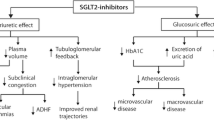
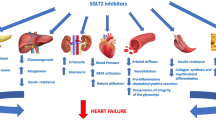
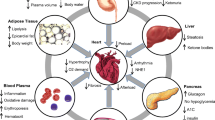
Abstract
In recent years, GLP-1 receptor agonists (GLP-1RA), and SGLT-2 inhibitors (SGLT-2i) have become available, which have become valuable additions to therapy for type 2 diabetes as they are associated with low risk for hypoglycemia and cardiovascular benefits. Indeed, SGLT-2i have emerged as a promising class of agents to treat heart failure (HF). By inhibiting SGLT-2, these agents lead to excretion of glucose in urine with subsequent lowering of plasma glucose, although it is becoming clear that the observed benefits in HF cannot be explained by glucose-lowering alone. In fact, multiple mechanisms have been proposed to explain the cardiovascular and renal benefits of SGLT-2i, including hemodynamic, anti-inflammatory, anti-fibrotic, antioxidant, and metabolic effects. Herein, we review the available evidence on the pathophysiology of the cardiological benefits of SGLT-2i. In diabetic heart disease, in both clinical and animal models, the effect of SGLT-2i have been shown to improve diastolic function, which is even more evident in HF with preserved ejection fraction. The probable pathogenic mechanisms likely involve damage from free radicals, apoptosis, and inflammation, and therefore fibrosis, many of which have been shown to be improved by SGLT-2i. While the effects on systolic function in models of diabetic heart disease and HF with preserved ejection fraction is limited and contrasting, it is a key element in patients with HF and reduced ejection fraction both with and without diabetes. The significant improvement in systolic function appears to lead to subsequent structural remodeling of the heart with a reduction in left ventricle volume and a consequent reduction in pulmonary pressure. While the effects on cardiac metabolism and inflammation appear to be consolidated, greater efforts are still warranted to further define the entity to which these mechanisms contribute to the cardiovascular benefits of SGLT-2i.
Similar content being viewed by others
Introduction
At present there are a number of classes of pharmacological agents used for type 2 diabetes (T2D), with multiple agents available in each class [1]. In recent years, new classes of agents have become available, namely GLP-1 receptor agonists (GLP-1RA), and SGLT-2 inhibitors (SGLT-2i). These newer agents have become valuable additions to therapy for T2D as they are associated with low risk for hypoglycemia as well as cardiovascular (CV) benefits [2]. As a result, treatment approaches are changing rapidly with preference now given to agents whose benefits extend beyond glucose-lowering [2]. To aid the prescriber, comprehensive algorithms are now available for diabetes management, which emphasize personalization of glycemic targets and CV risk [2,3,4].
Of note, T2D is one of the main risk factors for CV diseases. It is directly associated with diabetic cardiomyopathy [5] and increases risk of ischemic cardiomyopathy and heart failure (HF) [6]. In this regard, SGLT-2i have emerged as a promising class of agents to treat HF and have a prominent role in current guidelines [7], and some of these agents have been approved for treatment of HF.
Four major CV outcome trials (CVOTs) – EMPA-REG (empagliflozin), CANVAS (canagliflozin), DECLARE-TIMI 58 (dapagliflozin), and VERTIS CV (ertugliflozin), investigated the CV benefits of SGLT-2i in patients with T2D [8,9,10,11]. Reductions in the relative risk for hospitalizations for HF of 30%, 33%, 27%, and 35%, respectively, were seen in these four trials [8,9,10,11]. In addition, a meta-analysis of CVOTs on over 34,000 patients found that SGLT-2i reduced the risk of major adverse CV events by 11%, with benefits only for patients with atherosclerotic CV disease [12].
Faced with these promising results, dedicated trials in patients with HF were initiated [13,14,15]. Based on these results, the EMA approved dapagliflozin for the treatment of HF with reduced and preserved ejection fraction (HFrEF, HFpEF) and empagliflozin for symptomatic chronic HF. Moreover, a class effect has been suggested based on meta-analyses [16, 17].
By inhibiting SGLT-2, SGLT-2i lead to excretion of glucose in urine with subsequent lowering of plasma glucose [18]. However, it is unlikely that the observed benefits in HF can be explained by glucose-lowering alone. In fact, multiple mechanisms have been proposed to explain the CV and renal benefits of SGLT-2i, including hemodynamic, anti-inflammatory, anti-fibrotic, antioxidant, and metabolic effects [19,20,21]. Furthermore, several cardiac morphofunctional parameters have been shown to be modified by this drug class, and it is possible that different mechanisms can reverse physio-pathological alterations that are typical of diabetes and HF. Herein, we review the available evidence on the pathophysiology of the cardiological benefits of SGLT-2i in different groups of patients, namely those with diabetic cardiomyopathy, HFpEF, and HFrEF.
Materials and methods
A literature search was performed on PubMed for relevant articles and abstracts. The search string used was (“Sodium-Glucose Transporter 2 Inhibitors“[tiab] OR “Sodium-Glucose Transporter 2 Inhibitor“[tiab] OR “Sodium-Glucose Transporter 2 Inhibitors“[Mesh] OR “SGLT2-i“[tiab] OR “dapagliflozin“[tiab] OR “empagliflozin“[tiab] OR “canagliflozin“[tiab]) AND (“stroke volume“[mesh] OR “ejection fraction“[tiab] OR “Doppler“[tiab] OR “wedge“[tiab] OR “pulmonary artery pressure“[tiab] OR “end-diastolic volume“[tiab] OR “end-diastolic pressure“[tiab] OR “cardiac index“[tiab] OR “stroke volume“[tiab]) AND (mechanism*[tiab] OR pathway*[tiab]) AND (“Heart Failure“[Mesh] OR “heart failure”[tiab]). Papers were selected for inclusion in the present review according to their relevance, as judged by the authors. As a literature review, no ethics committee approval was needed.
Effects in diabetic cardiomyopathy: animal and human studies
A number of studies have examined the effects of SGLT-2i on cardiac structure and function in animals and humans with diabetes (Table 1).
