Abstract
Background
This study compared the risks of cardiovascular morbidity and mortality between patients with type 2 diabetes (T2D) with and without microvascular diseases, and between matched patients with microvascular diseases.
Methods
We identified newly diagnosed type 2 diabetes patients from National Health Insurance Research Database in Taiwan from January 1, 2008, to December 31, 2019. Propensity score matching was applied to construct matched pairs of patients with diabetic kidney disease, retinopathy, or neuropathy. Multivariable Cox proportional-hazard models were adopted to compare the risks of cardiovascular morbidity and mortality.
Results
Patients with microvascular disease had a significantly higher risk of cardiovascular morbidities and mortality than those without microvascular disease. Among the matched cohorts, patients with diabetic retinopathy had a significantly higher risk of stroke development than those with diabetic kidney disease (aHR 1.11, 95%CI 1.03–1.2). Diabetic neuropathy showed a significantly higher risk of stroke development than diabetic kidney disease (aHR 1.17, 95%CI 1.1–1.25) and diabetic retinopathy (aHR 1.12, 95%CI 1.03–1.21). Diabetic retinopathy had a significantly higher risk of incident heart failure than diabetic kidney disease (aHR 1.43, 95%CI 1.3–1.57), and diabetic neuropathy had a significantly lower risk of incident heart failure than diabetic retinopathy (aHR 0.79, 95%CI 0.71–0.87).
Conclusions
T2D patients with microvascular disease have a significantly higher risk of cardiovascular morbidities and mortality than those without microvascular disease. In the matched cohorts, diabetic neuropathy was significantly associated with stroke development, and diabetic retinopathy had a significant association with heart failure compared to other microvascular diseases.
Similar content being viewed by others
Background
According to the International Diabetes Federation (IDF) Diabetes Atlas 2021, there are 537 million adults (20–79 years) with diabetes mellitus (DM) worldwide; this prevalence rate is about 3.56 times that of 2000 (151 million), and the estimated prevalence will jump to a startling 783 million in 2045 [1]. Stroke, myocardial infarction, and heart failure are the main macrovascular complications of diabetes mellitus, which can lead to devastating premature death [2]. Diabetic kidney disease (DKD), retinopathy, and neuropathy are major microvascular complications of diabetes mellitus, which can lead to dialysis, blindness, and amputation [3, 4]. These major microvascular complications can affect the daily life of patients and impose a significant burden on the family and society [3, 4]. In recent years, aggressive glucose control has reduced macrovascular and microvascular diseases in many countries. However, the global burden of macrovascular and microvascular diseases is still alarming owing to improved life expectancy and the increased prevalence of diabetes mellitus [5].
Although there are some cardiovascular prediction models available for patients with type 2 diabetes (T2D), their predictive accuracy is moderate (most < 0.8) [6]. Some patients with diabetes mellitus who do not have a high-risk score still develop macrovascular disease, which suggests that there may be important risk factors not considered in the prediction models [6, 7]. Studies have shown that patients with diabetes mellitus and proteinuria or renal function impairment are prone to cardiovascular morbidity and mortality [3, 8], patients with diabetic retinopathy (DR) are likely to have cardiovascular diseases and heart failure [9], and patients with diabetic neuropathy (DN) are at risk of stroke and limb amputation [8, 10]. A cohort study also showed that patients with type 2 diabetes with more microvascular diseases have a higher associated risk of macrovascular diseases and mortality [7]. Diabetic microvascular diseases may have common pathogenic pathways with the development of macrovascular diseases, but it remains unclear whether the individual microvascular disease has a different association with the development of macrovascular diseases. Therefore, we conducted this nationwide cohort study to compare the risks of macrovascular disease development (1) between patients with type 2 diabetes with and without microvascular disease, (2) between matched patients of diabetic kidney disease versus retinopathy, diabetic kidney disease versus neuropathy, diabetic retinopathy versus neuropathy.
Methods
Data source
The Taiwanese government implemented the National Health Insurance (NHI) in 1995 to provide a mandatory comprehensive medical care plan. About 99% of 23 million citizens were enrolled in the NHI program by 2000 [11]. Patient information obtained during medical visits, including age, gender, residential area, diagnosis, procedures, and hospitalizations, is recorded in the National Health Insurance Research Database (NHIRD). Disease diagnosis of the NHIRD is according to the International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification (ICD-9/10-CM). NHIRD has a link with the National Death Registry to substantiate the cause of death. This study identified patients from the NHIRD. All information from patients and care providers was de-identified and encrypted before release to protect individual privacy. This study was approved by the Research Ethics Committee of China Medical University and Hospital (CMUH104-REC2-115-CR4), and the Research Ethics Committee approved a request to waive informed consent.
