Abstract
Background
This study aimed to explore the association between the triglyceride-glucose (TyG) index and the risk of in-hospital mortality in critically ill patients with sepsis.
Methods
This was a retrospective observational cohort study and data were obtained from the Medical Information Mart for Intensive Care-IV (MIMIC IV2.2) database. The participants were grouped into three groups according to the TyG index tertiles. The primary outcome was in-hospital all-cause mortality. Multivariable logistics proportional regression analysis and restricted cubic spline regression was used to evaluate the association between the TyG index and in-hospital mortality in patients with sepsis. In sensitivity analysis, the feature importance of the TyG index was initially determined using machine learning algorithms and subgroup analysis based on different subgroups was also performed.
Results
1,257 patients (56.88% men) were included in the study. The in-hospital, 28-day and intensive care unit (ICU) mortality were 21.40%, 26.17%, and 15.43% respectively. Multivariate logistics regression analysis showed that the TyG index was independently associated with an elevated risk of in-hospital mortality (OR 1.440 [95% CI 1.106–1.875]; P = 0.00673), 28-day mortality (OR 1.391; [95% CI 1.52–1.678]; P = 0.01414) and ICU mortality (OR 1.597; [95% CI 1.188–2.147]; P = 0.00266). The restricted cubic spline regression model revealed that the risks of in-hospital, 28-day, and ICU mortality increased linearly with increasing TyG index. Sensitivity analysis indicate that the effect size and direction in different subgroups are consistent, the results is stability. Additionally, the machine learning results suggest that TyG index is an important feature for the outcomes of sepsis.
Conclusion
Our study indicates that a high TyG index is associated with an increased in-hospital mortality in critically ill sepsis patients. Larger prospective studies are required to confirm these findings.
Similar content being viewed by others
Introduction
Sepsis, a condition characterized by a dysregulated immune response to infection, is a prominent contributor to global mortality [1]. Despite ongoing efforts, both the incidence and mortality of sepsis have demonstrated limited reductions over the past decades [2, 3]. This further emphasize the importance of identifying risk factors associated with sepsis outcomes, enabling early prevention strategies.
Insulin resistance (IR) is a pathological physiological state characterized by diminished sensitivity in the peripheral tissues to insulin, which is often prevalent in patients with sepsis and manifests as elevated insulin levels and reduced sensitivity. Acute glucose fluctuations may increase mortality risk in patients with sepsis [4]. The triglyceride–glucose (TyG) index has emerged as a surrogate marker of insulin resistance, some studies indicated that TyG index is associated with the progression of metabolic disorders [5, 6]. The extensive release of inflammatory factors and imbalanced oxidative stress are likely culprits for inducing insulin resistance in the context of sepsis. As such, it is imperative to further explore the association between the degree of insulin resistance and the prognosis of septic patients.
A multitude of cross-sectional and retrospective studies have revealed noteworthy links between the TyG index and all-cause mortality among critically ill patients afflicted with conditions such as ischemic stroke, chronic kidney disease, and cardiac arrest [7,8,9]. Currently, research investigating the connection between TyG index and sepsis outcomes in the literature is lacking. This study aimed to investigate the association between the TyG index and the clinical outcome of patients with sepsis, ultimately revealing the profound implications of insulin resistance on sepsis.
Methods
Study population
This was a retrospective observational cohort study with longitudinal follow-up of patients. Medical Information Mart for Intensive Care-IV (MIMIC-IV-2.2) is a freely accessible database that encompasses over 50,000 ICU admissions at the Beth Israel Deaconess Medical Center in Boston, Massachusetts, from 2008 to 2019 [10]. The MIMIC-IV database contains a wealth of information, including demographics, vital signs, test results, and diagnoses categorized using codes from both the International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10). To access this database, one of the authors (Rui Zheng) obtained the necessary certification and subsequently extracted the relevant variables for our study (certification number: 1797305). Individual patient consent was not needed due to the anonymized nature of the patient health information within this database.