Animal studies
The effects of dapagliflozin on myocardial function have been evaluated in a rat model with streptozotocin-induced diabetes, wherein the heart developed diabetic cardiomyopathy with pronounced fibrosis and a decline in diastolic and systolic function [22]. All of these effects were improved by treatment with dapagliflozin. Under high glucose conditions, cardiomyocytes showed significant activation of apoptosis, reactive oxygen species, and endoplasmic reticulum (ER) stress–associated proteins, which were attenuated by the coincubation with dapagliflozin.
Adingupu et al. studied metabolic changes and CV function in ob/ob-/- mice, a model for obesity and reduced glucose tolerance characterized by systolic microvascular dysfunction [23]. Compared to untreated animals, those administered empagliflozin showed improved cardiac contractility as well as coronary microvascular function as assessed by coronary flow velocity reserve (CFVR) and fractional area change.
Human studies
Cardiac contractility and CFVR were studied by Suhrs et al. in a randomized, placebo-controlled cross-over study in 13 patients with T2D treated with empagliflozin 25 mg or placebo for 12 weeks [24]. No substantial changes in CVFR were demonstrated, which may have been related to the short time of treatment.
Shih et al. examined changes in left ventricular function in patients with diabetes who did not have symptomatic HF following 6 months of treatment with dapagliflozin [22]. Both diastolic function and systolic function estimated with longitudinal strain improved despite no substantial changes in left ventricular ejection fraction (LVEF). Contrasting data have been obtained regarding reduction of ventricular mass. Changes in cardiac structure and function have been examined by cardiac magnetic resonance in patients with T2D treated with empagliflozin for 6 months and compared to a control group of patients with T2D not administered a SGLT-2i [25]. In patients receiving empagliflozin, a significant reduction in left ventricular end diastolic volume was seen, from a mean of 155 mL at baseline to 145 mL at 6 months, while no differences in left ventricular mass, ejection fraction, or heart rate were observed in either group. In contrast, Verma et al. found significant changes in left ventricular mass following treatment with empagliflozin [26]. In their study, 97 patients with T2D and CAD were randomized to empagliflozin or placebo for 6 months. Mean left ventricular mass decreased by 2.6 g/m2 with empagliflozin compared to 0.01 g/m2 in patients receiving placebo.
Kayano and colleagues investigated changes in diastolic function during exercise, measuring left ventricular filling pressure (LVFP) and right ventricular systolic pressure (RVSP) in 78 patients with T2D and poor glycemic control [27]. Participants were randomized to dapagliflozin or conventional add-on therapy for 6 months. Significant improvement of diastolic function was seen with decreases in both RVSP and LVFP at 6 months in patients treated with the SGLT-2i, while no changes were seen on the control group. No differences were observed in stroke volume index and cardiac index in either group.
Diastolic function has also been examined by Rau et al. who randomized 40 patients with T2D to empagliflozin or placebo for 3 months [28]. Empagliflozin had no effect on the systemic vascular resistance index, cardiac index, stroke volume index at any time point, and there was no difference in LVEF. However, empagliflozin was seen to significantly improve diastolic function (defined as reduction of early mitral inflow velocity relative to early diastolic left ventricular relaxation [E/e’]) that was significant after the first day of treatment.
Ikonomidis et al. investigated the effects of a number of glucose-lowering therapies on cardiac hemodynamic parameters [29]. Altogether, 160 patients with T2D were randomized to insulin, liraglutide, empagliflozin, or liraglutide + empagliflozin as add-on to metformin (40 patients in each group) for 12 months. At study end, perfused boundary region, a biomarker of endothelial integrity, pulse wave velocity (PWV), and systolic function estimated as global strain (longitudinal, circumferential, and radial) were improved in all subgroups of patients. However, those receiving liraglutide, empagliflozin, or the combination had a greater reduction of perfused boundary region, PWV, and systolic blood pressure compared to those receiving insulin, Global work index was improved in those receiving liraglutide alone or in combination (12.7% and 17.4%) compared with insulin or empagliflozin (3.1% and 2%). Patients receiving empagliflozin alone or in combination had a significantly greater decrease in PWV (10.1% and 13%) and central and brachial systolic blood pressure compared to insulin or liraglutide (PWV, 3.6% and 8.6%). Thus, the effects of an SGLT-2i were potentiated when used in combination with a GLP-1 RA.
Impedance cardiography is a non-invasive method that allows calculation of stroke volume, cardiac output, and other hemodynamic variables [30]. The technique has been used to study 33 patients with T2D and without established CV disease or HF who were randomized to either dapagliflozin or placebo for 12 weeks. At study end, no significant changes were seen in stroke volume, cardiac output, or cardiac index. Likewise, no changes were seen in any measures of systolic or circulatory function after treatment. The lack of effects on these parameters may be due to the short observation period and/or a sample size that is not sufficiently powered to detect differences in these measures.
Effects in heart failure with preserved ejection fraction: animal and human studies
Animal and human studies in heart failure with preserved ejection fraction are listed in Table 2.
Animal studies
Habibi et al. reported that empagliflozin improves diastolic function in obese female db/db mice [31]. In particular, these animals were fed chow with or without empagliflozin for 5 weeks. In addition to improvements in glycemic parameters, empagliflozin was associated with improved left ventricular filling pressure (< E/E’ ratio) and less interstitial fibrosis compared to control animals. Improved diastolic function with empagliflozin was also observed in a nondiabetic model of HFpEF [32]. In addition, studies in myocardial fibers from patients and rats with diastolic HFpEF have shown that empagliflozin reduced passive stiffness of myofilaments through increases in phosphorylation of myofilament regulatory proteins [33].
In a hypertensive model of HFpEF in rats, empagliflozin was associated with significantly decreased cardiac fibrosis in atrial and ventricular tissues, and the atrial and ventricular expression of several molecules including PPARα, natriuretic peptides, and TNF-α were normalized [34]. In a similar model, rats were administered dapagliflozin for 6 weeks [35]. As monitored by echo-Doppler and heart catheterization, significant improvement in diastolic function was seen. Treatment with dapagliflozin also reduced diastolic overload of Ca2+ and Na+. Dapagliflozin further inverted endothelial activation, and reduced cardiac inflammatory markers such as NFkB and IL-6 as well as pro-fibrotic signaling via TGF-b.
