Abstract
Background
Previous studies have mainly focused more on how diabetes affects the valve than the myocardium in aortic stenosis (AS). In the pressure-overloaded heart, myocardial fibrosis is an important driver of the progression from compensated hypertrophy to heart failure. Using comprehensive noninvasive imaging and plasma proteomics, we investigated whether and how diabetes aggravates the remodeling of the myocardium and its relation with prognosis in AS patients.
Methods
Severe AS patients were enrolled in two prospective cohorts for imaging and biomarker analysis. The imaging cohort (n = 253) underwent echocardiography and cardiac magnetic resonance, and the biomarker cohort (n = 100) blood sampling with multiplex proximity extension assay for 92 proteomic biomarkers. The composite outcome of hospitalization for heart failure admissions and death was assessed in the imaging cohort.
Results
Diabetic patients were older (70.4 ± 6.8 versus 66.7 ± 10.1 years) with more advanced ventricular diastolic dysfunction and increased replacement and diffuse interstitial fibrosis (late gadolinium enhancement % 0.3 [0.0–1.6] versus 0.0 [0.0–0.5], p = 0.009; extracellular volume fraction % 27.9 [25.7–30.1] versus 26.7 [24.9–28.5], p = 0.025) in the imaging cohort. Plasma proteomics analysis of the biomarker cohort revealed that 9 proteins (E-selectin, interleukin-1 receptor type 1, interleukin-1 receptor type 2, galectin-4, intercellular adhesion molecule 2, integrin beta-2, galectin-3, growth differentiation factor 15, and cathepsin D) were significantly elevated and that pathways related to inflammatory response and extracellular matrix components were enriched in diabetic AS patients. During follow-up (median 6.3 years), there were 53 unexpected heart failure admissions or death in the imaging cohort. Diabetes was a significant predictor of heart failure and death, independent of clinical covariates and aortic valve replacement (HR 1.88, 95% CI 1.06−3.31, p = 0.030).
Conclusions
Plasma proteomic analyses indicate that diabetes potentiates the systemic proinflammatory−profibrotic milieu in AS patients. These systemic biological changes underlie the increase of myocardial fibrosis, diastolic dysfunction, and worse clinical outcomes in severe AS patients with concomitant diabetes.
Similar content being viewed by others
Background
Aortic stenosis (AS) is initially a disease of the heart valve but its prognosis depends greatly on the health of the myocardium. Sustained pressure overload by AS induces ventricular hypertrophy and myocardial fibrosis that lead to ventricular decompensation [1, 2]. Myocardial fibrosis is commonly observed in two forms, diffuse interstitial and focal replacement fibrosis. Both forms of fibrosis can be imaged noninvasively with cardiac magnetic resonance (CMR) with gadolinium-based contrast agents: diffuse interstitial fibrosis is quantified by extracellular volume fraction (ECV) on T1 mapping and replacement fibrosis by late gadolinium enhancement (LGE). The former is partially reversible while the latter remains even after relief of pressure overload by aortic valve replacement (AVR) in AS [3]. Both increased ECV and LGE are associated with worse prognosis in patients with AS [4,5,6].
‘Diabetic cardiomyopathy’ was first described from autopsies of diabetic patients who manifested with heart failure but had no evidence of coronary problems, valvular disease or hypertension [7]. Subsequent investigations demonstrated that diabetic patients have increased myocardial fibrosis explained by multiple biological and molecular mechanisms [8]. Most studies on the interaction of diabetes with AS have focused on the progression of valvular stenosis [9,10,11]; however, there have been only few studies that addresses the impact of diabetes on myocardial health in AS patients [12,13,14].
Considering that diabetes is associated with worse prognosis in AS patients [15, 16], we hypothesized that diabetes would aggravate the degree of myocardial fibrosis in AS patients. The objective of this study was two-fold; first, to elucidate the prognostic impact of diabetes in AS patients and second, to dissect its underlying mechanisms using comprehensive noninvasive imaging and plasma proteomics.
Methods
Study population
This study utilized two prospective cohorts of AS patients: the imaging cohort for the assessment of the myocardial health using CMR and echocardiography with long term follow-up for clinical events, and a biomarker cohort for the assessment of enriched circulating proteins using multiplex proximity extension assay.
The imaging cohort consisted of 253 patients with moderate or severe AS prospectively enrolled from 2011 to 2015 at three tertiary medical centers in Korea. All participants in this cohort underwent comprehensive echocardiography and CMR. The biomarker cohort consisted of 100 patients with severe AS undergoing surgical AVR enrolled prospectively from 2018 to 2021 at Seoul National University Hospital (Additional file 1: Fig. S1). Detailed inclusion and exclusion criteria are in Additional file 1: Additional Methods. Two separate cohorts were used in the study because patients in the imaging cohort did not undergo blood sample collection and most of the patients in the biomarker cohort did not undergo CMR and were followed for less than one year (median follow-up 6.6 [IQR 3.9–12.2] months). The study complied with the Declaration of Helsinki, and the cohorts were approved by the institutional review boards of each institution, with all study subjects providing written informed consent before enrollment.