Study population and procedures
We identified patients with newly diagnosed type 2 diabetes from January 1, 2009, to December 31, 2018, and followed them up to December 31, 2019. The diagnosis of T2D was based on the ICD codings (Additional file 1: Table S1), with at least 3 outpatient visits within 1 year or one hospitalization [12]. Exclusion criteria were as follows: (1) missing information on age or sex; (2) age below 18 or above 80 years; (3) previous diagnosis of type 2 diabetes before January 1, 2009; (4) diagnosis of type 1 diabetes; (5) diagnosis of chronic kidney disease, dialysis, diabetic retinopathy, or diabetic neuropathy before the diagnosis of T2D; (6) diagnosis of coronary artery disease (CAD), stroke, atrial fibrillation, or heart failure before the index date; (7) death or follow-up for less than 180 days after the index date.
Patients with T2D diagnosis within one year were identified and categorized as (1) without microvascular disease, (2) diabetic kidney disease, including proteinuria, diabetic and hypertensive nephropathy, chronic kidney disease, renal failure, dialysis and renal replacement [13, 14], (3) diabetic retinopathy, including background and proliferative diabetic retinopathy, macular degeneration, retinal edema and detachment, vitreous hemorrhage, and vision loss [15], (4) diabetic neuropathy, including mononeuropathy and polyneuropathy, cranial nerve and peripheral nerve palsy, autonomic neuropathy, neuralgia, and Charcot’s arthropathy [16, 17], (5) diabetic kidney disease and retinopathy, (6) diabetic kidney disease and neuropathy, (7) diabetic retinopathy and neuropathy, (8) diabetic kidney disease, retinopathy, and neuropathy (Additional file 1: Table S2). We defined the 366th day after the diagnosis of type 2 diabetes as the index date of this study and assessed the outcomes during the follow-up period.
Demographics, comorbidities, and medications
The study included the following variables: age, gender, obesity, smoking, alcohol-related disorders, hypertension, dyslipidemia, peripheral arterial disease (PAD), chronic obstructive pulmonary disease (COPD), liver cirrhosis, connective tissue diseases, cancers, psychosis, depression, dementia, Charlson Comorbidity Index (CCI) score [18], antidiabetic agents, antihypertensive drugs, statin, and aspirin.
The outcomes of interest
The outcomes of interest were the development of CAD, stroke, heart failure, and cardiovascular death. Stroke, CAD, and heart failure were diagnosed by at least two outpatient visits within 1 year or one hospitalization during the study period [19, 20]. The adjudication of cardiovascular death was via the link with the National Death Registry. Each patient was tracked to the occurrence of outcomes, discontinuation of the NHI program, or the study observation end date of December 31, 2019, depending on which occurred first.
Statistical analyses
Multivariable-adjusted Cox proportional-hazard models were used to measure the risk of outcomes between patients with and without microvascular disease. Propensity score matching was used to construct matched pairs of patients with diabetic kidney disease and retinopathy, diabetic kidney disease and neuropathy, and diabetic retinopathy and neuropathy. We adopted the non-parsimonious multivariable logistic regression to appraise the propensity score for every patient, and 42 clinically relevant variables (Additional file 1: Tables S4–S6) were determined as independent variables. We adopted the nearest-neighbor algorithm to identify matched pairs and considered a standardized mean difference (SMD) < 0.1 to be a negligible difference between the matched pair of patients. We have performed subgroup analyses to see the influence of one, two or three microvascular diseases on composite major adverse cardiovascular events (CAD, stroke, heart failure and cardiovascular death) in the subgroups of patients with and without PAD, with and without insulin, aspirin and statin use.
The analyzed results were shown as hazard ratios (HRs) and 95% confidence intervals (CIs) for the compared groups. The two-tailed P value of less than 0.05 was considered statistically significant. SAS v9.4 (SAS Institute, Inc., Cary, NC, USA) was used for analysis.