A study in a hypertension/hyperlipidemia-induced HFpEF pig model, dapagliflozin administered for 9 weeks significantly diminished cardiac concentric remodeling, although no improvements in diastolic function were observed [36]. Moreover, elevations in the inflammatory cytokines IL-6 and TNF-α were seen in aortic tissue in control pigs that were inhibited by dapagliflozin. Thus, multiple benefits in the coronary epithelium were observed. In an obesity model of HFpHF in rats, empagliflozin was also found to significantly suppress increased levels of ICAM-1, VCAM-1, TNF-α, and IL-6 in myocardium [37]. In addition, higher levels of oxidative stress-dependent activation of eNOS and PKGIα oxidation were seen, which were attenuated by empagliflozin. Levels of NO, cGMP, and PKGIα in HFpEF were all increased during treatment with empagliflozin, which was associated with augmented phosphorylation of myofilament proteins. Moreover, oxidative stress and PKGIα polymerization was found to correlate with increased cardiomyocyte stiffness and diastolic dysfunction in patients with HFpEF.
Lastly, HMGB1 appears to serve as a driver of inflammation for CV disease [38]. In a mouse model of HFpEF, expression of HMGB1 was found to be increased in cardiac tissue of HFpEF mice, which is decreased by empagliflozin [39].
Human studies
In patients with T2D and stable HF with preserved EF, Soga et al. investigated the effect of dapagliflozin on left ventricular diastolic function [40]. Patients who had been administered at least 1 antidiabetic agent other than an SGLT-2i initiated treatment with dapagliflozin for 6 months. Left ventricular diastolic function ratio of (mitral inflow E to the mitral e’ annular velocities [E/e’]), significantly decreased from 9.3 to 8.5 after 6 months. Multivariate logistic regression analysis found that that dyslipidemia was the only independent factor associated with improvement in E/e’. Moreover, the change in E/e’ after 6 months for patients with dyslipidemia was significantly larger than that for patients without dyslipidemia. In the same patients, improvements in global longitudinal strain were also observed [41].
Effects in heart failure with reduced ejection fraction: animal and human studies
Animal studies
Several animal studies have attempted to shed additional light on the mechanisms behind the cardiac benefits of SGLT-2i in patients with HF (Table 3). In a diabetes mice model of ischemic cardiomyopathy, Ideishi et al. treated animals with vehicle, empagliflozin, linagliptin, and empagliflozin + linagliptin before inducing myocardial ischemia for 30 min [42]. Combination therapy was found to significantly preserve cardiac systolic function as monitored by left ventricular volume at the peak left ventricular ejection rate. Combination therapy also appeared to have an anti-fibrotic effect compared to the control group that was not dependent on blood glucose levels.
SGLT-2i inhibitors appear to be of benefit when given both before and after MI. For example, in another model of HFrEF due to ischemic cardiomyopathy, pressure-volume (P-V) relationship analysis was used to examine changes in intrinsic cardiac function in animals with experimental MI [43]. Following confirmation of infarct size at 1 week post-infarction, diabetic mice received either empagliflozin or vehicle for 6 weeks. Load-insensitive measures of cardiac function were found to be improved with empagliflozin compared to vehicle. In addition, load-independent measures of cardiac contractility, preload recruitable stroke work, and end-systolic pressure volume relationship were all greater in animals randomized to empagliflozin. There was also a reduction in left ventricular end-diastolic pressure with empagliflozin.
The effects of SGLT-2i have also been evaluated in non-diabetic experimental models. In one study, non-diabetic rats underwent sham surgery or permanent coronary artery ligation to induce MI [44]. The animals received empagliflozin-containing chow (2 weeks before or 2 weeks after surgery, or control chow). While empagliflozin had no effect on the size of the MI, the LVEF was significantly higher in both groups receiving empagliflozin either 2 weeks before or 2 weeks after surgery compared to vehicle. Empagliflozin also reduced cardiomyocyte hypertrophy, and decreased both interstitial fibrosis and oxidative stress in the myocardium. A number of metabolic changes were observed that were associated with an increase in cardiac ATP production. These included an increase in circulating ketone levels and myocardial expression of the ketone body transporter and key ketogenic enzymes.
Mechanistic insights have also been provided. In a nondiabetic rat model of ischemic HF, animals were administered, or not, empagliflozin immediately after induction of MI [45]. In animals treated with empagliflozin, collagen deposition was found to be significantly lower in both the scar and remote cardiac areas. Expression of TGF-β1 and Smad3 were both decreased by empagliflozin. Thus, empagliflozin decreased myocardial fibrosis possibly through inhibition of the TGF-β1/Smad3 pathway.
Cardiac contractile function and myocardial substrate utilization has been assessed in lean swine that received canagliflozin or placebo 24 h before temporary occlusion of the circumflex coronary artery. Invasive study protocol was performed at baseline, during.
Coronary occlusion and during 2 h of reperfusion [46]. At the onset of ischemia, canagliflozin was associated with large increases in left ventricular end-diastolic and systolic volumes which during reperfusion returned to baseline levels. Canagliflozin administration further increased end-diastolic volume, stroke volume, and stroke work vs. control animals during ischemia, and augmented cardiac work efficiency during ischemia. It was concluded that canagliflozin provides protection from ischemia that is independent of changes in utilization of myocardial substrate given that no changes seen in myocardial uptake of glucose, lactate, ketones, or free fatty acid were observed.