Diabetic status was defined as either: (i) treatment for diabetes at enrollment with lifestyle modification, oral hypoglycemic agents, or insulin, or (ii) HbA1c ≥ 6.5% in those not previously diagnosed with diabetes and with HbA1c levels available at the period of enrollment. Medication status at enrollment was assessed for the diabetes group. Ischemic heart disease (IHD) was defined as either a history of myocardial infarction or coronary intervention, or significant coronary artery stenosis (> 50%) on angiography prior to AVR, or coronary intervention or concomitant coronary artery bypass grafting performed together with AVR.
Echocardiographic evaluation
Echocardiography was performed using commercially available machines and the severity of AS was determined according to the contemporary guidelines [17]. The left ventricular (LV) chamber size, and systolic and diastolic function were also evaluated and categorized according to the latest guidelines [18, 19]. AS severity was defined based on aortic valve area (severe AS, aortic valve area ≤ 1 cm2; moderate AS, aortic valve area > 1 cm2 and ≤ 1.5 cm2) [20].
CMR analysis
In the imaging cohort, CMR was performed at a median interval of 11 days (interquartile range 2–29 days) from the date of echocardiography. The details of the scanners, field strengths, T1 mapping sequences, contrast agents, and summary of imaging analyses for each study center are presented in Additional file 1: Table S1 [6]. Briefly, CMR scans consisted of balanced steady-state free precession cine images, pre- and post-gadolinium T1 mapping, and LGE images. The chamber sizes and myocardial mass were quantified according to a standardized protocol [5]. The degree of diffuse interstitial fibrosis was assessed by calculating ECV from the pre- and post-gadolinium T1 values measured at the short-axis mid-ventricular septum and blood pool, and hematocrit from blood samples at the time of CMR [2, 21]. Infarct-related LGE was excluded while non-infarct LGE was included in the ECV assessment [5]. The presence and extent of LGE was assessed on short-axis images acquired by phase sensitive inversion recovery sequence; the regions of LGE were drawn semi-automatically as pixels of the myocardium with a signal intensity > 5 standard deviations of the normal remote myocardium and the LGE% was calculated by dividing the LGE area by the total LV myocardial area.
Plasma proteomics assay
Blood samples from the biomarker cohort were collected preoperatively in EDTA bottles, divided into plasma and buffy coat layers with centrifugation, and then stored at – 80 °C. For plasma proteomics analysis, deep frozen plasma samples were shipped to Olink Proteomics (Uppsala, Sweden) and the plasma levels of 92 protein biomarkers were measured using commercially available multiplex proximity extension assay kits (Cardiovascular III Panel, Additional file 1: Table S2). This high-throughput technique utilizes immunoassay with oligonucleotide-labeled antibodies followed by real-time polymerase chain reaction for simultaneous quantification of target proteins with high specificity and scalability [22]. After normalization, plasma levels were expressed for each protein in relative quantification units called normalized protein expression (NPX) using the Log2 scale (1 NPX difference equaling twofold change in protein concentration).
Clinical outcomes
The clinical outcomes of interest in this study were unexpected hospitalization for heart failure that necessitated intravenous diuretics and all-cause mortality. These outcomes were assessed in the imaging cohort by review of medical records, reports from family members, and official mortality data from Statistics Korea. Patients were followed from the date of CMR to the last clinical follow-up or death.
Statistical analysis
Continuous data are presented as mean ± standard deviation or median (interquartile range) depending on the normality of distribution, and categorical data as number (%). Characteristics were compared between the groups using the t-test (or Mann–Whitney test for non-normally distributed continuous variables) or the chi-square test. Comparisons of groups according to diabetes medication was conducted using the Kruskal–Wallis test. Variables associated with increased diffuse interstitial or replacement fibrosis were analyzed using logistic regression and the degree of association expressed in odds ratio (OR) with 95% confidence interval (CI). Multivariable models were constructed with the stepwise backward selection method using the Akaike information criterion or inclusion of clinically important variables such as age, sex, diabetes, hypertension, atrial fibrillation, IHD, and peak aortic velocity.
Comparison of plasma biomarker levels according to the diabetic status was performed using the Welch’s two-sample t-test, adjusting for multiple testing with the Benjamini–Hochberg method. The adjusted p-values represent the false discovery rate and p-values < 0.05 were considered significant. Logistic regression was used to assess the association of plasma biomarkers with diabetic status, adjusting for age, sex, hypertension, atrial fibrillation, IHD, and peak aortic velocity. Functional enrichment analyses were performed using g:Profiler with Gene Ontology terms.
Kaplan–Meier survival curves with log-rank tests were used to compare event-free survival according to the presence of diabetes. Cox proportional-hazards regression analyses were used to assess predictors of the endpoints and the effect size expressed as hazard ratio (HR) with 95% CI. The final multivariable model was constructed with stepwise backward selection from clinically important variables such as age, sex, diabetes, hypertension, atrial fibrillation, stroke, IHD, peak aortic velocity, LV ejection fraction by echocardiography, and AVR. Two-sided p-values < 0.05 were considered statistically significant. Analyses were conducted using R version 4.0 (Vienna, Austria) or SPSS version 25 (Chicago, USA).
