Abstract
Background
Insulin resistance and hyperinsulinemia in patients with type 2 diabetes (T2D) are adversely associated with the development and worsening of heart failure (HF). Herein, we sought to investigate the effect of canagliflozin on insulin concentrations and the associations of changes in insulin concentrations with HF-related clinical parameters in patients with T2D and HF.
Methods
This was a post-hoc analysis of the investigator-initiated, multicenter, open-label, randomized, controlled CANDLE trial for patients with T2D and chronic HF (UMIN000017669). The endpoints were the effects of 24 weeks of canagliflozin treatment, relative to glimepiride treatment, on insulin concentrations and the relationship between changes in insulin concentrations and clinical parameters of interest, including New York Heart Association (NYHA) classification. The effects of canagliflozin on those parameters were also analyzed by baseline insulin level.
Results
Among the participants in the CANDLE trial, a total of 129 patients (canagliflozin, n = 64; glimepiride, n = 65) who were non-insulin users with available serum insulin data both at baseline and week 24 were included in this analysis. Overall, the mean age was 69.0 ± 9.4 years; 75% were male; the mean HbA1c was 6.8 ± 0.7%; and the mean left ventricular ejection fraction was 59.0 ± 14.1%, with parameters roughly balanced between treatment groups. Canagliflozin treatment significantly reduced insulin concentrations at week 24 (p < 0.001), and the between-group difference (canagliflozin minus glimepiride) in those changes was − 3.52 mU/L (95% confidence interval, − 4.85 to − 2.19; p < 0.001). Decreases in insulin concentrations, irrespective of baseline insulin level, were significantly associated with improvement in NYHA class in patients treated with canagliflozin.
Conclusion
Our findings suggest that canagliflozin treatment in patients with T2D and HF ameliorated excess insulin overload, contributing to the improvement of clinical HF status.
Trial registration: University Medical Information Network Clinical Trial Registry, number 000017669, Registered on May 25, 2015.
Similar content being viewed by others
Introduction
Insulin resistance and hyperinsulinemia play a central role in the pathogenesis of obesity and metabolic syndrome, including type 2 diabetes (T2D), resulting in an increased risk of cardiovascular disease (CVD) [1,2,3]. Such insulin abnormalities can also adversely affect cardiac function and serve as independent risk factors for incident heart failure (HF) [4, 5]. Conversely, excess inflammation in the visceral adipose tissue evoked in HF induces systemic insulin resistance, and the resulting hyperinsulinemia exacerbates HF by continuous activation of insulin signaling in cardiac tissue [6, 7]. Thus, insulin abnormalities and HF form a vicious cycle, and accordingly represent strong candidate targets of HF therapy.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors have a unique mode of action to lower plasma glucose levels in an insulin-independent manner via an increase in urinary glucose excretion, which in turn can mitigate glucotoxicity and hyperinsulinemia [8]. Several experimental studies have also shown that SGLT2 inhibition attenuates systemic insulin resistance through complex actions in adipose tissues, liver, and skeletal muscles [9,10,11,12]. Clinical studies have demonstrated that SGLT2 inhibitor treatment induces favorable metabolic responses and improves insulin sensitivity in several tissues in patients with T2D [13,14,15,16].
Recent meta-analyses of cardiovascular (and/or renal) outcome trials (CVOT) with SGLT2 inhibitors showed that these agents reduce the risk of worsening HF and cardiovascular death in patients with T2D at high risk of cardiovascular events [17, 18]. More recent CVOTs also demonstrated that SGLT2 inhibitor therapy reduces the risk of HF-related clinical events specifically in patients with HF, irrespective of diabetes status [19, 20]. Given the close link between impaired insulin actions and HF, the correction of insulin resistance and hyperinsulinemia induced by SGLT2 inhibition likely contributed to the clinical benefits observed in those CVOTs [21]. However, the effects of SGLT2 inhibition on serum insulin concentrations and its relationship of SGLT2 inhibition with clinical impact remain poorly elucidated in those CVOTs, even in patients complicated with HF. Therefore, herein, we sought to investigate the effect of the SGLT2 inhibitor canagliflozin on serum insulin concentrations and the associations of such changes with HF-related clinical parameters of interest, using data obtained from the randomized CANDLE trial for patients with T2D and HF [22].