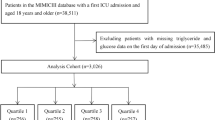
Patients diagnosed with sepsis according to the sepsis 3.0 diagnostic criteria were included in this study, which with infection and Sequential Organ Failure Assessment (SOFA) score ≥ 2 [11]. The method for obtaining patients with sepsis who meet the Sepsis 3.0 diagnostic criteria from the MIMIC database is consistent with previously published studies[12], details in Additional file 1-A. The exclusion criteria were as follows: (1) patients stayed in ICU < 48 h; (2) multiple admissions to the ICU for sepsis, for whom only data from the first admission were extracted; (3) insufficient data (such as triglycerides and fasting blood glucose); (4) patients with diabetic or acute pancreatitis; (5) patients receiving lipid-lowering drugs and antidiabetic treatment (Fig. 1).
Variable extraction
In this study, the information was extracted using PostgresSQL software (version 13.7.2) and Navicat Premium software (version 16) through the execution of a Structured Query Language (SQL). Data extracted from the MIMIC-IV database on the first 24 h of ICU admission included age, Gender, Body mass index (BMI), and SOFA score and each component of the SOFA score. Other relevant data, including laboratory test results, clinical outcomes, and comorbidities were obtained. All laboratory parameters extracted from the MIMIC-IV (2.2) database were measured on the first time after ICU admission. Follow-up started on the ICU admission date and ended on the date of death. The TyG index was calculated using the formula ln [fasting triglycerides (mg/dl) × fasting blood glucose (mg/dl)/2]. Low HDL (high-density lipoprotein) was defined as HDL-C < 40 mg/dL for males or < 50 mg/dL for females. AKI (acute kidney injury) were defined by both serum creatinine (Scr) and the volume of urine during the first 48 h after ICU admission. NLR (neutrophil to lymphocyte ratio) was defined as neutrophil to lymphocyte ratio.
There is no consensus regarding the standard percentage of missing values for excluding variables from the analysis. For our study, we set our threshold at 60%, considering that Zhang et al. [13] omitted variables with over 70% missing values in their analysis. Before each model fitting process, we assumed missing data were “missing at random” (MAR) [14, 15]. The “missForest” package in R studio was employed to impute the data [16, 17].
Primary outcome and secondary outcomes
The primary outcome of the present study was in-hospital all-cause mortality, and the secondary endpoints was ICU mortality and mortality within 28 days after admission to the ICU. Patient mortality information for discharged patients was accessed from the US Social Security Death Index.
Feature selection
Before investigating the association between the TyG index and in-hospital mortality in sepsis patients, we first employed machine learning algorithms for feature selection to determine their importance in the prognostic model. In this regard, a key approach is the Boruta algorithm, which is a widely utilized feature-selection method. The essence of this algorithm is based on two concepts: "shadow features" and "binomial distribution.". Boruta generated a set of feature replicas, referred to as shadow features, from the original dataset. If a feature's Z-score surpasses the maximum possible Z-score for shadow features, it is considered significant and retained; otherwise, it is excluded [18]. Additionally, we employed a random forest model for variable feature selection and employed the SHapley Additive extension (SHAP) package to visualize variable importance [19]. The SHAP package, implemented via the SHAP Python package (version 0.39.0), facilitates model interpretation to mitigate the inherent black-box nature of machine learning, thereby assisting clinicians in comprehending the outcomes provided by the models [20].
Statistical analysis
Categorical variables were evaluated using Fisher's exact or chi-square tests and are presented as counts (percentages). For continuous variables, the Wilcoxon rank-sum test, Student's t-test, or one-way analysis of variance was employed. To explore potential nonlinear relationships between TyG index levels and in-hospital, ICU, and 28-day mortality, a restricted cubic spline analysis was conducted. Four knots were positioned at the 5th, 35th, 65th, and 95th percentiles as recommended by Harrell [21, 22]. To evaluate the association between TyG index and the risk of in-hospital mortality, ICU mortality, and 28-day mortality, multivariate logistic regression analyses were performed. Odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were calculated to quantify the impact of the TyG index on these outcomes. Model 1 included only the TyG index, without any adjustments. In Model 2, age, Gender and BMI were included as the modified variables. Model 3 incorporated variables based on clinical expertise, prior literature [23], and feature importance selection results from the Boruta and random forest algorithms, as showed in Fig. 2. The variables included in this model were Age; Gender; BMI; SOFA score; Hemoglobin; Sodium; White Blood Cell (WBC); Red Cell Distribution width (RDW); Low-Density Lipoprotein (LDL); Prothrombin Time (PT); Partial Thromboplastin Time (PTT); Alanine Aminotransferase (ALT); Alkaline Phosphatase(ALP); Aspartate Aminotransferase(AST); C-Reactive Protein (CRP); NLR; Atrial Fibrillation; Hypertension; Myocardial Infarction; Congestive Heart Failure (CHF); Chronic Obstructive Pulmonary Disease (COPD); Coronary Artery Disease (CAD); Acute Kidney Injury (AKI); Low HDL. Variance inflation factors (VIFs) were examined to detect multicollinearity among variables. Furthermore, a subgroup analysis was conducted to validate the association between TyG index and in-hospital, ICU, and 28-day mortality within each subgroup. Kaplan–Meier analysis and multivariate Cox regression were also used as sensitivity analyses to explore the association between TyG index and 28-day and 90-day mortality endpoints. In patients with complete SOFA scores, the relationship between TyG index and in-hospital, ICU, and 28-day mortality was performed to affirm the robustness and stability of the obtained results. All statistical analyses were performed using R version 4.1.2 (R Foundation). Statistical significance was defined as a two-sided P-value of < 0.05.