Results
Demographic and clinical characteristics according to the presence of diabetes
There were no significant differences in the clinical characteristics and AS severity between the imaging and biomarker cohorts, except that the imaging cohort had slightly greater left atrial volume and more prevalent LV diastolic dysfunction compared to the biomarker cohort (Additional file 1: Tables S3 and S4). There was also no significant difference in the severity of diabetes assessed by diabetes medication status, HbA1c and FBS levels in the diabetic patients of the imaging and biomarker cohorts (Additional file 1: Table S4). Detailed information on the diabetes medication status of both cohorts are presented in Additional file 1: Table S5.
In the imaging cohort (n = 253), there were 66 patients with diabetes (26.1%). Among the diabetic patients, 47 (71.2%) were on oral medication only, 6 (9.1%) on insulin, and 13 (19.7%) not on any medication. Compared to non-diabetic patients, the diabetic patients were older (70.4 ± 6.8 vs. 66.7 ± 10.1 years, p = 0.001), had a higher prevalence of hypertension (72.7% vs. 55.1%, p = 0.018) and IHD (28.8% vs. 9.1%, p < 0.001), and tended to use more diuretics (Table 1).
In the biomarker cohort (n = 100), there were 27 patients with diabetes (27%), of whom 19 (70.4%) were on oral medication only, 4 (14.8%) on insulin, and 4 (14.8%) on no medication (Table 1). There were no statistical differences in the clinical or demographic parameters between the diabetic and non-diabetic patients in the biomarker cohort. White blood cell count or neutrophil/lymphocyte ratio was also not different between the diabetic and non-diabetic patients (Additional file 1: Table S6).
Increased risk of myocardial fibrosis on noninvasive imaging in diabetic AS patients
There was no significant difference in AS severity, aortic valve area and mean pressure gradient between diabetic and non-diabetic patients in the imaging cohort, although the peak aortic velocity was slightly lower in patients with diabetes (4.5 ± 0.9 vs. 4.8 ± 8.0 m/s, p = 0.036). However, compared to the non-diabetic patients, the diabetic patients had worse LV diastolic function (prevalence of LV diastolic dysfunction 79.7% vs. 53.5%, p = 0.001) (Fig. 1A), supported by lower e′ velocity, higher E/e′ and tricuspid regurgitation peak velocity, and a shorter mitral deceleration time (Table 2). Notably, the prevalence of grade 2 or 3 LV diastolic dysfunction was higher in the diabetic patients. When stratified by diabetes medication as a surrogate marker of chronicity and severity of diabetes, there was also a higher prevalence of LV diastolic dysfunction with needs for more intensive diabetes treatment (Fig. 1B, p-for-trend < 0.001). In the biomarker cohort as well, while there was no significant difference in AS severity, the diabetic patients had lower e′ velocity with a tendency towards more advanced LV diastolic dysfunction than the nondiabetic patients (Additional file 1: Table S7).
Myocardial fibrosis and left ventricular diastolic function in aortic stenosis patients according to diabetes and diabetes medication status. a, b Comparison of the degree of diastolic dysfunction in a patients with versus without diabetes and in b patients stratified by the diabetes medication status. c, d Comparison of late gadolinium enhancement (LGE) in c patients with versus without diabetes and in d patients stratified by the diabetes medication status. e, f Comparison of extracellular volume fraction (ECV) in e patients with versus without diabetes and in f patients stratified by the diabetes medication status. g, h Representative LGE and ECV images of cardiac magnetic resonance taken from g a non-diabetic aortic stenosis patient (no LGE; ECV 26.4%) versus h a diabetic aortic stenosis patient on oral hypoglycemic agent (midwall LGE in the septal and lateral wall; ECV 34.5%). DM, diabetes mellitus; ECV, extracellular volume; LGE, late gadolinium enhancement; LVDD, left ventricular diastolic dysfunction; OHA, oral hypoglycemic agents
On analysis of CMR in the participants of the imaging cohort, a significant increase of replacement fibrosis was observed in diabetic patients (Table 2). The LGE was present in 56% of diabetic patients compared to 40% in non-diabetic patients (p = 0.036), and LGE was more frequently present in patients with LGE with need for more intensive diabetes treatment (p-for-trend = 0.016) (Additional file 1: Fig. S2). The extent of LGE was also higher in the diabetic patients (Fig. 1C, LGE% in the entire population 0.3 [0.0–1.6] vs. 0.0 [0.0–0.5], p = 0.009) (LGE% in those with any LGE 1.2 [0.4–2.9] vs. 0.6 [0.2–1.5], p = 0.026). There was also a tendency for higher LGE% with more intensive diabetes treatment (p-for-trend = 0.070) (Fig. 1D, Additional file 1: Table S8). As for the degree of diffuse interstitial fibrosis, the ECV was higher in patients with diabetes (Fig. 1E, ECV% 27.9 [25.7–30.1] vs. 26.7 [24.9–28.5], p = 0.025). Similar to the analysis of LGE%, the ECV% was significantly higher with more intensive treatment of diabetes (p-for-trend = 0.003) (Fig. 1F, Additional file 1: Table S8). Exploratory analyses in the subsets of patients with HbA1c and FBS levels available (n = 162 and n = 137, respectively) also showed that the presence of LV diastolic dysfunction and parameters of fibrosis increased numerically with worse glycemic control, albeit with variable statistical significance (Additional file 1: Tables S9 and S10). The LV stroke volume and ejection fraction measured by CMR were lower in diabetic patients.