Results
We investigated the occurrence of microvascular disease within one year after the diagnosis of type 2 diabetes. There were 718,059 people without microvascular disease, 45,634 people had diabetic kidney disease, 15,778 had diabetic retinopathy, 51,887 had diabetic neuropathy, 11,763 had diabetic kidney disease and retinopathy, 36,850 had diabetic kidney disease and neuropathy, 10,917 had diabetic retinopathy and neuropathy, 8934 had diabetic kidney disease, retinopathy, and neuropathy (Additional file 1: Table S2). The mean follow-up time was 5.55 years.
Patients with diabetic kidney disease, retinopathy, or neuropathy had a significantly higher risk of coronary artery disease, stroke, heart failure, and cardiovascular death than those without microvascular disease (Fig. 1). Patients with two or three microvascular diseases had a further higher risk of coronary artery disease, stroke, and heart failure than those without microvascular disease (Fig. 1). The subgroup analyses showed that patients with one or two microvascular disease had a significantly higher risk of composite cardiovascular events than those without microvascular disease, which was particularly pronounced in those without PAD compared with those with PAD; people with two or three microvascular diseases had a significantly higher risk of composite cardiovascular events than those with no microvascular disease, which was particularly pronounced in those without aspirin compared with those with aspirin; people with one, two or three microvascular diseases had a significantly higher risk of composite cardiovascular events than those with no microvascular disease, which was further pronounced in those on insulin therapy compared with those not on insulin therapy (P for interaction < 0. 05; Additional file 1: Table S3).
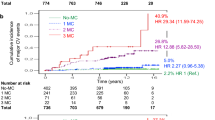
We used propensity-score matching to identify 14,651 pairs of patients with diabetic kidney disease and retinopathy (Fig. 2 and Additional file 1: Table S4), 24,996 pairs of patients with diabetic kidney disease and neuropathy (Additional file 1: Table S5), and 13,250 pairs of patients with diabetic retinopathy and neuropathy (Additional file 1: Table S6). Among the matched cohorts (Table 1), patients with diabetic neuropathy had a significantly higher risk of incident coronary artery disease than those with diabetic kidney disease (aHR 1.07). Patients with diabetic retinopathy had a significantly higher risk of stroke development than those with diabetic kidney disease (aHR 1.11). Diabetic neuropathy showed a significantly higher risk of stroke development than diabetic kidney disease (aHR 1.17). Diabetic neuropathy exhibited a significantly higher risk of stroke development than diabetic retinopathy (aHR 1.12). Diabetic retinopathy had a significantly higher risk of new-onset heart failure than diabetic kidney disease (aHR 1.43). Diabetic neuropathy showed a significantly lower risk of new-onset heart failure than diabetic retinopathy (aHR 0.79).
In the matched cohorts, diabetic neuropathy had a significantly higher cumulative incidence of incident coronary disease than diabetic kidney disease (Fig. 3). Diabetic neuropathy had a significantly higher cumulative incidence of stroke development than diabetic kidney disease and diabetic retinopathy. Diabetic retinopathy had a significantly higher cumulative incidence of stroke development than diabetic kidney disease. Diabetic retinopathy had a significantly higher cumulative incidence of new-onset heart failure than diabetic kidney disease and diabetic neuropathy.
Discussion
This nationwide cohort study showed that patients with microvascular disease have a significantly higher risk of cardiovascular disease and mortality than those without microvascular disease, and those with more microvascular diseases have a further higher risk of cardiovascular disease and mortality. In the matched cohorts, the three microvascular diseases had a similar impact on the risk of incident coronary artery disease and cardiovascular mortality; diabetic neuropathy had a higher effect on the risk of stroke than diabetic kidney disease and retinopathy; retinopathy had a higher impact on the development of heart failure than diabetic kidney disease and neuropathy.