Application of Machine Learning in Feature Selection. A Shapley Additive Explanations (SHAP) for the random forest model. A Distribution of the impact of each feature on the model output. Each dot represents a patient in a row. The colors of the dots represent the feature values: red represents larger values and blue represents lower values. B Feature selection for the relationship between various TyG indices and in-hospital mortality was analyzed using the Boruta algorithm. B The horizontal axis shows the name of each variable, whereas the vertical axis represents the Z-value of each variable. The box plot depicts the Z-value of each variable in the model calculation, with green boxes representing important variables, blue boxes representing tentative attributes, and yellow boxes representing unimportant variables. SOFA: Sequential Organ Failure Assessment; Activated Partial Thromboplastin Time WBC: White Blood Cell Count BMI: Body Mass Index LDL: Low-Density Lipoprotein PT: Prothrombin Time ALP: Alkaline Phosphatase RDW: Red Cell Distribution Width HDL: High-Density Lipoprotein. TyG: triglyceride glucose index ALT: Alanine Aminotransferase; COPD: Chronic Obstructive Pulmonary Disease CAD: Coronary Artery Disease; CHF: Congestive Heart Failure; HTN: hypertension
Results
Baseline characteristics
The inclusion and exclusion criteria resulted in the enrollment of 1,257 patients with sepsis from the MIMIC-IV database for this study. The in-hospital, 28-day, and ICU mortality were 21.40%, 26.17%, and 15.43% respectively. In Table 1, patients in the TyG T1 group were compared to those in the other groups, revealing that the latter were more likely to be younger and male, with a higher prevalence of obesity, kidney injury, Atrial Fibrillation, Low_HDL. Moreover, patients with a higher TyG index exhibited elevated SOFA score, LDL, Albumin, anion gap, Bicarbonate, blood urea nitrogen, creatinine, potassium, ALT, AST, ALP and NLR levels. Additionally, within the group characterized by a higher TyG index, longer hospital stays and ICU durations were observed. In Table 2, a comparison between the non-hospital survivor and survivor groups revealed that the latter exhibited greater severity of illness, with a higher median SOFA score (10 [IQR, 6–14] vs. 6 [IQR, 4–9]). The results showed that age, Hemoglobin, Platelets, WBC, Mean Cell Volume (MCV), RDW, Total Cholesterol (TC), HDL, LDL, Albumin, Anion gap, Blood Urea Nitrogen (BUN), Bicarbonate, International Normalized Ratio (INR), PT, ALP, AST, Total bilirubin, TyG, NLR were associated with in-hospital mortality. Non-Surviving patients were also more likely to be have a higher prevalence of atrial fibrillation, Low HDL and AKI.