Diabetes was significantly associated with increased diffuse interstitial and replacement fibrosis on univariable and multivariable analyses (Table 3). The presence of diabetes showed the strongest associations with the highest quartile of both increased interstitial or replacement fibrosis, i.e. ECV% (adjusted HR 1.9, p-value = 0.05) and LGE% (adjusted HR 3.0, p-value < 0.01).
Upregulation of the proinflammatory−profibrotic pathways in the plasma proteome of diabetic AS patients
The distribution of NPX values for each sample are shown in Additional file 1: Fig. S3. Among the 92 candidate proteins in the plasma proteomics analysis of the biomarker cohort, 9 proteins (E-selectin, interleukin-1 receptor type 1, interleukin-1 receptor type 2, galectin-4, intercellular adhesion molecule 2, integrin beta-2, galectin-3, growth differentiation factor 15 [GDF-15], and cathepsin D) were significantly upregulated in diabetic AS patients compared to non-diabetic AS patients (Fig. 2, Additional file 1: Table S11) (false discovery rate < 5% and minimal fold change of 1.15 [Log2(fold change) > 0.20])[23]. There were no proteins that were significantly downregulated in diabetic AS patients. These proteins biomarkers were independently associated with diabetic status after adjustment for age, sex, atrial fibrillation, IHD, peak aortic velocity, and LV ejection fraction, with odds ratios ranging from 3 to 13 per twofold increase in each protein level (Additional file 1: Table S12). Biomarker levels also did not differ significantly in diabetic patients according to metformin use (Additional file 1: Table S13). Pathway over-representation analyses of the upregulated plasma proteome indicated that pathways related to proinflammatory response and extracellular matrix components were enriched in the plasma of AS patients with concomitant diabetes (Fig. 3, Additional file 1: Table S14).
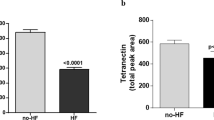
Significantly upregulated plasma proteins in AS patients with diabetes on proteomic analysis. a Volcano plot identifying the significantly upregulated proteins (annotated in red) in the plasma proteome of diabetic AS patients. b Scatter plots comparing the plasma levels of differentially expressed proteins in AS patients with and without diabetes. All comparisons were adjusted for multiple testing with the Benjamini & Hochberg method, with adjusted p-values representing the false discovery rate. The horizontal bars for each group indicate median and interquartile range. adj, adjusted; AS, aortic stenosis; DM, diabetes mellitus; SELE, E-selectin; IL-1RT1, interleukin-1 receptor type 1; CTSD, cathepsin D; Gal-4, galectin-4; Gal-3, galectin-3; IL-1RT2, interleukin-1 receptor type 2; GDF-15, growth differentiation factor 15; ICAM-2, intercellular adhesion molecule 2; ITGB2, integrin beta-2
Over-represented pathways in the plasma proteome of AS patients with diabetes. Functional enrichment analyses using the g:Profiler with Gene Ontology terms were performed with the proteomic analysis results and are presented in a Manhattan plot. The sizes of circles signify intersection size. adj, adjusted; AS, aortic stenosis; BP, biological process; CC, cellular component; GO, Gene Ontology domains; MF, molecular function
Exploratory pathway over-representation analyses with plasma biomarkers fulfilling a more lenient criteria of nominal p-value < 0.050 and minimal fold change of 1.15 showed similar results. A total of 16 biomarkers met this criteria (interleukin-18-binding protein, C–C motif chemokine 16, tissue-type plasminogen activator, scavenger receptor cysteine-rich type 1 protein M130, pulmonary surfactant-associated protein D, insulin-like growth factor-binding protein 1, and urokinase plasminogen activator surface receptor as well as the aforementioned 9 proteins; Additional file 1: Fig. S4A) and the tendency for enrichment of proinflammatory and profibrotic pathways was persistent (Additional file 1: Fig. S4B, Table S15).
Clinical outcomes according to the presence of diabetes
The participants in the imaging cohort were followed for a median 6.3 (interquartile range 5.2–7.2) years and nearly all patients (n = 232, 91.7%) received AVR during follow-up. Most patients received surgical AVR (n = 216, 93.1%). The proportion of patients receiving AVR (92.0% vs 90.9%, p = 0.991) or the type of AVR, either surgical or transcatheter (surgical AVR 94.8% vs 88.3%, p = 0.162), was not significantly different between the non-diabetic and diabetic AS patients. There were 53 events of unexpected admission for heart failure or death (20.9%) (Additional file 1: Table S16).