Methods
Study design and subjects
This was a post-hoc analysis of the CANDLE trial (UMIN000017669), an investigator-initiated, multicenter, prospective, randomized, open-label clinical trial in which the primary endpoint was the effect of 24 weeks of canagliflozin therapy, relative to glimepiride therapy, on N-terminal pro-brain natriuretic peptide (NT-proBNP) concentrations in patients with T2D and chronic HF (CHF) [22]. The CANDLE trial was approved by the institutional review boards of the individual sites and conducted in accordance with the Declaration of Helsinki. All participants provided written, informed consent prior to screening and randomization.
Details of the study design and inclusion and exclusion criteria have been reported previously [23]. Briefly, the key eligibility criteria were (i) adults, (ii) T2D, and (iii) CHF with New York Heart Association (NYHA) class I to III, with no change in NYHA class and background therapies for HF within 4 weeks prior to screening. Key exclusion criteria included type 1 diabetes, severe hepatic and/or renal dysfunction (estimated glomerular filtration rate [eGFR] < 45 mL/min/1.73m2 or on dialysis), NYHA class IV, history of diabetic ketoacidosis, diabetic coma, or hypoglycemic attack within 6 months prior to study enrollment, and history of CVD within 3 months prior to eligibility assessment.
Eligible participants were randomly allocated to receive either canagliflozin (100 mg daily) or glimepiride (starting dose 0.5 mg daily) add-on therapy at a ratio of 1:1 using a web-based minimization method balanced for age (< 65, ≥ 65 years), hemoglobin A1c (HbA1c) level (< 6.5%, ≥ 6.5%), and left ventricular ejection fraction (LVEF; < 40%, ≥ 40%) at the time of screening. All participants received the study therapy for 24 weeks. In participants assigned to the glimepiride group, adjustment of the glimepiride dose was allowed according to individual glycemic management and the local investigator’s judgment. Background medications for T2D, CHF, and other comorbidities were, in principle, unchanged during the study interval within clinically permissible ranges.
Measurements and endpoints
The details of the original outcome measures in the CANDLE trial have been described previously [23]. Briefly, vital sign recording and blood sample collection were mandatory at baseline and at week 24. Routine laboratory data, including glycemic parameters were, in principle, collected in the early morning when fasting and measured at each local site. Based on a previous report showing that fasting levels of plasma insulin were 11.2 ± 6.0 mU/L in Japanese patients with T2D [24], serum insulin values > 20 mU/L were considered inappropriate for fasting conditions and thereby excluded from the present analysis. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as serum insulin (mU/L) × plasma glucose (mg/dL)/405. NT-proBNP concentrations were measured at baseline and week 24 in a blinded manner at central commercial laboratories (SRL Inc., Tokyo, Japan). The percentage change in estimated plasma volume (ePV) from baseline to week 24 was calculate with the Strauss formula [25,26,27].
In the present analysis, we compared the effects of 24 weeks of canagliflozin therapy, relative to glimepiride, on serum insulin levels and other glycemic parameters. In addition, we assessed the relationship between changes in the insulin levels and other clinical parameters of interest, including NT-proBNP, obtained in the CANDLE trial. Furthermore, we compared the effects of two study therapies on those parameters by baseline serum insulin level. Among the prespecified full analysis set (FAS) of the CANDLE trial dataset, participants who were non-insulin users and had available serum insulin data (≤ 20 mU/L) both at baseline and week 24 were included in the present analysis.
Statistical analysis
Baseline demographics and clinical characteristics are expressed as number (%) for categorical variables and as mean ± standard deviation or median [interquartile range] for continuous variables where appropriate. Comparisons between the treatment groups were made using linear regression models for changes in serum insulin levels and glycemic parameters from baseline to week 24. Associations between changes from baseline to week 24 in serum insulin concentrations and clinical parameters of interest were assessed by calculating Pearson’s correlation coefficients. To investigate the influence of baseline serum insulin levels on treatment effects for those parameters at week 24, data were analyzed using linear regression models for NT-proBNP and linear mixed models for other parameters in subgroups according to baseline serum insulin level. The ratio (canagliflozin vs. glimepiride) of the proportional change from baseline to week 24 in NT-proBNP was estimated based on a natural logarithmic scale [28]. A p-value for the interaction between the study treatments and baseline serum insulin category on the NYHA class was calculated using an ordinal logistic regression model analysis.
All statistical analyses were performed using R software, version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) at a two-sided significance level of 0.05. No adjustments for multiplicity were considered in the present analyses.