TyG index and in-hospital, ICU, and 28-day mortality
Multivariate logistics regression analysis showed that the TyG index was independently associated with an elevated risk of in-hospital mortality (OR 1.440 [95% CI 1.106–1.875]; P = 0.00673), 28-day mortality (OR 1.391; [95% CI 1.52–1.678]; P = 0.01414) and ICU mortality (OR 1.597; [95% CI 1.188–2.147]; P = 0.00266). These results were further confirmed in the fully adjusted Model 3, specifically, the OR for in-hospital mortality in the highest TyG index tertile was 2.242, 95% CI: 1.448–3.472, and for ICU mortality, it was 2.564, 95% CI: 1.553–4.233, both compared with the lowest tertile. Additionally, the risk of in-hospital mortality, ICU mortality, and 28-day mortality demonstrated a consistent upward trend with increasing TyG index tertiles, with all trend p-values below 0.05 (Table 3). Moreover, the restricted cubic spline regression model was applied to reveal that the risks of in-hospital mortality, ICU mortality, and 28-day mortality increased linearly with increasing TyG index (Fig. 3). For stability of results, the Kaplan–Meier analysis plot showed a significant difference among various TyG index groups of 28-day and 90-day mortality (Additional file 1: Fig. S1). Among patients with complete components of the SOFA score, the relationship between the TyG index group and in-hospital, ICU, and 28-day mortality was consistent with core results (Additional file 2: Table S2) and the association between TyG index groups and mortality in patients with sepsis for Cox Regression is stable (Additional file 2: Table S3).
The relationship for the levels of TyG index with in-hospital mortality, ICU mortality, and 28-day mortality. a–c OR (95% CIs) for in-hospital, in-ICU, and 28-day mortality according to TyG index tertile after adjusting for Adjusted for Age; Gender; BMI; SOFA score; Hemoglobin; Sodium; WBC; RDW; LDL; PT; PTT; ALT; ALP; AST; CRP; NLR; Atrial Fibrillation; Hypertension; Myocardial Infarction; CHF; COPD; CAD; AKI; Low HDL. Error bars indicate 95% CIs. The first tertile is the reference. d Restricted cubic spline for hospital mortality. e Restricted cubic spline for ICU mortality. f Restricted cubic spline for 28-day mortality. OR: odds ratio; CI: confidence interval; ICU: intensive care unit; TyG: triglyceride-glucose
Subgroup analysis
Furthermore, to confirm the relationship between TyG index and in-hospital mortality, ICU mortality, and 28-day mortality, stratified analyses were conducted based on age, Gender, BMI, SOFA score, hypertension, myocardial infarction, and congestive heart failure. In Fig. 4, There is a significant relationship between TyG and ICU mortality for both males (OR = 1.367, 95% CI 1.024–1.826) and females (OR = 1.989, 95% CI 1.389–2.848) in full adjusted model, as well as for individuals with myocardial infarction (OR = 1.571, 95% CI 1.225–2.016) and those without myocardial infarction (OR = 1.716, 95% CI 1.023–2.878). The relationship between the TyG index and mortality remains consistent in direction. Regardless of the outcome variable being in-hospital mortality, ICU mortality, or even 28-day mortality, the results of the stratified analysis consistently demonstrated a similar association of TyG index values across most sub-populations.
Discussion
To our knowledge, this study represents the first investigation into the relationship between the TyG index and all-cause mortality among sepsis patients. In this study, we found a significant association between an elevated TyG index and increased in-hospital mortality among patients with sepsis. This conclusion aligns with ICU and 28-day mortality in patients with sepsis, which shown a consistent linear relationship. This association remained robust, even after adjusting for multiple clinical and laboratory variables. Our results extended the application of the TyG index to the realm of cardiovascular disorders, indicating its potential value as a decision-making tool for clinicians managing patients with sepsis.
In recent years, TyG index has been proposed as a potential marker for metabolic disorders, atherosclerotic conditions, cardiovascular disease and COVID-19 [24,25,26,27]. Numerous clinical studies have examined the association between an elevated TyG index and higher morbidity and mortality associated with critically ill patients and infectious diseases across various populations. Yang et al. found that the TyG index was an independent risk factor for in-hospital and ICU mortality in patients after cardiac arrest [7]. Lee et al. observed that the TyG index might be helpful in predicting short-term functional outcomes in critically ill stroke patients undergoing reperfusion therapy [28]. Additionally, for individuals with coronary artery disease, the TyG index could contribute to predicting adverse cardiovascular events [29, 30]. For general population, a high TyG index has been found to be associated with an increased incidence of respiratory symptoms, an elevated risk of chronic lung disease, and a reduction in lung function [31]. These studies collectively suggest the potential of the association between TyG index and clinical outcomes in critically ill and infection-related patients. Other studies have reported that for each unit increase in the TyG index, the risk of in-hospital mortality in critically ill patients increases by nearly 30% or more [8, 9]. We observed that for each unit increase in the TyG index, the risk of in-hospital mortality for sepsis patients increased by 44.0%, our study's conclusions align with previous researches, indicating an association between elevated TyG index and increased mortality.