The incidence of the composite clinical events was significantly higher in diabetic AS patients compared to the non-diabetic AS subjects (Fig. 4A); all-cause mortality was also higher in diabetic AS subjects (Fig. 4B). Diabetes was a significant predictor of heart failure and all-cause death (HR 2.09, 95% CI 1.20–3.63, p = 0.009), independent of age, sex, atrial fibrillation, IHD, LV ejection fraction, and AVR (Table 4).
In the analysis of patients who underwent AVR (n = 232), the AS patients with concomitant diabetes also had worse clinical outcomes (Additional file 1: Fig. S5, Table S17), again suggesting that diabetes has a pervasive systemic effect on the myocardial health even after relief of pressure overload by AVR. Diabetes was associated with clinical events independently of the type of AVR and other clinical variables (Additional file 1: Table S18).
Discussion
Herein, we demonstrated that AS patients with diabetes compared to non-diabetic patients had increased diffuse interstitial and replacement fibrosis by CMR analysis of the myocardium. An in-depth investigation of plasma proteomics demonstrated that factors related to proinflammatory response and extracellular matrix components were enriched in diabetic AS patients. These diabetic AS patients had a significantly higher incidence of heart failure and death than the non-diabetic patients. These results suggest that diabetes is associated with effects that potentiate systemic proinflammatory and profibrotic milieu in the pressure-overloaded myocardium, which translates into worse clinical outcomes even after AVR.
Diabetes mellitus is a systemic disease that affects the myocardium directly [8]. Patients with otherwise uncomplicated diabetes show reduced cardiopulmonary performance [24, 25], which is associated with increased myocardial fibrosis [26]. Microvascular function is also impaired in diabetes [27], and therefore, the myocardium may be more vulnerable to ischemic insult [28]. Studies utilizing CMR in the general population have suggested that diffuse interstitial and replacement fibrosis are increased in diabetic compared to non-diabetic subjects, and both forms of fibrosis are associated with heart failure and mortality events [29,30,31].
There have been few studies on how diabetes impacts myocardial remodeling in AS. We found that the degree of myocardial fibrosis and diastolic dysfunction is significantly more advanced in diabetic AS patients. An invasive histological study using myocardial biopsies in 60 AS patients undergoing AVR suggested that patients with concomitant AS and diabetes had more myocardial fibrosis and higher cardiomyocyte stiffness [13]. Using noninvasive imaging to examine the entire myocardium, we show that the histologic evidence from the previous study [13] holds true in a larger AS population.
In AS patients, diabetes is independently associated with an increase in both mid-term and long-term mortality in those undergoing AVR, as well as in those with asymptomatic AS patients managed conservatively [15, 16, 32]. Herein, we suggest the missing link between diabetes and outcome in AS by demonstrating the association between diabetes and myocardial fibrosis. Moreover, the need for more intensive diabetes treatment as a marker of diabetes severity was associated with a greater degree of myocardial fibrosis and LV diastolic dysfunction, especially in insulin-treated diabetic patients. Previous studies have also shown that the prognosis of diabetic AS patients even after AVR are significantly worse especially in those treated with insulin [16, 32]. This suggests that factors other than the stenotic valve in AS is responsible for the worse prognosis in diabetic AS patients and that relief of the stenotic valve may not be enough for optimal outcome.
In the pressure-overloaded heart, myocardial fibrosis is an important driver of the progression from compensated hypertrophy to heart failure with diastolic and systolic dysfunction, findings demonstrated in both histological and imaging studies [1, 33, 34]. In the current study, AS patients with diabetes had greater replacement and diffuse interstitial fibrosis compared to non-diabetic counterparts. Although the difference in both measures of fibrosis between the diabetics versus non-diabetics may seem small, this difference is clinically significant as shown by previous investigations demonstrating 11% and 10% higher mortality with each 1% increase of %LGE and %ECV, respectively [4, 5]. Furthermore, there is a characteristic non-linear and threshold effect of %LGE and %ECV on outcome in AS [6], suggesting that even a small increase of fibrosis can lead to significantly worse outcomes. Diabetes is also associated with poor LV mass regression after AVR [14], suggesting that it continues to affect myocardial remodeling after relief of pressure overload.