Coronary artery disease is the major macrovascular complication in patients with T2D, and about 1–2 in 3 persons with T2D die of cardiovascular disease [2, 21]. Systemic reviews of clinical studies showed that diabetes mellitus with proteinuria increased composite cardiovascular events by about 1.3–4.4 times and cardiovascular death by about 1.2-2.0 times. Renal function impairment increased cardiac events about 2.2 times [8]. Diabetic retinopathy led to a 1.6-4.4-fold increase in the risk of cardiovascular events and a 1.5-5.6-fold increase in the risk of cardiovascular death [8, 9, 22]. Studies on the association between diabetic neuropathy and cardiovascular disease are relatively few. Several studies showed that QT variables were associated with a 0.94-3.3-fold increased risk of cardiovascular death [8]. Brownrigg et al. demonstrated that diabetic nephropathy, retinopathy, and peripheral neuropathy were associated with a consistently higher risk of composite major cardiovascular events and cardiovascular death than no microvascular disease in patients with T2D [7]. Our study also showed that patients with microvascular disease exhibited a significantly higher risk of incident coronary artery disease and cardiovascular death than those without microvascular disease. Patients with more microvascular diseases showed a further higher risk of cardiovascular morbidity and mortality than those with no microvascular disease. The cumulative microvascular diseases may represent a greater generalized vascular abnormality and herald the development of coronary artery disease and cardiovascular death. The impact of the three matched microvascular diseases on coronary artery disease and cardiovascular death seemed similar. Our results are consistent with the results of Brownrigg’s cohort study. Microvascular diseases may provide a more accurate estimation of cardiovascular risk score [7].
In recent years, aggressive glucose control, coronary artery disease, and stroke have gradually decreased, but the incidence of heart failure has increased rather than decreased in the United States [23]. Previous studies revealed that patients with proteinuria exhibited a 1.34-2.45-fold increased risk of heart failure [8]. Patients with diabetic retinopathy showed a 2.71-fold increased risk of heart failure [8, 9]. A large cohort of patients with type 1 diabetes demonstrated that persons with cardiac autonomic neuropathy demonstrated a significantly higher risk of left ventricular dysfunction than those without cardiac autonomic neuropathy [24]. Brownrigg’s cohort study showed that peripheral neuropathy, diabetic nephropathy, and retinopathy were similarly associated with a higher risk of hospitalization for heart failure than those without microvascular disease [7]. The present study showed that patients with the three microvascular diseases exhibited a significantly higher risk of new-onset heart failure than those without microvascular disease, which was consistent with the results of Brownrigg’s study. However, when our three microvascular diseases were matched and compared, we found that diabetic retinopathy had a significantly higher risk of heart failure than diabetic kidney disease and neuropathy, contrary to Brownrigg’s study. The reason could be that our study identified microvascular diseases within one year of T2D diagnosis, but Brownrigg detected microvascular diseases long after T2D diagnosis. The potential explanations for the association of diabetic retinopathy and heart failure are as follows: first, the occurrence of retinopathy may indicate that the dysfunction of coronary microvasculature providing blood circulation to the myocardium causes structural and functional changes in the heart, leading to diabetic cardiomyopathy and heart failure. Studies have demonstrated that a diabetic myocardium is often associated with microaneurysms and perfusion defects [25]. Second, retinopathy may also represent a broad systemic microvascular disease, with increased impedance burden on the heart, compromised cardiac performance, and increased predisposition to heart failure. Patients diagnosed with retinopathy should undergo cardiac function tests to detect heart failure and receive timely preventive treatment.
Patients with T2D have two times higher risk of stroke than those without T2D [26]. Clinical studies showed that patients with diabetes mellitus and nephropathy had a 1.7–2.3 times higher risk of stroke [8]. Patients with retinopathy had a 1.11–7.35 times higher risk of stroke [21]. Patients with cardiac autonomic neuropathy exhibited a 1.7–2.7 folds higher risk of stroke than those without cardiac autonomic neuropathy [8, 10, 27, 28]. Our study showed that patients with microvascular disease demonstrated a significantly higher risk of stroke development than those without microvascular disease. After propensity score matching of the three microvascular diseases, our study indicated that diabetic neuropathy had a higher impact on stroke development than retinopathy and nephropathy. Diabetic neuropathy is a syndrome that includes somatic and autonomic neuropathy of the peripheral nervous system [4, 29]. The plausible mechanisms to explain the intense association of neuropathy and stroke development are as follows: first, diabetic neuropathy may lead to the dysfunction of the parasympathetic and sympathetic innervation of cerebral vasculatures, resulting in the defective autoregulation of cerebral blood flow and render the brain vessels more vulnerable to injury and occlusion [10, 27, 28]. Second, neuropathy may cause unstable baroreflex and heart rhythms and favor atrial fibrillation with subsequent stroke development [28].
