Abstract
Background
Sleep duration is associated with mortality. However, prior studies exploring whether sleep duration predicts subsequent long-term mortality in patients with diabetes are limited. This study aims to examine whether metabolic factors affect the associations between baseline sleep duration and subsequent risks of all-cause, expanded, and non-expanded cardiovascular disease (CVD) mortalities among patients with type 2 diabetes (T2D).
Methods
A total of 12,526 T2D patients aged 30 years and older, with a follow-up period ≥ 3 years, were identified from the Diabetes Case Management Program of a medical center in Taiwan. Sleep duration was measured using computerized questionnaires by case managers, and the time frame for this question was 1 month prior to the interview date. Sleep duration in relation to subsequent mortality from all causes, expanded CVD, and non-expanded CVD was examined using Cox proportional hazard models.
Results
Within 10 years of follow-up, 2918 deaths (1328 CVD deaths and 1590 non-CVD deaths) were recorded. A J-shaped association was observed for all-cause, expanded CVD, and non-expanded CVD mortalities, and the lowest risks were observed for patients with 5–7 h of sleep. The significant joint effects included sleep duration of more or less than 7 h with age ≥ 65 years [adjusted HRs: 4.00 (3.49–4.60)], diabetes duration ≥ 5 years [1.60 (1.40–1.84)], age at diabetes diagnosis ≤ 45 years [1.69 (1.38–2.07)], insulin use [1.76 (1.54–2.03)], systolic blood pressure/diastolic blood pressure > 130/85 mmHg [1.24 (1.07–1.43)], triglyceride ≥ 150 mg/dL [1.38 (1.22–1.56)], HbA1c ≥ 7% [1.31 (1.13–1.52)], and body mass index < 27 kg/m2 [1.31 (1.17–1.45)] for all-cause mortality.
Conclusion
A J-shaped association was observed between sleep duration and all-cause and expanded CVD mortality, and a sleep duration of 5–7 h had the lowest mortality risk. Sleep duration also showed significant synergistic interactions with diabetes duration but shared an antagonistic interaction with age and obesity.
Similar content being viewed by others
Background
Sleep occupies approximately one-third of the day of most people. According to the National Sleep Foundation (NSF) in the USA, sleep is essential for a person’s health and wellbeing [1]. NSF recommends 7–9 and 7–8 h of sleep for adults aged 18–64 years and older adults ≥ 65 years, respectively [1]. Sleep disorders have been linked with a number of health problems, including obesity [2, 3], diabetes [4], cardiometabolic disease [2, 5,6,7], and mortality [8,9,10]. Itani and Jike et al. used the same methodology to explore the effects of sleep duration on different health outcomes in adults aged ≥ 20 years [11, 12]. They found that deviations from optimal sleep duration pose a substantial threat to health. A long sleep duration is significantly associated with pooled adjusted risk ratios (RRs) of 1.08 for obesity, 1.24 for coronary heart disease, 1.25 for cardiovascular disease (CVD), 1.26 for diabetes mellitus, and 1.39 for mortality [12]. The corresponding pooled adjusted RRs for short sleepers compared with normal sleepers are 1.38, 1.26, 1.16, 1.37, and 1.12 [11]. Previous studies provided different definitions of long and short sleep, but most studies defined long sleep as having a duration greater than 8 or 9 h per day and short sleep as having a duration less than 5 or 6 h per day [11, 12].
Good-quality sleep with adequate hours plays an effective role in cognitive performance [13] and preventing chronic health conditions, CVD, and even immature death [14]. Sleep difficulties have been reported as a long-COVID 19 sequelae [15]. Sleep is a critical component of healthy lifestyles, especially among patients with diabetes [16]. It is important for persons with type 2 diabetes (T2D) because they are susceptible to developing micro- and macro-vascular diseases [17], and sleep disorders affect blood sugar and insulin levels [18, 19]. A recent study reported sleep disturbance is significantly associated with an increased risk of CVD and all-cause mortality in patients with new-onset T2D [20], and a long sleep duration (≥ 9 h/day) has been shown to be associated with an increased all-cause mortality in persons with T2D [21]. A higher level of HbA1c can be found in patients with diabetes with short and long sleep durations than in those with medium sleep duration [22]. A review article linked the epidemiologic and lab evidence of physiological mechanisms by which insufficient sleep and sleep disorders increase the risk of diabetes [23]. A cross-sectional study reported that people with less than 5 h of sleep had two times higher prevalence of T2D than those with 7–9 h of sleep [24]. An epidemiologic cohort study found that sleep problems are associated with an increased likelihood of incident T2D [25]. Two population-based studies reported that short sleep duration is more common among persons with diabetes (37%) [26] than among the general population (24%) [7]. Furthermore, T2D may affect sleep. Higher prevalence rates of insomnia (50% vs. 31%) and sleeping pill use (26% vs. 6%) and lower sleep quality level were found in persons with diabetes than in those without diabetes [27]. However, the effects of sleep duration on other adverse health outcomes among patients with T2D are unclear. Related research on persons with T2D focused on short-term outcomes, such as glucose level. A recent study has found that variability in sleep duration within a week is the most important modifiable factor associated with sleep for HbA1c level in diabetes care [22], followed by the mean total sleep duration within a week. However, limited evidence is available regarding the effects of sleep duration on long-term outcomes, such as mortality, in patients with T2D.