Results
Participants
The flow chart of study participants is shown in Fig. 1. Among the FAS population (canagliflozin, n = 113; glimepiride, n = 120), two subjects were using insulin at baseline and were excluded from the analysis. In addition, 67 subjects were excluded due to lack of serum insulin data at baseline or week 24, and 35 subjects were excluded for serum insulin level > 20 mU/L. Finally, a total of 129 subjects (canagliflozin, n = 64; glimepiride, n = 65) were included in the present analysis. Baseline demographic and clinical characteristics of the analysis population are shown in Table 1. Regarding the HF-related parameters, the level of NT-proBNP was modest (median 228.0 [interquartile range 72.0− 421.0] pg/mL). Most patients had preserved LVEF and low/mild NYHA classes. Ischemia was the cause of HF in about half of the subjects. Regarding the T2D-related parameters, mean HbA1c was 6.8 ± 0.7%, and about half of the subjects had been receiving DPP-4 inhibitors, while about 40% of the subjects had not been taking any glucose-lowering agents at baseline.
Comparison of glycemic and insulin indices between groups
The mean changes from baseline to week 24 in HbA1c were 0.12% (95% confidence interval [CI], − 0.06 to 0.30) in the canagliflozin group and − 0.30% (95% CI, − 0.48 to –0.11) in the glimepiride group (between-group difference [canagliflozin minus glimepiride] 0.42% [95% CI, 0.16 to 0.68]; p = 0.002). The mean changes in glucose levels were –6.84 mg/dL (95% CI, − 14.03 to 0.34) in the canagliflozin group and − 13.08 mg/dL (95% CI, − 20.20 to − 5.95) in the glimepiride group (between-group difference 6.23 mg/dL [95% CI, − 3.89 to 16.35]; p = 0.227). These results were similar to those observed in the overall CANDLE trial population [22].
The mean insulin levels at baseline were 8.4 ± 3.9 mU/L in the canagliflozin group and 8.4 ± 4.7 mU/L in the glimepiride group. Serum insulin concentrations at week 24 were significantly reduced in the canagliflozin group and increased in the glimepiride group, with a between-group difference of − 3.52 mU/L (95% CI, − 4.85 to − 2.19; p < 0.001, Fig. 2A). Canagliflozin treatment also reduced HOMA-IR, while glimepiride treatment did not, with a between-group difference − 0.95 (95% CI, − 1.54 to − 0.36; p = 0.002, Fig. 2B).
Association between changes in serum insulin concentrations and clinical parameters of interest
Pearson’s correlations between changes in serum insulin concentrations and clinical parameters of interest from baseline to week 24 are shown in Table 2. In the canagliflozin group, changes in serum insulin concentrations were significantly correlated with those in systolic blood pressure (SBP), but not in other parameters, such as body mass index, lipid profiles, and NT-proBNP. Changes in serum insulin concentrations were also significantly associated with categorical changes in the NYHA class in the canagliflozin group, but not in the glimepiride group (Fig. 3A). This was also observed in the analyses to assess the association between changes in HOMA-IR and NYHA class (Fig. 3B).
Associations between changes in insulin indices and NYHA class. The data are expressed as the median (interquartile range) change from baseline to week 24 in serum insulin concentrations A and HOMA-IR B in subgroups stratified by the categorical changes in NYHA class at week 24. HOMA-IR homeostasis model assessment of insulin resistance, NYHA New York Heart Association
Effects of baseline serum insulin concentration on clinical measures of interest
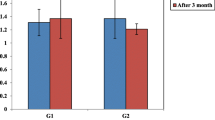
Between-group differences in changes from baseline to week 24 on clinical measures of interest in subgroups stratified by baseline serum insulin concentration (median 7.5 mU/L) are shown in Table 3. The treatment effects on those parameters did not differ between subgroups with baseline serum insulin concentration < 7.5 mU/L and ≥ 7.5 mU/L (all p for interaction > 0.09). The treatment effects on categorical changes in the NYHA class at week 24 were also similar according to the median serum insulin concentration at baseline (p for interaction = 0.095, Fig. 4, left). These findings were also similar when applying the HOMA-IR (Additional file 1 and Fig. 4, right).