Our results suggest an association between a high TyG index and the severity and outcomes of sepsis. Sepsis can lead to insulin resistance and the disruption of lipid metabolism accompanied by uncontrolled hyperglycemia and glycemic variability during the acute phase of sepsis. The prognosis of sepsis is closely tied to the severity of inflammatory responses, which are significantly correlated with insulin resistance. Our results demonstrate that the TyG index is positively correlated with disease severity scores. The TyG index reflects the severity of disease in patients with sepsis and provides insights that can contribute to the clinical management of sepsis. Clinicians should be aware of patient blood glucose management and monitor changes in insulin resistance indicators.
The exact biological mechanisms underlying the relationship between the TyG index and sepsis prognosis remain unclear. The TyG index is associated with insulin resistance (IR), insulin resistance has been widely demonstrated to be closely related to endothelial dysfunction, oxidative stress, immune dysregulation, coagulation imbalance, and inflammatory response [32,33,34], all of which are also closely associated with the occurrence and progression of sepsis. From the baseline data, we observed significant differences in SOFA scores among patients in different TyG index groups, indicating a close association between the TyG index and disease severity. Changes in insulin resistance during the acute phase of sepsis could reflect the inflammatory state or severity of sepsis. A potential explanation for the role of the TyG index as an indicator of cardiovascular disease could be that the TyG index serves as a reflection of IR in patients. IR, in turn, can contribute to the development of cardiovascular diseases by enhancing vascular stiffness and diminishing the bioavailability of nitric oxide (NO) [35, 36]. An elevated TyG index is associated with cardiovascular diseases, and the presence of cardiovascular diseases in sepsis is a risk factor contributing to adverse patient outcomes. Although cardiovascular diseases were present in the various TyG index level groups in this study, no differences were observed.
In sensitivity analysis, our study found that the linear relationship between the TyG index and in-hospital mortality in sepsis patients remained consistent in the younger age group, female patients, those with lower BMI, non-hypertensive individuals, and those without congestive heart failure. This result might be attributed to the fact that advanced age, male, higher BMI, and hypertension are traditionally recognized as unfavorable risk factors for sepsis prognosis. Furthermore, factors such as Gender, obesity, and cardiovascular disease can contribute to insulin resistance, potentially leading to an underestimation of the association between the TyG index and sepsis outcomes. Meanwhile, in subgroup analyses and regarding the 28-day mortality and ICU mortality, we observed that after stratifying by SOFA score and gender, the results were not statistically significant. After stratified analysis, the sample size decreases, leading to a reduction in effect size, which can be one of the reasons for non-significant results. However, the consistent direction of all results indicates the stability and reliability of the core outcomes. In patients with complete SOFA scores, the relationship between the TyG index group and in-hospital ICU, and 28-day mortality remained stable. In Cox regression analysis, the relationship between 28-day and 90-day mortality rates in the TyG index group was consistent with core results. Furthermore, we performed a feature analysis utilizing SHAP and Brouta plots, and the importance of the TyG index as a feature was evaluated within the outcome prediction model. Consequently, a machine learning prognostic model for sepsis could be established in the future by focusing on the TyG index.
However, it is important to acknowledge the limitations of this study. First, our analysis was retrospective and derived from observational data, precluding the definitive establishment of causality. Nonetheless, we employ a range of rigorous statistical methods to yield robust and credible outcomes. Second, the TyG index was not dynamically monitored, and sepsis itself could influence lipid metabolism and blood glucose fluctuations. The TyG index obtained from the first-time glucose and triglyceride measurements may not comprehensively represent insulin resistance in the body. Third, some confounding factors, including metabolic syndrome parameters, Acute Physiology and Chronic Health Evaluation II (APACHE II), nutritional state parameters, and inflammatory markers, were not thoroughly considered. This may have an impact on the results. Fourth, clinicians were following different guidelines with different definitions of sepsis from 2008 to 2019, which could potentially impact the study results. Fifth, when patients receive enteral or parenteral nutrition, it may impact lipid and glucose levels, potentially leading to an increase in the TyG index. However, with the large sample size included in our study, this effect is likely mitigated. Further research is needed to investigate the key mechanisms of insulin resistance in patients with sepsis.