In an investigation in healthy normoglycemic rats, at 30 min after the administration of empagliflozin, increases in ventricular systolic pressure, mean pressure, and the max dP/dt were observed [47]. Empagliflozin given for one week was found to increase cardiac output, stroke volume, and fractional shortening. At 7 days after induction of MI through ligation of the left coronary artery, empagliflozin improved global longitudinal strain compared to vehicle (-21.0% vs. -16.6%, respectively). Analysis of infarcted tissues showed that empagliflozin decreased the expression of matrix metalloproteinase 9 (MMP9) and also regulated the cardiac transporters SERCA2a and NHE1. In vitro, empagliflozin decreased the activity of MMP2 and MMP9 and also prevented apoptosis. mTOR signaling has also been implicated in the cardioprotective effects of SGLT-2i.
mTOR has also been implicated in a mouse model of cardiac dysfunction induced by sunitinib that was used to study the protective effects of empagliflozin [48]. Mice treated with sunitinib showed decreased LVEF compared to control animals (50.2% vs. 84.9%, respectively) that was attenuated with administration of empagliflozin (76.18%). In cardiomyocytes studied in vitro, it was found that sunitinib led to cell death and dysfunction of AMPK-mTOR signaling that were reversed by the addition of empagliflozin. The PI3K/AKt/mTOR signaling pathway was also implicated in another in vitro study in which H9C2 cells were exposed to hypoxia/reoxygenation to simulate ischemia/reperfusion injury [49]. Co-administration of rosuvastatin and dapagliflozin not only significantly enhanced cell viability, but upregulated the expression of p-PI3K, p-Akt, p-mTOR, and Bcl-2.
In a model in rats in which mitral regurgitation (MR) induced HFrEF, animals were given no treatment or dapagliflozin for 6 weeks [50]. Dapagliflozin partially rescued MR-induced dysfunction that included partial restoration of LVEF and the end-systolic pressure volume relationship. Animals administered dapagliflozin also showed significantly diminished cardiac fibrosis, cardiac apoptosis, and expression of stress-associated proteins in the endoplasmic reticulum.
In another animal model of non-ischemic HFrEF, transverse aortic constriction (TAC) was used to induce cardiac remodeling [51]. Dapagliflozin was then administered to some of the animals for 4 weeks. Compared to those not receiving dapagliflozin, treatment with the SGLT-2i decreased myocardial hypertrophy, cardiomyocyte apoptosis, perivascular and myocardial interstitial fibrosis. Dapagliflozin also inhibited phosphorylation of the P38 and JNK proteins and inhibited phosphorylation of FoxO1, which regulate cellular pathways involved in the development of cardiac failure, including fibrosis, apoptosis, inflammatory responses, and cell proliferation, in untreated mice that was reversed by administration of dapagliflozin.
Inflammation has been clearly linked in the CV benefits of SGLT-2i. In the same model of TAC-induced HFrEF, treatment with inhibitor empagliflozin confirmed the effects on lessened left ventricular enlargement [52]. Furthermore, empagliflozin reduced the expression of Tnfrsf12a, a TNF superfamily receptor with proinflammatory, and prohypertrophic effects.
In an animal model of diabetes using male BTBR ob/ob mice, dapagliflozin, ticagrelor, or the combination for were administered for 12 weeks, both agents decreased progression of diabetic cardiomyopathy as shown by amelioration in left ventricular end-systolic and end-diastolic volumes as well as LVEF [53]. Both drugs also diminished activation of the NOD-like receptor 3 inflammasome. Compared to controls, significant decreases myocardial TNF-α and IL-6 were seen with both drugs alone or in combination. In nondiabetic mice with established HFrEF, Byrne et al. reported that the empagliflozin diminished activation of the NLRP3 inflammasome [54]. In addition, the same effects on the NLRP3 inflammasome were also observed in animals with HFpEF. Since a calcium ionophore blocked the ability of empagliflozin to reduce inflammation, it was suggested that empagliflozin exerts its benefit in a calcium dependent manner.
Lastly, changes in myocardial energetics were also observed in a nondiabetic swine model of HFrEF [55]. In contrast to control animals, pigs treated with empagliflozin showed increased uptake of ketone bodies, free fatty acids, and branched chain amino acids instead of glucose, as well as enhanced expression of the enzymes used for their metabolism. Thus, increases in myocardial ATP were observed together with improved myocardial work efficiency. In the same model of TAC-induced HFrEF, Li et al. reported that empagliflozin increased glucose and fatty acid oxidation in failing hearts, while reducing glycolysis [56]. This attenuated adverse cardiac remodeling and progression of heart failure reestablishing activation of adenosine monophosphate-activated protein kinase.
Human studies
SGLT-2i have been extensively studied in patients with HFrEF with or without diabetes. Cardiac energetics have been shown to be improved by SGLT-2i in patients with T2D [57]. After treatment, improvements were observed in LVEF, global longitudinal strain, and mean myocardial cell volume. In addition, empagliflozin was associated with significant improvement in the phosphocreatine-to-ATP ratio, from 1.52 to 1.76. The authors suggested that empagliflozin gives rise to a “cardiac energy-deficient” state that also serves to help reverse myocardial cellular remodeling and improve cardiac function.
Chan et al. published the results of a study in which they enrolled 665, 119, and 132 patients with T2D and preserved (≥ 50%), moderately reduced (40–50%), and reduced baseline LVEF (< 40%) who were receiving with SGLT-2i [58]. In addition, 212 patients receiving a DPP-4i were enrolled. After a median of 230 days, in those with reduced LVEF and treated with a SGLT-2i, LVEF improved from 29.4 to 42.2% and decrease in left ventricle end systolic volume (LVESV) decreased from 133.2 mL to 117.4 mL. In those with moderately reduced LVEF, the LVEF improved from 44.8 to 49.7%, while LVESV decreased from 90.7 mL to 80.0 mL. In contrast, in patients with preserved LVEF, there was no improvement in LVEF or LVESV following treatment with a SGLT-2i. In patients who had impaired LVEF (< 50%) at baseline and receiving a DPP-4i, there were no changes LVEF or LVESV. Thus, LV systolic function improved in patients with T2D and severe and moderately reduced LVEF after treatment with an SGLT-2i.
The SUGAR-DM-HF trial randomized 105 patients with T2D/prediabetes and HFrEF (77.1% with NYHA II and 22.9% with NYHA III) to empagliflozin or placebo and followed for 36 weeks [59]. In patients treated with empagliflozin, favorable reverse LV remodeling was observed: the LV end-systolic volume index was significantly reduced by 6.0 mL/m2 compared to placebo, and the LV end-diastolic volume index by 8.2 mL/m2 (P = 0.0042). No difference was seen in LV global longitudinal strain.
Ilyas et al. investigated the effects of two weeks of therapy with dapagliflozin in 19 patients with T2D and HFrEF in a placebo-controlled crossover trial [60]. While reductions in blood pressure were seen as expected, no functional effects on cardiac function were observed considering chamber size, ventricular systolic function, and filling pressure. However, it must be highlighted that the time on the SGLT-2i was limited to two weeks.