This leads us to question whether and how diabetes changes the systemic milieu and ultimately, the myocardium. Circulating protein biomarkers provide important clues to pathophysiological mechanisms of diseases, and high-throughput proteomic methods can measure a multitude of proteins simultaneously. In previous plasma proteome studies of AS patients, higher GDF-15 was associated with poor LV reverse-remodeling and increased mortality after AVR [35, 36]. In plasma proteome analysis of patients with heart failure, diabetic patients had higher circulating GDF-15 and galectin-4 levels [23], and over-representation of pathways related to inflammation, cardiac remodeling, and fibrosis [23, 37]. We found that E-selectin, interleukin-1 receptor type 1, interleukin-1 receptor type 2, galectin-3, galectin-4, intercellular adhesion molecule 2, integrin beta-2, GDF-15, and cathepsin D levels were significantly upregulated in diabetic AS patients, proteins which have been implicated in inflammation, cardiac fibrosis and remodeling, atherosclerosis, and heart failure [38,39,40,41,42,43,44]. Furthermore, over-representation analyses of the plasma proteome demonstrated that pathways related to neutrophil activation, interleukin-1 and amplification of inflammation, leukocyte migration, and extracellular matrix were enriched in diabetic AS patients. Our study supports that upregulation of signals related to inflammation and extracellular matrix expansion are important pathophysiological processes systemically mediated by diabetes in AS patients, which in turn, may aggravate the degree of myocardial fibrosis and lead to worse outcomes.
Our study suggests that clinicians should be aware of the significantly higher clinical events in AS patients with diabetes. According to our analysis, diabetes not only damages the stenotic valve [9,10,11], but also, the health of the myocardium, with its effect pervading even after AVR. In particular, diabetes patients treated with insulin may be at the highest risk. Our findings suggest that the changes in the myocardium by AS are already more advanced in diabetes patients and it can be assumed that the systemic proinflammatory−profibrotic milieu will continue with diabetes even after AVR. This also suggests that AVR may not be the final treatment for AS when the patient has diabetes. Considering the systemic proinflammatory−profibrotic environment by diabetes, our findings call for further studies on whether anti-inflammatory approaches may be beneficial in AS patients with concomitant diabetes, to prevent adverse myocardial remodeling and to improve clinical outcomes. Exciting options testing this idea have been developed with promising outcomes, such as the monoclonal antibody targeting interleukin-1β [45]. The anti-diabetic medications alleviate inflammation by differing degrees [46], which may also affect the diabetes-related myocardial remodeling and fibrosis, especially in the pressure-overloaded myocardium of AS patients. Additionally, whether individualizing the treatment strategy would be more beneficial in severe AS patients with concomitant diabetes should be evaluated in future trials.
This study has some limitations that needs to be considered. First, because of the shortage of resources, two separate imaging and biomarker cohorts were used for analysis, and analysis of the direct association of plasma proteins with noninvasive measures of fibrosis on CMR could not be performed. Second, information on the diabetes control status assessed by HbA1c or FBS values was not available for all patients and information on the duration of diabetes was unavailable. Thus, we used the diabetes medication status as a surrogate marker of the severity of diabetes, but its limitations must be acknowledged. Also, it should be considered that the number of patients on insulin was small to make any definite statements regarding the relationship between myocardial fibrosis and the type of medications for diabetes. Lastly, we used a select biomarker panel of 92 proteins, and future studies utilizing a more comprehensive set of proteins may provide more pathophysiological information as well as therapeutic targets.
Conclusions
In conclusion, plasma proteome analyses indicate that diabetes is associated with increased systemic proinflammatory−profibrotic responses in patients with AS. These biological changes underlie the increase of myocardial fibrosis, advanced LV diastolic dysfunction, and ultimately, worse clinical outcomes observed in AS patients with concomitant diabetes.
Availability of data and materials
Individual-level participant data used in this study can be provided to researchers upon reasonable request to the corresponding author. A detailed proposal for how the data will be used is required.
Abbreviations
- AS:
-
Aortic stenosis
- AVR:
-
Aortic valve replacement
- CI:
-
Confidence interval
- CMR:
-
Cardiac magnetic resonance
- ECV:
-
Extracellular volume fraction
- HR:
-
Hazard ratio
- IHD:
-
Ischemic heart disease
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricular
- NPX:
-
Normalized protein expression
- OR:
-
Odds ratio
References
Chin CWL, Everett RJ, Kwiecinski J, Vesey AT, Yeung E, Esson G, Jenkins W, Koo M, Mirsadraee S, White AC, et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging. 2017;10(11):1320–33.
Lee HJ, Lee H, Kim SM, Park JB, Kim EK, Chang SA, Park E, Kim HK, Lee W, Kim YJ, et al. Diffuse myocardial fibrosis and diastolic function in aortic stenosis. JACC Cardiovasc Imaging. 2020;13(12):2561–72.
Treibel TA, Kozor R, Schofield R, Benedetti G, Fontana M, Bhuva AN, Sheikh A, Lopez B, Gonzalez A, Manisty C, et al. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J Am Coll Cardiol. 2018;71(8):860–71.
Musa TA, Treibel TA, Vassiliou VS, Captur G, Singh A, Chin C, Dobson LE, Pica S, Loudon M, Malley T, et al. Myocardial scar and mortality in severe aortic stenosis. Circulation. 2018;138(18):1935–47.
