocrinology. 2009;150(10):4521–30. https://doi.org/10.1210/en.2009-0252.
Drew BG, Rye K-A, Duffy SJ, Barter P, Kingwell BA. The emerging role of HDL in glucose metabolism. Nat Rev Endocrinol. 2012;8(4):237–45. https://doi.org/10.1038/nrendo.2011.235.
Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes. 2001;50(Suppl 1):S118-121. https://doi.org/10.2337/diabetes.50.2007.s118.
Barter PJ, Brewer HB Jr, Chapman MJ, et al. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23(2):160–7. https://doi.org/10.1161/01.atv.0000054658.91146.64.
Föger B, Ritsch A, Doblinger A, et al. Relationship of plasma cholesteryl ester transfer protein to HDL cholesterol: studies in normotriglyceridemia and moderate hypertriglyceridemia. Arterioscler Thromb Vasc Biol. 1996;16(12):1430–6. https://doi.org/10.1161/01.atv.16.12.1430.
Li N, Fu J, Koonen DP, et al. Are hypertriglyceridemia and low HDL causal factors in the development of insulin resistance? Atherosclerosis. 2014;233(1):130–8. https://doi.org/10.1016/j.atherosclerosis.2013.12.013.
Hazzard WR. Atherogenesis: why women live longer than men. Geriatrics. 1985;40(1):42–51.
Dayspring T, Pokrywka G. Impact of triglycerides on lipid and lipoprotein biology in women. Gend Med. 2010;7(3):189–205. https://doi.org/10.1016/j.genm.2010.05.002.
Kolovou GD, Anagnostopoulou KK, Kostakou PM, et al. Primary and secondary hypertriglyceridaemia. Curr Drug Targets. 2009;10(4):336–43. https://doi.org/10.2174/138945009787846452.
Ko S-H, Kim H-S. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients. 2020;12(1):202. https://doi.org/10.3390/nu12010202.
Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–9.
Maskarinec G, Eeber E, Grandinetti A, et al. Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes. 2009;58(8):1732–8. https://doi.org/10.2337/db08-1685.
Katsuki A, Sumida Y, Urakawa H, et al. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care. 2003;26(8):2341–4. https://doi.org/10.2337/diacare.26.8.2341.
Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. https://doi.org/10.1056/NEJMoa0807646.
Preiss D, Seshasai SRK, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556–64. https://doi.org/10.1001/jama.2011.860.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content. MF and HO reviewed and edited the manuscript. HO and MI researched data and contributed to the discussion. MH, KK, and HM contributed to the discussion. HO designed this study and contributed to the discussion. HY wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Panasonic Health Insurance Organization's local ethics committee (permission number: 2021–001) and was carried out in conformity with the principles of the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
Hamaguchi M received grants from Ono Pharma Co. Ltd., AstraZeneca K.K., Oishi Kenko inc., Nippon Boehringer Ingelheim Co. Ltd., Yamada Bee Farm. Fukui M received grants from Oishi Kenko inc., Ono Pharma Co. Ltd., Yamada Bee Farm, Kissei Pharma Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Mitsubishi Tanabe Pharma Corp., Sanofi K.K., Daiichi Sankyo Co. Ltd., Astellas Pharma Inc., Kyowa Kirin Co. Ltd., MSD K.K., Sumitomo Pharma Co. Ltd., Novo Nordisk Pharma Ltd., Kowa Pharma Co. Ltd., Sanwa Kagagu Kenkyusho CO., Ltd., Taisho Pharma Co., Eli Lilly, Japan, K.K., Terumo Corp., Nippon Chemiphar Co. Ltd., Tejin Pharma Ltd., Abbott Japan Co. Ltd., Medical Co., Johnson & Johnson K.K, TERUMO Co.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table 1.
Unadjusted hazard ratios for incidence of diabetes during the 10-year follow-up period.
Additional file 2: Table 2.
The area under the curve and optimal cut-off values according to sex and BMI category.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuge, H., Okada, H., Hamaguchi, M. et al. Triglycerides/HDL cholesterol ratio and type 2 diabetes incidence: Panasonic Cohort Study 10. Cardiovasc Diabetol 22, 308 (2023). https://doi.org/10.1186/s12933-023-02046-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-02046-5