In patients without diabetes and HFrEF, Santos-Gallego carried out a study in which 84 participants were randomized to empagliflozin or placebo for 6 months [61]. Empagliflozin was associated with a significant decrease in LV end-diastolic volume compared to placebo (-25.1 mL vs. -1.5 mL, respectively) and LV end-systolic volume (-26.6 mL vs. -0.5 mL, respectively). Moreover, compared to placebo significant reduction in LV mass was seen (-17.8 g vs. 4.1 g, respectively) as well as improvement in LVEF (6.0 vs. -0.1, respectively). Compared to placebo, empagliflozin also improved peak O2 consumption, oxygen uptake efficiency slope, and the 6-min walk test.
In a post hoc analysis of the randomized Empire HF trial involving 190 patients with HFrEF (12.6% with T2D), empagliflozin was associated with significantly reduced LVESV (-4.3 mL/m2), left ventricular end-diastolic volume (-5.5 mL/m2), and left atrial volume (-2.5 mL/m2) compared with placebo after 12 weeks, although no change in LVEF was seen [62]. As noted by the authors, further study is warranted to examine the effects of empagliflozin beyond 12 weeks. Considering longer treatment times, Hwang et al. followed 304 patients with T2D for a median of 13 months [63]. Changes in cardiac function were investigated in 4 groups of patients: group 1 (without HF nor SGLT-2i), group 2 (without HF and received SGLT-2i), group 3 (with HF but no SGLT-2i), and group 4 (with HF and received SGLT-2i). Patients with HF being treated with an SGLT-2i showed a significant decrease in left ventricular end-diastolic dimension in addition to a significant improvement in the LVEF. LV mass index and diastolic parameters were also improved in HF patients and administered an SGLT-2i. The improvements in cardiac function seen in patients receiving an SGLT-2i were less prominent in patients without HF and in those with HFpEF. The effects of dapagliflozin on echocardiographic parameters have also been examined in 30 patients with diabetes and HFrEF [64]. The addition of dapagliflozin improved both systolic and diastolic function.
The effects of empagliflozin on the ratio of pulmonary capillary wedge pressure (PCWP) to cardiac index (CI) at peak exercise have been studied in 70 patients with HFrEF (mean LVEF 26%, 17% with T2D) [65]. While no significant effect on peak PCWP/CI was observed, PCWP was significantly reduced (-2.40 mm Hg) without improvement of CI. Reductions were seen in patients with and without T2D. Mullens et al. investigated the effects of dapagliflozin on systolic pulmonary artery pressure (PAP) with an implantable system that provides real-time remote monitoring of pressure [66]. Changes in PAP were monitored in 9 patients with HFrEF before and after 7 days of therapy with dapagliflozin. Mean PAP decreased from 42 mmHg to 38 mmHg during therapy with dapagliflozin. Of note, the reduction in PAP occurred within the first 2 days of treatment and remained stable throughout the entire study period.
A secondary analysis of patients enrolled in the EMPA-TROPISM trial investigating empagliflozin in patients without diabetes and HFrEF evaluated the effect of SGLT2i in cardiac remodeling [67]. Empagliflozin treatment was significantly associated with a reduction in epicardial adipose tissue (-5.14 mL vs. -0.75 mL), interstitial myocardial fibrosis estimated by T1 mapping (-1.25% vs. 0.24%) and cardiomyocyte volume (-11.08 mL vs. 0.80 mL). Proteomic analysis furthermore demonstrated that empagliflozin was associated with a significant reduction in several markers of inflammation, including E-selectin and TNFRSF10C.
Discussion
Effects in patients with diabetic cardiomyopathy
Diabetes mellitus, even in the absence of other risk factors such as arterial hypertension, obesity, and coronary artery diseaseis known to be associated with high risk for CV complications. The main cardiac morphofunctional alterations associated with diabetes are hypertrophy and diastolic dysfunction [68]. A number of mechanisms have been proposed to explain these effects, and many have been implicated to rationalize the cardiac benefits of SGLT-2i in patients with diabetes as seen herein.
In a study of the effects of SGLT-2i in patients with or without established CV disease no significant changes in cardiac stroke volume of output were evident [30]. Furthermore, although the number of studies analyzing systolic function in patients with diabetes is limited, SGLT-2i would not seem to directly affect this parameter [24, 30].
In contrast, diastolic function does appear to be directly influenced by SGLT-2i [22, 25, 28]. In addition, significant decreases in left ventricular mass were documented following treatment with empagliflozin [26]. This is potentially relevant considering that increased left ventricular mass and diastolic dysfunction have both been associated with associated with endothelial dysfunction [69]. An animal study documented that empagliflozin improved coronary microvascular dysfunction [23], even if no substantial changes were observed in a study in humans considering CVFR, although the treatment period may have been too short to expect any significant changes [24]. It is thus possible that SGLT-2i, by improving endothelial dysfunction, can inhibit negative cardiac remodeling and thus improve diastolic function.
Effects in heart failure with preserved EF
The benefits of SGLT-2i have also been confirmed in the context of HFpEF both with and without diabetes. Animal models have largely confirmed these findings with improvement in LV diastolic function in animals with and without diabetes [31, 32]. Similar to studies in HFrEF, benefits in several cardiac inflammatory markers have been observed during administration of an SGLT-2i in HFpEF [34,35,36,37,38,39]. SGLT-2i thus appear to regulate inflammatory processes, which are a known pathological element in HFpEF, and may also reduce fibrosis [70]. Among the mechanisms involved in the development of HFpEF, metabolic dysfunction and inflammation are now known to have major roles [71]. Metabolic alterations and oxidative stress are capable of activating low-grade inflammation which in turn activates pathways involved in hypertrophy and fibrosis and therefore diastolic dysfunction. SGLT-2i have been shown to modulate several inflammatory pathways by reducing the level of circulating cytokines and reduce oxidative stress [34, 36,37,38,39]. Even if limited, studies in patients with diabetes and HFpEF suggest that diastolic function is improved by administration of an SGLT-2i [40, 41].