Conclusions
The elevated TyG index is strongly associated with increased in-hospital all-cause mortality in patients with sepsis. Our results suggest that the TyG index assists in the early detection of insulin resistance in patients with sepsis, thus enhancing risk assessment and directing subsequent interventions. However, additional prospective studies are required to validate these findings.
Availability of data and materials
Raw data supporting the obtained results are available at the corresponding author.
References
Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated sepsis current. Estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–72.
Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. Incidence and trends of sepsis in US Hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318:1241–9.
Epidemiological trends of sepsis in the twenty-first century (2000–2013): an analysis of incidence, mortality, and associated costs in Spain - PubMed.https://pubmed.ncbi.nlm.nih.gov/29433513/. Accessed 18 Aug 2023.
Li X, Zhang D, Chen Y, Ye W, Wu S, Lou L, et al. Acute glycemic variability and risk of mortality in patients with sepsis: a meta-analysis. Diabetol Metab Syndr. 2022;14:59.
Faerch K, Vaag A, Holst JJ, Hansen T, Jørgensen T, Borch-Johnsen K. Natural history of insulin sensitivity and insulin secretion in the progression from normal glucose tolerance to impaired fasting glycemia and impaired glucose tolerance: the Inter99 study. Diabetes Care. 2009;32:439–44.
Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–85.
Boshen Y, Yuankang Z, Xinjie Z, Taixi L, Kaifan N, Zhixiang W, et al. Triglyceride-glucose index is associated with the occurrence and prognosis of cardiac arrest: a multicenter retrospective observational study. Cardiovasc Diabetol. 2023;22:190.
Cai W, Xu J, Wu X, Chen Z, Zeng L, Song X, et al. Association between triglyceride-glucose index and all-cause mortality in critically ill patients with ischemic stroke: analysis of the MIMIC-IV database. Cardiovasc Diabetol. 2023;22:138.
Liao Y, Zhang R, Shi S, Zhao Y, He Y, Liao L, et al. Triglyceride-glucose index linked to all-cause mortality in critically ill patients: a cohort of 3026 patients. Cardiovasc Diabetol. 2022;21:128.
Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. 2023;10:1.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–10.
Hu W, Chen H, Ma C, Sun Q, Yang M, Wang H, et al. Identification of indications for albumin administration in septic patients with liver cirrhosis. Crit Care. 2023;27:300.
Machine learning for the prediction of volume responsiveness in patients with oliguric acute kidney injury in critical care - PubMed. https://pubmed.ncbi.nlm.nih.gov/30961662/. Accessed 18 Aug 2023.
Strategies for dealing with missing data in clinical trials: from design to analysis - PubMed. https://pubmed.ncbi.nlm.nih.gov/24058309/. Accessed 28 Aug 2023.
Addressing missing data in clinical studies of kidney diseases - PubMed. https://pubmed.ncbi.nlm.nih.gov/24509298/. Accessed 28 Aug 2023.
MissForest--non-parametric missing value imputation for mixed-type data - PubMed. https://pubmed.ncbi.nlm.nih.gov/22039212/. Accessed 28 Aug 2023.
Zhang Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann Transl Med. 2016;4:30.
Evaluation of variable selection methods for random forests and omics data sets - PubMed. https://pubmed.ncbi.nlm.nih.gov/29045534/. Accessed 26 Aug 2023.
From Local Explanations to Global Understanding with Explainable AI for Trees - PubMed. https://pubmed.ncbi.nlm.nih.gov/32607472/. Accessed 26 Aug 2023.
Nohara Y, Matsumoto K, Soejima H, Nakashima N. Explanation of machine learning models using shapley additive explanation and application for real data in hospital. Comput Meth Prog Bio. 2022;214: 106584.
Harrell FE, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–202.
Regression models for ordinal responses: a review of methods and applications - PubMed. https://pubmed.ncbi.nlm.nih.gov/9447413/. Accessed 26 Aug 2023.