Changes from baseline in NYHA class at week 24 in subgroups stratified by baseline median serum insulin concentrations (left) and HOMA-IR (right). The numbers next to the bars indicate the frequency of cases in which NYHA improved or worsened at week 24. Between-subgroup differences in the treatment effect on NYHA class are analyzed as Pfor interaction. HOMA-IR, homeostasis model assessment of insulin resistance; NYHA, New York Heart Association
Discussion
Major findings in the present analysis of data from the randomized CANDLE trial in patients with T2D and CHF were as follows: (1) 24 weeks of treatment with canagliflozin, relative to glimepiride, significantly reduced serum insulin concentrations and HOMA-IR, (2) the decrease in insulin concentrations was significantly correlated with reductions in SBP (3) the decreases in insulin concentrations, and even HOMA-IR, were also significantly associated with improvement in the NYHA class in patients treated with canagliflozin, and (4) those effects of canagliflozin treatment on clinical measures did not differ according to baseline levels of insulin and HOMA-IR. These findings suggest that canagliflozin-induced attenuation of excess insulin overload explains, in part, the clinical benefits on HF-related outcomes observed in recent CVOTs with SGLT2 inhibitors.
Systemic insulin resistance also causes chronic activation of local insulin signaling and energy disturbances in cardiac tissues, resulting in the development and deterioration of HF [4,5,6,7]. Thus, insulin abnormalities and insulin resistance are key drivers of the development of HF in T2D, and thereby represent possible therapeutic targets [29,30,31]. However, there is little clinical evidence on whether therapeutic interventions for insulin resistance can improve HF-related status and outcomes [32]. Currently, two conventional glucose-lowering agents, metformin and thiazolidinedione, are well known to improve insulin resistance and cause cardiovascular benefits [33, 34]. Metformin treatment is associated with clinical benefits in patients with T2D and HF [35], although thiazolidinedione is not recommended in patients with or at risk of HF due to enhanced sodium reabsorption at the renal proximal tubule and a resultant increased risk of incident HF [36, 37]. However, the potential risk of HF in patients with T2D is still higher than that in a non-T2D population, which imposes an excess risk of morbidity and mortality [38, 39].
SGLT2 inhibitors are glucose-lowering agents that increase urinary glucose excretion [8]. This unique mode of action of SGLT2 inhibitors mitigates glucose toxicity independently of insulin secretion, thereby protecting pancreatic beta-cell function and relieving excess insulin overload. To date, several experimental and clinical studies have demonstrated an improvement in insulin resistance with SGLT2 inhibition [9,10,11,12,13,14,15,16]. Recent CVOTs with SGLT2 inhibitors demonstrated a consistent reduction in the risk of HF-related events in patients with T2D at high risk of CVD or HF irrespective of diabetes status [17,18,19,20]. These findings indicate that the therapeutic effects of SGLT2 inhibitors on HF-related outcomes are, at least in part, beyond glycemic control. Intriguingly, it is speculated that a modest increase of ketone body levels via SGLT2 inhibition plays beneficial roles in cardiac energetic alterations and amelioration of insulin resistance [21]. Thus, an improvement in insulin resistance accompanied by SGLT2 inhibitor treatment is likely to, at least in part, mediate the reduction in the risk of HF-related events.
In a recent substudy from the Empire HF trial for patients with left ventricular systolic dysfunction (LVEF ≤ 40%) with or without T2D [40], Jensen et al. for the first time revealed that 12 weeks of empagliflozin treatment, relative to placebo, improved both hepatic and peripheral insulin resistance, accompanied by significant reductions in body weight and lean mass. Results of the Empire HF trial previously demonstrated that empagliflozin also reduced estimated extracellular volume, ePV, and pulmonary capillary wedge pressure [26, 41], suggesting key mechanisms of SGLT2 inhibition underlying early and sustained clinical benefits for HF-related events. In the present study from the CANDLE trial, we also found that canagliflozin treatment alleviated hyperinsulinemia and insulin resistance in patients with T2D and HF almost exhibiting HF with preserved ejection fraction (HFpEF). Importantly, we also previously reported that canagliflozin treatment reduced ePV in the overall CANDLE trial population, and even in the HFpEF subpopulation [22]. These findings suggest that SGLT2 inhibitor treatment provides favorable hemodynamic and metabolic alterations in patients with HF, irrespective of LVEF category. This may support the robust clinical benefits seen in CVOTs for both HF phenotypes, systolic dysfunction and HFpEF, although profound pathophysiological mechanisms and molecular actions of SGLT2 inhibitors are likely to differ between phenotypes [31]. Given the multifaceted mechanisms potentially underlying such clinical benefits of SGLT2 inhibition [42, 43], however, whether improvement of hyperinsulinemia and insulin resistance via SGLT2 inhibition directly affects clinical manifestations and prognosis in patients with HF remains poorly understood.