Recent clinical evidence has suggested that sleep duration affects mortality [9, 28,29,30,31,32,33] in various populations. Most prior studies were conducted in the general population [9, 31, 32] and middle-aged and elderly [28, 29]. Three recent studies have examined the associations between sleep duration and mortality in patients with diabetes [21, 30, 33]. Although they all found a J-shaped association between sleep duration and all-cause mortality in patients with T2D, two studies reported increased risk of death in short (≤ 5 h) and long (≥ 9 h) sleep durations [30, 33], and one study did not observe the significant risk of death in short sleep duration [21]. In addition to the inconsistent findings on the independent effect of sleep, there existed conflict results on the effect modification of sleep duration with diabetes-related factors. Both Wang et al. [33] and Gu et al. [21] explored the interaction of sleep duration with age at diagnosis, diabetes duration, and type of anti-diabetes treatment. Wang’s study conducted in the US, reported significant interaction of the aforementioned three factors and sleep duration on mortality [33]. On the contrary, Gu’s study conducted in Korea, did not observe such interaction effects [21]. Furthermore, the interactions of sleep duration with some metabolic factors of hyperlipidemia and obesity have not been assessed. Therefore, the present study aimed to examine the associations of baseline sleep duration with subsequent risks of all-cause, expanded CVD, and non-expanded CVD mortality among Chinese persons with T2D interacted with age, diabetes duration, early onset of diabetes mellitus, insulin use, hypertension, hyperlipidemia, HbA1c, and obesity. Three additive interaction measures of relative excess risk due to interaction (RERI), the proportion attributable to interaction (AP), and the synergy index (S index) were reported to indicate the direction of interaction.
Methods
Study participants and data sources
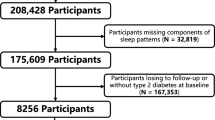
A retrospective cohort study was carried out among persons who registered in the Diabetes Care Management Program (DCMP) of China Medical University Hospital (CMUH). DCMP is a case management program set up by the National Health Insurance Administration in 2001. Participants included those who were diagnosed with T2D in accordance with the criteria of American Diabetes Association (International Classification Disease, Ninth Revision, Clinical Modification, abbreviated as ICD-9-CM; code 250). The eligibility criterion of the retrospective cohort study was all enrollees in the DCMP registry between November 2001 and April 2016. Under the inclusion criteria, this cohort was open or dynamic, i.e., each eligible person was allowed to enter this study at different time points. Persons who can provide at least a 3-year follow-up period were also included to ensure that the follow-up period was sufficiently long. The exclusion criteria included persons with gestational diabetes (ICD-9-CM code 648.83) or type 1 diabetes (ICD-9-CM code 250. × 1/ × 3, n = 448), persons under the age of 30 years (n = 504), and persons who followed up < 3 years to rule out the possibility of reverse causality (n = 1007). The exclusion criteria enhanced the homogeneity of the study population to increase the internal validity of the study findings. A total of 16,414 enrolled patients with T2D met the above criteria for a retrospective cohort study. Finally, 12,526 patients were included in the analysis after those without information on sleep and baseline characteristics were excluded (Additional file 1: Fig. S1).
The computerized database of persons with T2D registered in the DCMP of CMUH provided information on lifestyle behaviors, vital signs, annual eye examinations, annual self-care education and assessment, and laboratory test results. The DCMP contained information on lifestyle behaviors, such as sleep duration, alcohol drinking, smoking, dietary habits, and regular exercise. Laboratory tests involved HbA1c, fasting plasma glucose (FPG), serum creatinine, triglyceride (TG), high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), and total cholesterol (TC). The medication used included insulin, oral hypoglycemic agents, cholesterol lowering agents (e.g., statins [HMG-CoA reductase inhibitors]), and hypertension medications (e.g., calcium channel blockers). This study was approved by the Ethical Review Board of CMUH (CMUH109-REC1-197).
Measurements
The enrollees underwent medical tests for vital signs, anthropometric measurements, blood, urine, and lifestyle behaviors. Medical history of previous or current disease status was also obtained by a case management nurse through a standardized computerized questionnaire upon entering the DCMP. Vital signs, anthropometric measurements, medical history, and lifestyle behaviors were recorded annually. The variables used in this study are shown below.