Effects in heart failure with reduced EF
Diabetes and HFrEF are risk factors for each other and can bidirectionally independently worsen outcomes. Indeed, diabetes is a common concurrent diagnosis with HFrEF and increases the risk of HF by two- to four-fold [72]. At the same time, patients with HFrEF and diabetes have worse prognosis compared to those without diabetes [73]. SGLT-2i have been shown to significantly reduce MACE in patients with HFrEF and at the same time to reverse the most important morphofunctional alterations typical of HFrEF [74].
SGLT-2i appear to be associated with positive LV remodeling and with improvement in the LVEF along with reduced end-systolic volume [58, 59], although such changes have not been consistently seen across studies [62]. In this regard, it should be highlighted that the duration of therapy with an SGLT-2i was not consistent in these various analyses, which may help to explain some of the apparent discrepancies reported such as the 2-week study by Ilyas et al. [60].
Metabolic changes have also been noted in addition to improvement in LVEF, which may lead to benefits in cardiomyocyte contractility [57].
Effects in HFrEF without diabetes
The effects of SGLT-2i have been more amply demonstrated in the context of HFrEF in the absence of diabetes. SGLT-2i have been shown to improve systolic function [61, 63] and myocardial oxygen consumption [61] and reduce LV mass [61, 62]. They have also been demonstrated to reduce PCWP.
Considering cardiac hemodynamics, SGLT-2i seem to be associated with improvements in systolic function, and as a consequence pulmonary hypertension, that appear to be rapid, in contrast to the effects on LVEF remodeling and mass [65, 66]. SGLT-2i have been further shown to prevent post-infarct cardiac remodeling through reduction of end-diastolic pressure [43] and improve LVEF, along with reduced cardiomyocyte hypertrophy and improved cardiac ATP production [44,45,46,47, 49,50,51]. These effects may possibly be due, at least in part, to improvement in cardiac energetics by increasing ketone bodies and promoting beta-oxidation with consequent improvements in cardiomyocyte contractility [55,56,57].
Conclusions
Large CVOTs initially confirmed the CV benefits of SGLT-2i in patients with T2D [8,9,10,11]. However, the observed CV benefits cannot be explained solely by the effects of SGLT-2i on glycemic control and many studies have been carried out to better understand their mechanism of action. The present review aimed to focus on the direct effects of SGLT-2i on cardiac structure and function in different pathological contexts, and highlights the pleiotropic effects of this class of drugs.
In diabetic heart disease, in both clinical and animal models, the effect of SGLT-2i have been shown to improve diastolic function, which is even more evident in HFpEF where it represents a key pathogenetic element. The probable pathogenic mechanisms likely involve damage from free radicals, apoptosis, and inflammation, and therefore fibrosis, many of which have been shown to be improved by SGLT-2i. While the effects on systolic function in models of diabetic heart disease and HFpEF is limited and contrasting, it is a key aspect in HFrEF, both with and without diabetes. The significant improvement in systolic function appears to lead to subsequent structural remodeling of the heart with a reduction in the LV volume and a consequent reduction in pulmonary pressure [41, 43, 58, 59, 61, 63, 65, 66, 75]. The effects on cardiac metabolism and inflammation appear to be consolidated, although greater study is needed to further define the entity to which these mechanisms contribute to the CV benefits of SGLT-2i.
Data Availability
Not applicable.
Abbreviations
- CV:
-
Cardiovascular
- CVOTs:
-
Cardiovascular outcome trials
- ER:
-
Endoplasmic reticulum
- GLP-1RA:
-
GLP-1 receptor agonists
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with preserved ejection fraction
- HFrEF:
-
Heart failure with reduced ejection fraction
- LFEF:
-
Left ventricular ejection fraction
- LV:
-
Left ventricle
- LVFP:
-
Left ventricular filling pressure
- PWV:
-
Pulse wave velocity
- RSVP:
-
Right ventricular systolic pressure
- SGLT-2i:
-
SGLT-2 inhibitors
- T2D:
-
Type 2 diabetes
References
White JR. Jr. A brief history of the development of diabetes medications. Diabetes Spectr. 2014;27(2):82–6.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A Consensus Report by the american Diabetes Association (ADA) and the European Association for the study of diabetes (EASD). Diabetes Care. 2022;45(11):2753–86.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm – 2019 executive Summary. Endocr Pract. 2019;25(1):69–100.
Mannucci E, Candido R, Delle Monache L, et al. Italian guidelines for the treatment of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2022;32(4):770–814.
Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: an update of Mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38.
Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83.
Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the management of patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e72–e227.
Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 diabetes. N Engl J Med. 2020;383(15):1425–35.
Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57.
Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9.
McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008.
Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with Empagliflozin in Heart failure. N Engl J Med. 2020;383(15):1413–24.
Lin Y, Cai Z, Yuan J, et al. Effect of pharmacological treatment on outcomes of heart failure with preserved ejection fraction: an updated systematic review and network meta-analysis of randomized controlled trials. Cardiovasc Diabetol. 2022;21(1):237.
Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819–29.
Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–28.
Giaccari A. Sodium-glucose co-transporter inhibitors: medications that mimic fasting for cardiovascular prevention. Diabetes Obes Metab. 2019;21(10):2211–8.
Garla VV, Butler J, Lien LF. SGLT-2 inhibitors in Heart failure: guide for Prescribing and Future Perspectives. Curr Cardiol Rep. 2021;23(6):59.
Grubic Rotkvic P, Cigrovski Berkovic M, Bulj N, et al. Sodium-glucose cotransporter 2 inhibitors’ mechanisms of action in heart failure. World J Diabetes. 2020;11(7):269–79.
Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61(10):2108–17.
Shih JY, Lin YW, Fisch S, et al. Dapagliflozin suppresses ER stress and improves subclinical myocardial function in diabetes: from Bedside to Bench. Diabetes. 2021;70(1):262–7.
Adingupu DD, Gopel SO, Gronros J, et al. SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob(-/-) mice. Cardiovasc Diabetol. 2019;18(1):16.
Suhrs HE, Nilsson M, Bove KB, et al. Effect of empagliflozin on coronary microvascular function in patients with type 2 diabetes mellitus-A randomized, placebo-controlled cross-over study. PLoS ONE. 2022;17(2):e0263481.