Hyperinsulinemia up-regulates the expression of cardiac and renal sodium-hydrogen exchanger (NHE) isoforms, leading to cardiac dysfunction and renal sodium retention and excess body fluid burden [31, 44]. Recent experimental studies revealed that SGLT2 inhibition blocked NHE activation [45,46,47], and that is considered a promising mechanism underlying the clinical benefits of SGLT2 inhibitors for HF [31, 48]. Additionally, in our study, canagliflozin treatment alleviated hyperinsulinemia and insulin resistance, and those changes were associated with SBP reduction and improvement in NYHA class. This suggests that SGLT2 inhibitor treatment improved the clinical manifestations of HF via correction of hyperinsulinemia and insulin resistance located upstream of HF concomitant with T2D. To our knowledge, this is the first clinical report to show an SGLT2 inhibitor-mediated association between improvement in insulin resistance and relief of HF symptoms. However, our finding in this post-hoc analysis of the CANDLE trial may be still a hypothesis-generating. Further studies are needed to investigate whether the improvement in insulin homeostasis with SGLT2 inhibitor treatment has a direct impact on improving cardiac function and prognosis in patients with HF.
This present study has several potential limitations. First, this was a post-hoc analysis of the CANDLE trial that was not designed or powered to evaluate the effects of canagliflozin treatment on insulin-related parameters. Especially, the number of non-insulin users in the present analysis was small, although most participants (97%) to the entire CANDLE trial were non-insulin users. Second, although the study protocol specified fasting blood sampling, some insulin data were unlikely indicative of fasting sampling. To minimize the possibility of non-fasting blood sampling, the present analysis excluded subjects with insulin levels ≥ 20 mU/L, based on a previous report that fasting levels of plasma insulin were 11.2 ± 6.0 mU/L in Japanese patients with T2D [24]. Third, in general it would be quite reasonable that the HOMA-IR resultantly reduces after SGLT2 inhibitor treatment that decreases both serum insulin and glucose levels. It is currently uncertain whether HOMA-IR is a suitable method to assess insulin resistance in individuals receiving treatment with an SGLT2 inhibitor [49]. However, in the CANDLE trial, no data on other insulin resistance indices were assessed with gold-standard methods, such as the hyperinsulinemic-euglycemic clamp test, the Matsuda-DeFronzo index [50], and adipose insulin resistance index [51]. Further research would be therefore needed to assess the effects of SGLT2 inhibitor on those indices and their association with clinical HF status. Fourth, we have no detailed clinical information on events that would affect insulin levels, such as changes in glucose-lowering agents or addition of insulin treatment, during the follow-up period. Fifth, we were not able to evaluate an association of reductions in insulin levels and improvements in insulin resistance with the risk of HF-related events in the CANDLE trial. Finally, the CANDLE trial included Japanese patients with clinically stable T2D and HF, mostly of the HFpEF phenotype; therefore, generalizability of our findings to other clinical situations and populations is uncertain.
Conclusion
Our findings suggest that in patients with T2D and HF, canagliflozin treatment ameliorated excess insulin overload and insulin resistance, contributing to the improvement in clinical HF status. This may explain, in part, the clinical benefits of SGLT2 inhibitors on HF-related outcomes.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author upon reasonable request (tanakaa2@cc.saga-u.ac.jp).
Abbreviations
- CHF:
-
Chronic heart failure
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- CVOT:
-
Cardiovascular outcome trial
- eGFR:
-
Estimated glomerular filtration rate
- ePV:
-
Estimated plasma volume
- FAS:
-
Full analysis set
- HF:
-
Heart failure
- HFpEF:
-
Heart failure with preserved ejection fraction
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- LVEF:
-
Left ventricular ejection fraction
- NHE:
-
Sodium-hydrogen exchanger
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- NYHA:
-
New York Heart Association
- SBP:
-
Systolic blood pressure
- SGLT2:
-
Sodium-glucose cotransporter 2
- T2D:
-
Type 2 diabetes
References
Burchfiel CM, Sharp DS, Curb JD, Rodriguez BL, Abbott RD, Arakaki R, Yano K. Hyperinsulinemia and cardiovascular disease in elderly men: the Honolulu Heart Program. Arterioscler Thromb Vasc Biol. 1998;18(3):450–7.
Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and risk of cardiovascular disease: a meta-analysis. Circulation. 1998;97(10):996–1001.
DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53(7):1270–87.