Ye Z, An S, Gao Y, Xie E, Zhao X, Guo Z, et al. Association between the triglyceride glucose index and in-hospital and 1-year mortality in patients with chronic kidney disease and coronary artery disease in the intensive care unit. Cardiovasc Diabetol. 2023;22:110.
Zhao Q, Cheng Y-J, Xu Y-K, Zhao Z-W, Liu C, Sun T-N, et al. Comparison of various insulin resistance surrogates on prognostic prediction and stratification following percutaneous coronary intervention in patients with and without type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20:190.
Zhou Y, Pan Y, Yan H, Wang Y, Li Z, Zhao X, et al. Triglyceride glucose index and prognosis of patients with ischemic stroke. Front Neurol. 2020;11:456.
Zhang R, Shi S, Chen W, Wang Y, Lin X, Zhao Y, et al. Independent effects of the triglyceride-glucose index on all-cause mortality in critically ill patients with coronary heart disease: analysis of the MIMIC-III database. Cardiovasc Diabetol. 2023;22:10.
Chang Y, Jeon J, Song T-J, Kim J. Association of triglyceride-glucose index with prognosis of COVID-19: a population-based study. J Infect Public Heal. 2022;15:837–44.
Lee M, Kim C-H, Kim Y, Jang MU, Mo HJ, Lee S-H, et al. High Triglyceride glucose index is associated with poor outcomes in ischemic stroke patients after reperfusion therapy. Cerebrovasc Dis. 2021;50:691–9.
Jin J-L, Cao Y-X, Wu L-G, You X-D, Guo Y-L, Wu N-Q, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10:6137–46.
Tai S, Fu L, Zhang N, Yang R, Zhou Y, Xing Z, et al. Association of the cumulative triglyceride-glucose index with major adverse cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2022;21:161.
Wu TD, Fawzy A, Brigham E, McCormack MC, Rosas I, Villareal DT, et al. Association of triglyceride-glucose index and lung health: a population-based study. Chest. 2021;160:1026–34.
Markus MRP, Rospleszcz S, Ittermann T, Baumeister SE, Schipf S, Siewert-Markus U, et al. Glucose and insulin levels are associated with arterial stiffness and concentric remodeling of the heart. Cardiovasc Diabetol. 2019;18:145.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302.
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122.
Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:74.
Insulin resistance, cardiovascular stiffening and cardiovascular disease - PubMed. https://pubmed.ncbi.nlm.nih.gov/33766485/. Accessed 18 Aug 2023.
Acknowledgements
None.
Funding
This work was supported by a grant from Research Incubation Project of the First Affiliated Hospital of Wenzhou Medical University (Grant NO. FHY2019048, WZ); Foundation of Wenzhou City Science & Technology Bureau (Y20210713,ZY2021028); Wenzhou High-level Innovation Team for Critical Care and Intelligent Treatment (Grant No. 88923001); Zhejiang Provincial Outstanding Talent Project of Ten Thousand Talents Program (2020R51005).
Author information
Authors and Affiliations
Contributions
HX, JP: conceptualization, methodology, software; SQ, CL: data curation, writing-original draft preparation; YS: visualization, investigation; RZ, SQ: writing—reviewing and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Additional methods. Figure1S: The Kaplan–Meier analysis plot showed a significant difference among various TyG index groups of 28-day mortality (A.B) and 90-day mortality (C.D).
Additional file 2: Table S1.
Each component of the SOFA score categorized by TyG index. Table S2. The association between TyG index groups and in-hospital, ICU and 28-day mortality in patient with all component of the SOFA score (n = 733). Table S3. The association between TyG index groups 28-day and 90-day mortality for Cox Regression. Table S4. The association between TyG index on 28-day and 90-day mortality for Cox Regression in patient with all component of the SOFA score (n = 733). Table S5. Missing rate for demographics and clinical variables extracted from the database during the observation period. Table S6. Subgroup analyses for the association of TyG index with in-hospital death, ICU death, and 28-day death.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zheng, R., Qian, S., Shi, Y. et al. Association between triglyceride-glucose index and in-hospital mortality in critically ill patients with sepsis: analysis of the MIMIC-IV database. Cardiovasc Diabetol 22, 307 (2023). https://doi.org/10.1186/s12933-023-02041-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-02041-w