Cohen ND, Gutman SJ, Briganti EM, et al. Effects of empagliflozin treatment on cardiac function and structure in patients with type 2 diabetes: a cardiac magnetic resonance study. Intern Med J. 2019;49(8):1006–10.
Verma S, Mazer CD, Yan AT, et al. Effect of Empagliflozin on Left Ventricular Mass in patients with type 2 diabetes Mellitus and Coronary Artery Disease: the EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation. 2019;140(21):1693–702.
Kayano H, Koba S, Hirano T, et al. Dapagliflozin Influences Ventricular Hemodynamics and Exercise-Induced Pulmonary hypertension in type 2 diabetes Patients- A Randomized Controlled Trial. Circ J. 2020;84(10):1807–17.
Rau M, Thiele K, Hartmann NK, et al. Empagliflozin does not change cardiac index nor systemic vascular resistance but rapidly improves left ventricular filling pressure in patients with type 2 diabetes: a randomized controlled study. Cardiovasc Diabetol. 2021;20(1):6.
Ikonomidis I, Pavlidis G, Thymis J, et al. Effects of Glucagon-Like Peptide-1 receptor agonists, sodium-glucose Cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes Mellitus after 12-Month treatment. J Am Heart Assoc. 2020;9(9):e015716.
Bonora BM, Vigili de Kreutzenberg S, Avogaro A, et al. Effects of the SGLT2 inhibitor dapagliflozin on cardiac function evaluated by impedance cardiography in patients with type 2 diabetes. Secondary analysis of a randomized placebo-controlled trial. Cardiovasc Diabetol. 2019;18(1):106.
Habibi J, Aroor AR, Sowers JR, et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol. 2017;16(1):9.
Connelly KA, Zhang Y, Visram A, et al. Empagliflozin improves diastolic function in a nondiabetic rodent model of heart failure with preserved ejection fraction. JACC Basic Transl Sci. 2019;4(1):27–37.
Pabel S, Wagner S, Bollenberg H, et al. Empagliflozin directly improves diastolic function in human heart failure. Eur J Heart Fail. 2018;20(12):1690–700.
Lee HC, Shiou YL, Jhuo SJ, et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc Diabetol. 2019;18(1):45.
Cappetta D, De Angelis A, Ciuffreda LP et al. Amelioration of diastolic dysfunction by dapagliflozin in a non-diabetic model involves coronary endothelium. Pharmacol Res. 2020;157104781.
Zhang N, Feng B, Ma X, et al. Dapagliflozin improves left ventricular remodeling and aorta sympathetic tone in a pig model of heart failure with preserved ejection fraction. Cardiovasc Diabetol. 2019;18(1):107.
Kolijn D, Pabel S, Tian Y, et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase galpha oxidation. Cardiovasc Res. 2021;117(2):495–507.
Wahid A, Chen W, Wang X et al. High-mobility group box 1 serves as an inflammation driver of cardiovascular disease. Biomed Pharmacother. 2021;139111555.
Zhang XL, Wang TY, Chen Z, et al. HMGB1-Promoted Neutrophil Extracellular Traps Contribute to Cardiac Diastolic Dysfunction in mice. J Am Heart Assoc. 2022;11(4):e023800.
Soga F, Tanaka H, Tatsumi K, et al. Impact of Dapagliflozin on the left ventricular diastolic function in Diabetic patients with heart failure complicating Cardiovascular Risk factors. Intern Med. 2021;60(15):2367–74.
Tanaka H, Soga F, Tatsumi K, et al. Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovasc Diabetol. 2020;19(1):6.
Ideishi A, Suematsu Y, Tashiro K, et al. Combination of Linagliptin and Empagliflozin preserves cardiac systolic function in an Ischemia-Reperfusion Injury mice with diabetes Mellitus. Cardiol Res. 2021;12(2):91–7.
Connelly KA, Zhang Y, Desjardins JF, et al. Load-independent effects of empagliflozin contribute to improved cardiac function in experimental heart failure with reduced ejection fraction. Cardiovasc Diabetol. 2020;19(1):13.
Yurista SR, Sillje HHW, Oberdorf-Maass SU, et al. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail. 2019;21(7):862–73.
Daud E, Ertracht O, Bandel N, et al. The impact of empagliflozin on cardiac physiology and fibrosis early after myocardial infarction in non-diabetic rats. Cardiovasc Diabetol. 2021;20(1):132.
Baker HE, Kiel AM, Luebbe ST, et al. Inhibition of sodium-glucose cotransporter-2 preserves cardiac function during regional myocardial ischemia independent of alterations in myocardial substrate utilization. Basic Res Cardiol. 2019;114(3):25.
Goerg J, Sommerfeld M, Greiner B et al. Low-dose Empagliflozin improves systolic heart function after myocardial infarction in rats: regulation of MMP9, NHE1, and SERCA2a. Int J Mol Sci. 2021;22(11).
Ren C, Sun K, Zhang Y et al. Sodium-glucose CoTransporter-2 inhibitor Empagliflozin ameliorates Sunitinib-Induced Cardiac Dysfunction via Regulation of AMPK-mTOR Signaling pathway-mediated autophagy. Front Pharmacol. 2021;12664181.
Gong L, Wang X, Pan J, et al. The co-treatment of rosuvastatin with dapagliflozin synergistically inhibited apoptosis via activating the PI3K/AKt/mTOR signaling pathway in myocardial ischemia/reperfusion injury rats. Open Med (Wars). 2021;15(1):47–57.
Lin YW, Chen CY, Shih JY, et al. Dapagliflozin improves Cardiac Hemodynamics and mitigates Arrhythmogenesis in Mitral Regurgitation-Induced Myocardial Dysfunction. J Am Heart Assoc. 2021;10(7):e019274.
Shi L, Zhu D, Wang S, et al. Dapagliflozin attenuates Cardiac Remodeling in mice Model of Cardiac pressure overload. Am J Hypertens. 2019;32(5):452–9.
Yerra VG, Batchu SN, Kabir G, et al. Empagliflozin disrupts a Tnfrsf12a-Mediated feed Forward Loop that promotes left ventricular hypertrophy. Cardiovasc Drugs Ther. 2022;36(4):619–32.