Marfella R, Sardu C, Mansueto G, Napoli C, Paolisso G. Evidence for human diabetic cardiomyopathy. Acta Diabetol. 2021;58(8):983–8.
Ingelsson E, Sundström J, Arnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294(3):334–41.
Shimizu I, Yoshida Y, Katsuno T, Tateno K, Okada S, Moriya J, Yokoyama M, Nojima A, Ito T, Zechner R, et al. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab. 2012;15(1):51–64.
Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, Tateno K, Moriya J, Yokoyama M, Nojima A, et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J Clin Investig. 2010;120(5):1506–14.
Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60(2):215–25.
Obata A, Kubota N, Kubota T, Iwamoto M, Sato H, Sakurai Y, Takamoto I, Katsuyama H, Suzuki Y, Fukazawa M, et al. Tofogliflozin improves insulin resistance in skeletal muscle and accelerates lipolysis in adipose tissue in male mice. Endocrinology. 2016;157(3):1029–42.
Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, Mayoux E, Kaneko S, Ota T. SGLT2 Inhibition by Empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing m2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–49.
Xu L, Nagata N, Chen G, Nagashimada M, Zhuge F, Ni Y, Sakai Y, Kaneko S, Ota T. Empagliflozin reverses obesity and insulin resistance through fat browning and alternative macrophage activation in mice fed a high-fat diet. BMJ Open Diabetes Res Care. 2019;7(1): e000783.
Petito-da-Silva TI, Souza-Mello V, Barbosa-da-Silva S. Empaglifozin mitigates NAFLD in high-fat-fed mice by alleviating insulin resistance, lipogenesis and ER stress. Mol Cell Endocrinol. 2019;498: 110539.
Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle HJ. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Investig. 2014;124(2):499–508.
Cusi K, Bril F, Barb D, Polidori D, Sha S, Ghosh A, Farrell K, Sunny NE, Kalavalapalli S, Pettus J, et al. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21(4):812–21.
Matsuba R, Matsuba I, Shimokawa M, Nagai Y, Tanaka Y. Tofogliflozin decreases body fat mass and improves peripheral insulin resistance. Diabetes Obes Metab. 2018;20(5):1311–5.
Fuchigami A, Shigiyama F, Kitazawa T, Okada Y, Ichijo T, Higa M, Hiyoshi T, Inoue I, Iso K, Yoshii H, et al. Efficacy of dapagliflozin versus sitagliptin on cardiometabolic risk factors in Japanese patients with type 2 diabetes: a prospective, randomized study (DIVERSITY-CVR). Cardiovasc Diabetol. 2020;19(1):1.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet (London, England). 2019;393(10166):31–9.
McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, Pratley R, Greenberg M, Wang S, Huyck S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA cardiology. 2021;6(2):148–58.
Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet (London, England). 2020;396(10254):819–29.
Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
Hattori Y. Insulin resistance and heart failure during treatment with sodium glucose cotransporter 2 inhibitors: proposed role of ketone utilization. Heart Fail Rev. 2020;25(3):403–8.
Tanaka A, Hisauchi I, Taguchi I, Sezai A, Toyoda S, Tomiyama H, Sata M, Ueda S, Oyama JI, Kitakaze M, et al. Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: a randomized trial (CANDLE). ESC heart failure. 2020;7(4):1585–94.
Tanaka A, Inoue T, Kitakaze M, Oyama J, Sata M, Taguchi I, Shimizu W, Watada H, Tomiyama H, Ako J, et al. Rationale and design of a randomized trial to test the safety and non-inferiority of canagliflozin in patients with diabetes with chronic heart failure: the CANDLE trial. Cardiovasc Diabetol. 2016;15:57.
Shimabukuro M, Higa N, Asahi T, Yamakawa K, Oshiro Y, Higa M, Masuzaki H. Impaired glucose tolerance, but not impaired fasting glucose, underlies left ventricular diastolic dysfunction. Diabetes Care. 2011;34(3):686–90.
Dekkers CCJ, Sjostrom CD, Greasley PJ, Cain V, Boulton DW, Heerspink HJL. Effects of the sodium-glucose co-transporter-2 inhibitor dapagliflozin on estimated plasma volume in patients with type 2 diabetes. Diabetes Obes Metab. 2019;21(12):2667–73.
Jensen J, Omar M, Kistorp C, Tuxen C, Gustafsson I, Køber L, Gustafsson F, Faber J, Malik ME, Fosbøl EL, et al. Effects of empagliflozin on estimated extracellular volume, estimated plasma volume, and measured glomerular filtration rate in patients with heart failure (Empire HF Renal): a prespecified substudy of a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2021;9(2):106–16.