This study also has some limitations. First, this dataset lacked information on renal function, proteinuria, blood glucose, biochemical, neurological, fundus, imaging, and pathology findings. Although previous studies validated the algorithm for using ICD codes to define vascular diseases, it may have been unable to detect minor and early vascular lesions, leading to underestimation. Second, this research lacked complete information on dietary habits, exercise, family history, and alcohol consumption, which may have affected the results of this study. However, baseline demographics, obesity, smoking, alcohol-related disorders, comorbidities, antidiabetic drugs, and cardiovascular drugs were well-matched to maximally balance the variables of patients without and with various microvascular diseases and increase comparability. We used comorbidities, CCI scores, insulin, number of oral antidiabetic and antihypertensive drugs as surrogate markers of diabetes severity. Third, a retrospective cohort study cannot exclude unmeasured or unknown residual confounding, and rigorous prospective studies are warranted to justify our results.
Conclusions
Our study shows that microvascular diseases early in the diagnosis of type 2 diabetes are closely associated with the development of coronary artery disease, stroke, heart failure, and cardiovascular death. Furthermore, people with one, two or three microvascular diseases signify an even higher risk of cardiovascular morbidity and mortality than those without microvascular disease. We also found that these three microvascular diseases may be similarly associated with coronary artery diseases and cardiovascular death; but diabetic retinopathy was significantly associated with new-onset heart failure, and diabetic neuropathy had a significant association with stroke development. Further research may be needed to elucidate whether there are causal relationships and plausible mechanisms between retinopathy and heart failure, neuropathy and stroke.
Data availability
Data of this study are available from the National Health Insurance Research Database (NHIRD) published by Taiwan National Health Insurance (NHI) Administration. The data utilized in this study cannot be made available in the paper, the supplemental files, or in a public repository due to the ‘‘Personal Information Protection Act’’ executed by Taiwan government starting from 2012. Requests for data can be sent as a formal proposal to the NHIRD Office (https://dep.mohw.gov.tw/DOS/cp-2516-3591-113.html) or by email to stsung@mohw.gov.tw.
Abbreviations
- T2D:
-
type 2 diabetes
- DM:
-
diabetes mellitus
- DKD:
-
Diabetic kidney disease
- DR:
-
diabetic retinopathy
- DN:
-
diabetic neuropathy
- CAD:
-
coronary artery disease
- PAD:
-
peripheral arterial disease
- COPD:
-
chronic obstructive pulmonary disease
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
American Diabetes Association Professional Practice Committee. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:S144-74.
American Diabetes Association Professional Practice Committee, American Diabetes Association Professional Practice Committee. Chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;11:45:S175–84.
American Diabetes Association Professional Practice Committee, American Diabetes Association Professional Practice Committee. 12. Retinopathy, neuropathy, and foot care: standards of medical care in diabetes-2022. Diabetes Care. 2022;45:185–94.
The International Diabetes Federation (IDF) Group. 2022 International Diabetes Federation. Brussels: IDF; 2022. https://www.idf.org/our-activities/care-prevention/cardiovascular-disease/cvd-report.html#sub-content-tab-nav. Accessed 18 Nov 2022.
van Dieren S, Beulens JW, Kengne AP, Peelen LM, Rutten GEHM, Woodward M, et al. Prediction models for the risk of cardiovascular disease in patients with type 2 diabetes: a systematic review. Heart. 2012;98:360–9.
Brownrigg JR, Hughes CO, Burleigh D, Karthikesalingam A, Patterson BO, Holt PJ, et al. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population-level cohort study. Lancet Diabetes Endocrinol. 2016;4:588–97.
Rosenson RS, Fioretto P, Dodson PM. Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis. 2011;218:13–8.
Cheung N, Wong TY. Diabetic retinopathy and systemic vascular complications. Prog Retin Eye Res. 2008;27:161–76.
Ko SH, Song KH, Park SA, Kim SR, Cha BY, Son HY, et al. Cardiovascular autonomic dysfunction predicts acute ischaemic stroke in patients with type 2 diabetes mellitus: a 7-year follow-up study. Diabet Med. 2008;25:1171–7.
Cheng TM. Taiwan’s new national health insurance program: genesis and experience so far. Health Aff (Millwood). 2003;22:61–76.
Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104:157–63.
Hsieh CY, Cheng CL, Lai EC, Wang MC, Chen CH, Li CY, et al. Identifying renal dysfunction in stroke patients using diagnostic codes in the Taiwan National Health Insurance Research Database. Int J Stroke. 2015;10:E5.
Lin KD, Hsu CC, Ou HY, Wang CY, Chin MC, Shin SJ. Diabetes-related kidney, eye, and foot disease in Taiwan: an analysis of nationwide data from 2005 to 2014. J Formos Med Assoc. 2019;118:103–10.