Everett RJ, Treibel TA, Fukui M, Lee H, Rigolli M, Singh A, Bijsterveld P, Tastet L, Musa TA, Dobson L, et al. Extracellular myocardial volume in patients with aortic stenosis. J Am Coll Cardiol. 2020;75(3):304–16.
Kwak S, Everett RJ, Treibel TA, Yang S, Hwang D, Ko T, Williams MC, Bing R, Singh T, Joshi S, et al. Markers of myocardial damage predict mortality in patients with aortic stenosis. J Am Coll Cardiol. 2021;78(6):545–58.
Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30(6):595–602.
Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. 2018;122(4):624–38.
Katz R, Wong ND, Kronmal R, Takasu J, Shavelle DM, Probstfield JL, Bertoni AG, Budoff MJ, O’Brien KD. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113(17):2113–9.
Kopytek M, Zabczyk M, Mazur P, Undas A, Natorska J. Accumulation of advanced glycation end products (AGEs) is associated with the severity of aortic stenosis in patients with concomitant type 2 diabetes. Cardiovasc Diabetol. 2020;19(1):92.
Kopytek M, Mazur P, Zabczyk M, Undas A, Natorska J. Diabetes concomitant to aortic stenosis is associated with increased expression of NF-kappaB and more pronounced valve calcification. Diabetologia. 2021;64(11):2562–74.
Lindman BR, Arnold SV, Madrazo JA, Zajarias A, Johnson SN, Perez JE, Mann DL. The adverse impact of diabetes mellitus on left ventricular remodeling and function in patients with severe aortic stenosis. Circ Heart Fail. 2011;4(3):286–92.
Falcao-Pires I, Hamdani N, Borbely A, Gavina C, Schalkwijk CG, van der Velden J, van Heerebeek L, Stienen GJ, Niessen HW, Leite-Moreira AF, et al. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124(10):1151–9.
Nakamura T, Toda K, Kuratani T, Miyagawa S, Yoshikawa Y, Fukushima S, Saito S, Yoshioka D, Kashiyama N, Daimon T, et al. Diabetes mellitus impairs left ventricular mass regression after surgical or transcatheter aortic valve replacement for severe aortic stenosis. Heart Lung Circ. 2016;25(1):68–74.
Lancellotti P, Magne J, Dulgheru R, Clavel MA, Donal E, Vannan MA, Chambers J, Rosenhek R, Habib G, Lloyd G, et al. Outcomes of patients with asymptomatic aortic stenosis followed up in heart valve clinics. JAMA Cardiol. 2018;3(11):1060–8.
Abramowitz Y, Vemulapalli S, Chakravarty T, Li Z, Kapadia S, Holmes D, Matsouaka RA, Wang A, Cheng W, Forrester JS, et al. Clinical impact of diabetes mellitus on outcomes after transcatheter aortic valve replacement: insights from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2017;10(11): e005417.
Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller F Jr, Otto CM. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30(4):372–92.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.E14.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–60.
Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):e72–227.
Lee H, Park JB, Yoon YE, Park EA, Kim HK, Lee W, Kim YJ, Cho GY, Sohn DW, Greiser A, et al. Noncontrast myocardial T1 mapping by cardiac magnetic resonance predicts outcome in patients with aortic stenosis. JACC Cardiovasc Imaging. 2018;11(7):974–83.
Assarsson E, Lundberg M, Holmquist G, Bjorkesten J, Thorsen SB, Ekman D, Eriksson A, Rennel Dickens E, Ohlsson S, Edfeldt G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE. 2014;9(4): e95192.
Tromp J, Voors AA, Sharma A, Ferreira JP, Ouwerkerk W, Hillege HL, Gomez KA, Dickstein K, Anker SD, Metra M, et al. Distinct pathological pathways in patients with heart failure and diabetes. JACC Heart Fail. 2020;8(3):234–42.
Nesti L, Pugliese NR, Sciuto P, De Biase N, Mazzola M, Fabiani I, Trico D, Masi S, Natali A. Mechanisms of reduced peak oxygen consumption in subjects with uncomplicated type 2 diabetes. Cardiovasc Diabetol. 2021;20(1):124.
Nesti L, Pugliese NR, Sciuto P, Natali A. Type 2 diabetes and reduced exercise tolerance: a review of the literature through an integrated physiology approach. Cardiovasc Diabetol. 2020;19(1):134.
Dennis M, Howpage S, McGill M, Dutta S, Koay Y, Nguyen-Lal L, Lal S, Wu T, Ugander M, Wang A, et al. Myocardial fibrosis in type 2 diabetes is associated with functional and metabolomic parameters. Int J Cardiol. 2022;363:179–84.
Gallinoro E, Paolisso P, Candreva A, Bermpeis K, Fabbricatore D, Esposito G, Bertolone D, Fernandez Peregrina E, Munhoz D, Mileva N, et al. Microvascular dysfunction in patients with type II diabetes mellitus: invasive assessment of absolute coronary blood flow and microvascular resistance reserve. Front Cardiovasc Med. 2021;8: 765071.