Tanaka A, Shimabukuro M, Teragawa H, Okada Y, Takamura T, Taguchi I, Toyoda S, Tomiyama H, Ueda S, Higashi Y, et al. Reduction of estimated fluid volumes following initiation of empagliflozin in patients with type 2 diabetes and cardiovascular disease: a secondary analysis of the placebo-controlled, randomized EMBLEM trial. Cardiovasc Diabetol. 2021;20(1):105.
Tanaka A, Toyoda S, Imai T, Shiina K, Tomiyama H, Matsuzawa Y, Okumura T, Kanzaki Y, Onishi K, Kiyosue A, et al. Effect of canagliflozin on N-terminal pro-brain natriuretic peptide in patients with type 2 diabetes and chronic heart failure according to baseline use of glucose-lowering agents. Cardiovasc Diabetol. 2021;20(1):175.
Abel ED. Insulin signaling in the heart. Am J Physiol Endocrinol Metab. 2021;321(1):E130-e145.
Riehle C, Abel ED. Insulin Signaling and Heart Failure. Circ Res. 2016;118(7):1151–69.
Packer M. Differential pathophysiological mechanisms in heart failure with a reduced or preserved ejection fraction in diabetes. JACC Heart failure. 2021;9(8):535–49.
Son TK, Toan NH, Thang N, Le Trong TH, Tien HA, Thuy NH, Van Minh H, Valensi P. Prediabetes and insulin resistance in a population of patients with heart failure and reduced or preserved ejection fraction but without diabetes, overweight or hypertension. Cardiovasc Diabetol. 2022;21(1):75.
Schramm TK, Gislason GH, Vaag A, Rasmussen JN, Folke F, Hansen ML, Fosbøl EL, Køber L, Norgaard ML, Madsen M, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32(15):1900–8.
Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–31.
Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, Vanderloo SE, McAlister FA. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. 2013;6(3):395–402.
Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11(8):861–6.
Hernandez AV, Usmani A, Rajamanickam A, Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis and meta-regression analysis of placebo-controlled randomized clinical trials. Am J Cardiovasc Drugs. 2011;11(2):115–28.
Birkeland KI, Bodegard J, Eriksson JW, Norhammar A, Haller H, Linssen GCM, Banerjee A, Thuresson M, Okami S, Garal-Pantaler E, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020;22(9):1607–18.
Zareini B, Blanche P, D’Souza M, Elmegaard Malik M, Nørgaard CH, Selmer C, Gislason G, Kristensen SL, Køber L, Torp-Pedersen C, et al. Type 2 diabetes mellitus and impact of heart failure on prognosis compared to other cardiovascular diseases: a nationwide study. Circ Cardiovasc Qual Outcomes. 2020;13(7): e006260.
Jensen J, Omar M, Kistorp C, Tuxen C, Gustafsson I, Køber L, Gustafsson F, Faber J, Forman JL, Møller JE, et al. Metabolic effects of empagliflozin in heart failure: a randomized, double-blind, and placebo-controlled trial (Empire HF Metabolic). Circulation. 2021;143(22):2208–10.
Omar M, Jensen J, Frederiksen PH, Kistorp C, Videbæk L, Poulsen MK, Möller S, Ali M, Gustafsson F, Køber L, et al. Effect of Empagliflozin on Hemodynamics in Patients With Heart Failure and Reduced Ejection Fraction. J Am Coll Cardiol. 2020;76(23):2740–51.
Tanaka A, Node K. Emerging roles of sodium-glucose cotransporter 2 inhibitors in cardiology. J Cardiol. 2017;69(3):501–7.
Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: jacc state-of-the-art review. J Am Coll Cardiol. 2020;75(4):422–34.
Packer M. Activation and inhibition of sodium-hydrogen exchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation. 2017;136(16):1548–59.
Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, et al. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na(+)/H(+) exchanger, lowering of cytosolic Na(+) and vasodilation. Diabetologia. 2018;61(3):722–6.
Trum M, Riechel J, Lebek S, Pabel S, Sossalla ST, Hirt S, Arzt M, Maier LS, Wagner S. Empagliflozin inhibits Na(+) /H(+) exchanger activity in human atrial cardiomyocytes. ESC heart failure. 2020;7(6):4429–37.