Newton KM, Wagner EH, Ramsey SD, McCulloch D, Evans R, Sandhu N, et al. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol. 1999;52:199–207.
Christensen DH, Knudsen ST, Nicolaisen SK, Andersen H, Callaghan BC, Finnerup NB, et al. Can diabetic polyneuropathy and foot ulcers in patients with type 2 diabetes be accurately identified based on ICD-10 hospital diagnoses and drug prescriptions? Clin Epidemiol. 2019;11:311–21.
Kuo S, Yang CT, Chen HY, Ou HT. Valuing health states of people with type 2 diabetes: analyses of the nationwide representative linked databases. J Diabetes Investig. 2021;12:1749–58.
Meduru P, Helmer D, Rajan M, Tseng CL, Pogach L, Sambamoorthi U. Chronic illness with complexity: implications for performance measurement of optimal glycemic control. J Gen Intern Med. 2007;22:408–18.
Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YHK. Validation of acute myocardial infarction cases in the National Health Insurance Research Database in Taiwan. J Epidemiol. 2014;24:500–7.
Lin YS, Chen TH, Chi CC, Lin MS, Tung TH, Liu CH, et al. Different implications of heart failure, ischemic stroke, and mortality between non-valvular atrial fibrillation and atrial flutter-a view from a national cohort study. J Am Heart Assoc. 2017;6:e006406.
Chan JCN, Lim LL, Wareham NJ, Shaw JE, Orchard TJ, Zhan P, et al. The Lancet commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2021;396:2019–82.
Pearce I, Simó R, Lövestam-Adrian M, Wong DT, Evans M. Association between diabetic eye disease and other complications of diabetes: implications for care. A systematic review. Diabetes Obes Metab. 2019;21:467–78.
Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–23.
Pop-Busui R, Cleary PA, Braffett BH, Martin CL, Herman WH, Low PA, et al. Association between cardiovascular autonomic neuropathy and left ventricular dysfunction: DCCT/EDIC study (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications). J Am Coll Cardiol. 2013;61:447–54.
Cheung N, Wang JJ, Rogers SL, Brancati F, Klein R, Sharrett AR, et al. Diabetic retinopathy and risk of heart failure. J Am Coll Cardiol. 2008;51:1573–8.
Töyry JP, Niskanen LK, Länsimies EA, Partanen KP, Uusitupa MI. Autonomic neuropathy predicts the development of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke. 1996;27:1316–18.
Cohen JA, Estacio RO, Lundgren RA, Esler AL, Schrier RW. Diabetic autonomic neuropathy is associated with an increased incidence of strokes. Auton Neurosci. 2003;108:73–8.
Lo SF, Chen WL, Muo CH, Chen PC, Chen SY, Kuo CL, et al. Microvascular parameters help to predict stroke risk in the asian diabetic population in Taiwan: a population based case-control study. Front Neurol. 2018;9:719.
Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88.
Acknowledgements
We thank the Health Data Science Center, China Medical University Hospital, for the administrative, technical, and funding support.
Funding
This study was supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004), MOST Clinical Trial Consortium for Stroke (MOST 109-2321-B-039-002), China Medical University Hospital (DMR-110-222), Tseng-Lien Lin Foundation, Taichung, Taipei Veterans General Hospital (V105C-204, V110C-175), and the Ministry of Science and Technology, R.O.C (MOST 110-2314-B-075-027-MY3), Taiwan. These funding agencies had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation. The writing and preparation of this paper were not funded by any organization, and data analysis was not undertaken by employees of funders or any author who received the funding. The funders did not offer writing support. The corresponding authors had complete access to all data in the study and the final responsibility for the decision to publish it.
Author information
Authors and Affiliations
Contributions
CCH and CMH were the guarantors of the study. FSY and CMH participated in the study design. JCCW, CCH and YHS participated in study coordination and data collection. YHS and CCH participated in data analysis; all authors contributed to the interpretation of the results and discussion. FSY, JCCW, CCH and CMH participated in manuscript writing; all authors participated in revising the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yen, FS., Wei, J.CC., Shih, YH. et al. Impact of individual microvascular disease on the risks of macrovascular complications in type 2 diabetes: a nationwide population-based cohort study. Cardiovasc Diabetol 22, 109 (2023). https://doi.org/10.1186/s12933-023-01821-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-01821-8