Chen H, Tran D, Yang HC, et al. Dapagliflozin and Ticagrelor have Additive Effects on the attenuation of the activation of the NLRP3 inflammasome and the Progression of Diabetic Cardiomyopathy: an AMPK-mTOR interplay. Cardiovasc Drugs Ther. 2020;34(4):443–61.
Byrne NJ, Matsumura N, Maayah ZH, et al. Empagliflozin blunts worsening Cardiac Dysfunction Associated with reduced NLRP3 (nucleotide-Binding domain-like receptor protein 3) inflammasome activation in Heart failure. Circ Heart Fail. 2020;13(1):e006277.
Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. J Am Coll Cardiol. 2019;73(15):1931–44.
Li X, Lu Q, Qiu Y, et al. Direct cardiac actions of the Sodium glucose co-transporter 2 inhibitor Empagliflozin improve myocardial oxidative phosphorylation and attenuate pressure-overload heart failure. J Am Heart Assoc. 2021;10(6):e018298.
Thirunavukarasu S, Jex N, Chowdhary A, et al. Empagliflozin Treatment is Associated with improvements in Cardiac energetics and function and reductions in Myocardial Cellular volume in patients with type 2 diabetes. Diabetes. 2021;70(12):2810–22.
Chan YH, Hsu TJ, Wang CL, et al. Sodium glucose cotransporter-2 inhibitor was associated with an improvement in left ventricular systolic function in patients with type 2 diabetes mellitus with impaired left ventricular systolic function. ESC Heart Fail. 2020;7(5):2784–96.
Lee MMY, Brooksbank KJM, Wetherall K, et al. Effect of Empagliflozin on Left ventricular volumes in patients with type 2 diabetes, or Prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. 2021;143(6):516–25.
Ilyas F, Jones L, Tee SL, et al. Acute pleiotropic effects of dapagliflozin in type 2 diabetic patients with heart failure with reduced ejection fraction: a crossover trial. ESC Heart Fail. 2021;8(5):4346–52.
Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, et al. Randomized Trial of Empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77(3):243–55.
Omar M, Jensen J, Ali M, et al. Associations of Empagliflozin with Left ventricular volumes, Mass, and function in patients with heart failure and reduced ejection fraction: a Substudy of the Empire HF Randomized Clinical Trial. JAMA Cardiol. 2021;6(7):836–40.
Hwang IC, Cho GY, Yoon YE, et al. Different effects of SGLT2 inhibitors according to the presence and types of heart failure in type 2 diabetic patients. Cardiovasc Diabetol. 2020;19(1):69.
Maragkoudakis S, Marketou M, Katsi V et al. The early effect of dapagliflozin on strain and tissue doppler parameters of diastolic function in diabetic patients with heart failure with reduced ejection fraction. Arch Med Sci Atheroscler Dis. 2021;6e176–e181.
Omar M, Jensen J, Frederiksen PH, et al. Effect of Empagliflozin on Hemodynamics in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2020;76(23):2740–51.
Mullens W, Martens P, Forouzan O, et al. Effects of dapagliflozin on congestion assessed by remote pulmonary artery pressure monitoring. ESC Heart Fail. 2020;7(5):2071–3.
Requena-Ibanez JA, Santos-Gallego CG, Rodriguez-Cordero A, et al. Mechanistic insights of Empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. JACC Heart Fail. 2021;9(8):578–89.
Gollmer J, Zirlik A, Bugger H. Established and emerging mechanisms of Diabetic Cardiomyopathy. J Lipid Atheroscler. 2019;8(1):26–47.
Zizek B, Poredos P. Increased left ventricular mass and diastolic dysfunction are associated with endothelial dysfunction in normotensive offspring of subjects with essential hypertension. Blood Press. 2007;16(1):36–44.
Simmonds SJ, Cuijpers I, Heymans S et al. Cellular and Molecular differences between HFpEF and HFrEF: a step ahead in an Improved Pathological understanding. Cells. 2020;9(1).
Schiattarella GG, Rodolico D, Hill JA. Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovasc Res. 2021;117(2):423–34.
Echouffo-Tcheugui JB, Zhang S, Florido R, et al. Duration of diabetes and Incident Heart failure: the ARIC (Atherosclerosis Risk in Communities) study. JACC Heart Fail. 2021;9(8):594–603.
Dunlay SM, Givertz MM, Aguilar D, et al. Type 2 diabetes Mellitus and Heart failure: a Scientific Statement from the American Heart Association and the heart failure society of America: this statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019;140(7):e294–e324.
Abdelmasih R, Abdelmaseih R, Thakker R, et al. Update on the Cardiovascular benefits of sodium-glucose co-transporter-2 inhibitors: mechanism of action, available agents and Comprehensive Review of Literature. Cardiol Res. 2021;12(4):210–8.
Tanaka H, Hirata KI. Potential impact of SGLT2 inhibitors on left ventricular diastolic function in patients with diabetes mellitus. Heart Fail Rev. 2018;23(3):439–44.
Acknowledgements
Editorial and medical writing assistance was provided by Alessia Scotton (Editorial Project Manager, EDRA S.p.A, Milan, Italy) and Patrick Moore, PhD, and supported by an unconditioned grant from AstraZeneca.
Funding
None.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design, data collection, analysis and interpretation of results, draft manuscript preparation: Panico and Pintaudi. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
C. Panico: no conflicts of interest. E. Bonora: lecture or advisory board fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dome, Mundipharma, Novo Nordisk, Sanofi, Takeda. A. Camera: no conflicts of interest. N.C. Chilelli: no conflicts of interest. G. Da Prato: no conflicts of interest. G. Favacchio: no conflicts of interest. V. Grancini: no conflicts of interest. V. Resi; no conflicts of interest. M. Rondinelli: no conflicts of interest. E. Zarra: no conflicts of interest. B. Pintaudi: lectures and congresses, Novo Nordisk, Eli Lilly; advisory board MSD.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Panico, C., Bonora, B., Camera, A. et al. Pathophysiological basis of the cardiological benefits of SGLT-2 inhibitors: a narrative review. Cardiovasc Diabetol 22, 164 (2023). https://doi.org/10.1186/s12933-023-01855-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-01855-y