Paolisso P, Foa A, Bergamaschi L, Donati F, Fabrizio M, Chiti C, Angeli F, Toniolo S, Stefanizzi A, Armillotta M, et al. Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA. Cardiovasc Diabetol. 2021;20(1):33.
Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014;35(10):657–64.
Kwong RY, Sattar H, Wu H, Vorobiof G, Gandla V, Steel K, Siu S, Brown KA. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118(10):1011–20.
Khan MA, Yang EY, Nguyen DT, Nabi F, Hinojosa J, Jabel M, Nagueh SF, Graviss EA, Shah DJ. Examining the relationship and prognostic implication of diabetic status and extracellular matrix expansion by cardiac magnetic resonance. Circ Cardiovasc Imaging. 2020;13(7): e011000.
Ram E, Kogan A, Levin S, Fisman EZ, Tenenbaum A, Raanani E, Sternik L. Type 2 diabetes mellitus increases long-term mortality risk after isolated surgical aortic valve replacement. Cardiovasc Diabetol. 2019;18(1):31.
Hein S, Arnon E, Kostin S, Schonburg M, Elsasser A, Polyakova V, Bauer EP, Klovekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107(7):984–91.
Lee SP, Lee W, Lee JM, Park EA, Kim HK, Kim YJ, Sohn DW. Assessment of diffuse myocardial fibrosis by using MR imaging in asymptomatic patients with aortic stenosis. Radiology. 2015;274(2):359–69.
Kim JB, Kobayashi Y, Moneghetti KJ, Brenner DA, O’Malley R, Schnittger I, Wu JC, Murtagh G, Beshiri A, Fischbein M, et al. GDF-15 (growth differentiation factor 15) is associated with lack of ventricular recovery and mortality after transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2017;10(12): e005594.
Lindman BR, Breyley JG, Schilling JD, Vatterott AM, Zajarias A, Maniar HS, Damiano RJ Jr, Moon MR, Lawton JS, Gage BF, et al. Prognostic utility of novel biomarkers of cardiovascular stress in patients with aortic stenosis undergoing valve replacement. Heart. 2015;101(17):1382–8.
Sharma A, Demissei BG, Tromp J, Hillege HL, Cleland JG, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Davison BA, et al. A network analysis to compare biomarker profiles in patients with and without diabetes mellitus in acute heart failure. Eur J Heart Fail. 2017;19(10):1310–20.
Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM Jr, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96(12):4219–25.
Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126(9):1260–80.
van der Hoeven NW, Hollander MR, Yildirim C, Jansen MF, Teunissen PF, Horrevoets AJ, van der Pouw Kraan TC, van Royen N. The emerging role of galectins in cardiovascular disease. Vascul Pharmacol. 2016;81:31–41.
Glogowska-Ligus J, Dabek J, Zych-Twardowska E, Tkacz M. Expression analysis of intercellular adhesion molecule-2 (ICAM-2) in the context of classical cardiovascular risk factors in acute coronary syndrome patients. Arch Med Sci. 2013;9(6):1035–9.
Israeli-Rosenberg S, Manso AM, Okada H, Ross RS. Integrins and integrin-associated proteins in the cardiac myocyte. Circ Res. 2014;114(3):572–86.
Wesseling M, de Poel JHC, de Jager SCA. Growth differentiation factor 15 in adverse cardiac remodelling: from biomarker to causal player. ESC Heart Fail. 2020;7(4):1488–501.
Hoes MF, Tromp J, Ouwerkerk W, Bomer N, Oberdorf-Maass SU, Samani NJ, Ng LL, Lang CC, van der Harst P, Hillege H, et al. The role of cathepsin D in the pathophysiology of heart failure and its potentially beneficial properties: a translational approach. Eur J Heart Fail. 2020;22(11):2102–11.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31.
Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39(Suppl 2):S244-252.
Acknowledgements
We thank all patients who generously took the time and donated samples to participate in this study.
Funding
This work was supported by grants from the National Research Foundation of Korea (Ministry of Science and ICT) (2019R1A2C2084099) and the Seoul National University Hospital research fund (26-2020-0060). The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report.
Author information
Authors and Affiliations
Contributions
HJL and SPL conceived the study, collected the data and performed analyses, and wrote the manuscript. HJL directly accessed and verified the underlying data reported in the manuscript. CSP contributed to data analysis and discussion. JBP, HKK, and YJK contributed to the discussion and reviewed/edited the manuscript. SL and SJP collected the data and reviewed/edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study complied with the Declaration of Helsinki, and the cohorts were approved by the institutional review boards of Seoul National University Hospital, Asan Medical Center, and Samsung Medical Center, with all study subjects providing written informed consent before enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional methods, additional figures S1–S5, additional table S1–S10.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, HJ., Park, C.S., Lee, S. et al. Systemic proinflammatory−profibrotic response in aortic stenosis patients with diabetes and its relationship with myocardial remodeling and clinical outcome. Cardiovasc Diabetol 22, 30 (2023). https://doi.org/10.1186/s12933-023-01763-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-023-01763-1