Borges-Júnior FA, Silva Dos Santos D, Benetti A, Polidoro JZ, Wisnivesky ACT, Crajoinas RO, Antônio EL, Jensen L, Caramelli B, Malnic G et al: Empagliflozin inhibits proximal tubule NHE3 activity, preserves GFR, and restores euvolemia in nondiabetic rats with induced heart failure. JASN 2021, 32(7):1616–1629.
Packer M. Role of the sodium-hydrogen exchanger in mediating the renal effects of drugs commonly used in the treatment of type 2 diabetes. Diabetes Obes Metab. 2018;20(4):800–11.
So A, Sakaguchi K, Okada Y, Morita Y, Yamada T, Miura H, Otowa-Suematsu N, Nakamura T, Komada H, Hirota Y, et al. Relation between HOMA-IR and insulin sensitivity index determined by hyperinsulinemic-euglycemic clamp analysis during treatment with a sodium-glucose cotransporter 2 inhibitor. Endocr J. 2020;67(5):501–7.
Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol. 2011;11:158.
Søndergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD: How to measure adipose tissue insulin sensitivity. J Clin Endocrinol Metab 2017, 102(4):1193-1199.
Acknowledgements
The authors thank all the participants, investigators, board members, and medical staff members involved in the CANDLE trial.
Funding
The work was funded by Mitsubishi Tanabe Pharma Corporation. The funders of the trial had no role in the study design, data collection, analysis or interpretation, or writing of the report.
Author information
Authors and Affiliations
Consortia
Contributions
AT, MS, IT, AS, ST, HW, JA, and KN contributed to the study conception, design, and operation. Funding for the study was acquired by KN, who was the Principal Investigator for the CANDLE trial. The data analyses and interpretations were performed by AT, TI, and KN. TI was responsible for the statistical analyses. The first draft of the manuscript was written by AT, and all authors reviewed subsequent drafts of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The ethical committees of the participating institutions approved the study protocol. Written, informed consent for participation in the study was obtained from all subjects. This trial was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Consent for publication
All authors have read and approved the submission of the manuscript. The manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language. If the manuscript is accepted, we will approve it for publication in Cardiovascular Diabetology.
Competing interests
AT received honoraria from Boehringer Ingelheim and research funding from GlaxoSmithKline and Takeda. TI received lecture fees from JCR Pharmaceuticals and Kyowa Kirin and outsourcing fees from Organization for Clinical Medicine Promotion. MS received honoraria from Mitsubishi Tanabe. ST received honoraria from Ono, Bayer, Novartis, Otsuka, Daiichi Sankyo, and AstraZeneca and research funding from Boston Scientific. HW has received honoraria for lectures from Mitsubishi Tanabe, Sumitomo Dainippon, Sanwa Kagaku Kenkyusyo, Takeda, Sanofi, Kowa, MSD, Nippon Boehringer Ingelheim, Eli Lilly, Novo Nordisk, AstraZeneca, Ono, Astellas, Kyowa Kirin, Terumo, Taisho, Abbott Japan, and Kissei and research grants from Mitsubishi Tanabe, Takeda, Nippon Boehringer Ingelheim, Kissei, Novo Nordisk, Kyowa Kirin, Eli Lilly, Taisho, Astellas, Ono, Sanofi, MSD, Kowa, LifeScan Japan, Teijin, Daiichi Sankyo, Sumitomo Dainippon, and Sanwa Kagaku Kenkyusyo. JA received speaking honoraria from Mitsubishi Tanabe, Boehringer Ingelheim, and Astra Zeneca. KN has received honoraria from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim Japan, Daiichi Sankyo, Eli Lilly Japan, Kowa, Mitsubishi Tanabe, Mochida, MSD, Novartis, Ono, Otsuka, Takeda, and Tsumura, research grants from Asahi Kasei, Astellas, Boehringer Ingelheim Japan, Fuji, Mitsubishi Tanabe, Mochida, Novartis, and Teijin and scholarship from Bayer, Daiichi Sankyo, Takeda, Teijin, and Japan Lifeline. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Between-group differences in changes at week 24 for clinical measures of interest in subgroups stratified by baseline HOMA-IR.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tanaka, A., Imai, T., Shimabukuro, M. et al. Association between serum insulin levels and heart failure-related parameters in patients with type 2 diabetes and heart failure treated with canagliflozin: a post-hoc analysis of the randomized CANDLE trial. Cardiovasc Diabetol 21, 151 (2022). https://doi.org/10.1186/s12933-022-01589-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01589-3