Sleep duration and sleep-related variables
Sleep duration was assessed with the question “What are typical hours of sleep in a 24-h period?” The time frame for this question was a month prior to the interview date. This study used the baseline sleep duration that was measured on the index date, i.e., the entry date for a subject to the DCMP by a care manager with a computerized questionnaire. Various cutoff points for sleep duration have been reported in several studies [9, 28,29,30,31,32,33], and the cutoff points for six classes were adopted as follows: ≤ 4, 5–6, 7, 8, 9–10, and > 10 h. The cutoff points were determined with a slight modification from two meta-analyses of prospective studies [9, 31]. The diagnostic criteria of obstructive sleep apnea (OSA) and sleep disorders were ascertained through electronic outpatient records by the ICD-9-CM diagnosis codes for diagnosis of OSA (ICD-9-CM codes 327.23, 780.51, 780.53, and 780.57) and sleep disorders (291.82, 292.85, 307.4, 307.41, 307.42, 307.45, 307.46, 307.47, 307.48, 307.49, 327.11, 327.12, 327.2, 327.21, 327.24, 327.26, 327.27, 327.29, 327.3, 327.31, 327.32, 327.33, 327.34, 327.35, 327.36, 327.37, 327.39, 327.42, 327.43, 327.52, 327.53, 327.59, 327.8, 333.94, 347.0, 347.00, 347.01, 347.10, 347.11, 780.5, 780.55, 780.56, 780.58, and 780.59). The hypnotic-sedative medications comprised benzodiazepine (BZD), non-BZD (or Z-drug), anti-depressant (trazodone, mirtazapine, and doxepin), and anti-psychotics (sulpiride, olanzapine, clothiapine, chlorpromazine, risperidone, and quetiapine).
Anthropometric measurements
Anthropometric measurements included weight, height, body mass index (BMI), systolic blood pressure (SBP)/diastolic blood pressure (DBP), and pulse pressure. The weight and height of an individual were measured using an auto-anthropometer (super-view, HW-666). The participants were asked to remove their shoes and wear light clothing during the measurement. BMI was calculated as weight (kg)/(height)2 (m2). Blood pressure was measured for SBP and DBP by using an electronic device (OMRON, HEM-770A, Japan).
Laboratory examination
A 12-h overnight fasting was required before blood collection, and the samples were sent for analysis within 4 h after blood collection. The serum levels of creatinine, LDL-C, HDL-C, TG, and TC were measured by a biochemical auto-analyzer (Beckman Coulter Synchron System, AU5800, Fullerton, CA, USA) at the Clinical Laboratory Department of a medical center. Whole-blood HbA1c level was measured through boronate-affinity high-performance liquid chromatography (Premier Hb9210™, Trinity Biotech Plc, IDA business Park, Ireland) assay (reference range, 4.6–6.5%) with inter- and intra-assay coefficient variations (CVs) of 1.50–1.62% and 0.85–1.62%, respectively. The linearity was from 3.8% HbA1c to 18.5% HbA1c to ensure accuracy for the whole population with diabetes. FPG was measured in blood obtained using NaF tubes, each of which contained 5 mg of sodium fluoride to inhibit glucose metabolism and 4 mg of potassium oxalate to chelate calcium and prevent coagulation. Inter- and intra-assay CVs were both at 4%. TC and TG were measured in serum. TG levels were determined by an enzymatic colorimetric method with inter- and intra-assay CVs of 6.8% and 5%, respectively. LDL-C and HDL-C levels were measured using a direct method with inter- and intra-assay CVs of 4.5% and 3% for LDL-C, respectively, and 4.5% for HDL-C. Estimated glomerular filtration rate (eGFR) was derived based on serum creatinine levels in accordance with the following Chronic Kidney Disease Epidemiology Collaboration equation [34]: eGFR (mL/min/1.73 m2) = 141 \(\times\) min (Scr/κ,1)α \(\times\) max (Scr/κ,1)−1.209 \(\times\) 0.993age \(\times\) 1.018 (if female) \(\times\) 1.159 (if black); Scr: serum creatinine, κ: 0.7 for females and 0.9 for males, α: − 0.329 for females and − 0.411 for males. In this equation, min stands for the minimum of Scr/κ or 1, and max stands for the maximum of Scr/κ or 1.
Medication-related variable
The variables for pharmacologic agent use were derived from the DCMP dataset. The anti-diabetic drugs contained insulin and oral hypoglycemic agents, such as metformin, sulfonylurea, thiazolidinedione, meglitinide, and biguanide. Other medication-related variables consisted of hypertension, cardiovascular, and hyperlipidemia medications. All of these medications were each divided into two groups (yes and no).
The medications for hypertension included angiotensin-converting enzyme inhibitor, angiotensin II receptor blockers, calcium channel blockers, β-blockers, α-blockers, and diuretics. The medications for CVD included antiarrhythmic, anti-coagulants, antiplatelet, digoxin, and nitrates. The medications for hyperlipidemia consisted of fibrates (including bezafibrate, clofibrate, gemfibrozil, and fenofibrate), statins (including atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin), and ezetimibe.
Comorbidities and diabetic complications
Baseline metabolic morbidities consisted of hypertension and hyperlipidemia. Baseline diabetic acute complications included hyperglycemic hyperosmolar nonketotic coma (HHNK), severe hypoglycemia, and diabetic ketoacidosis (DKA). Chronic complications were stroke, coronary artery diseases, severe hypoglycemia, and peripheral neuropathy. All of these diabetic comorbidities were each divided into two groups (yes and no).
Outcome measures
The primary outcomes were total deaths and deaths from expanded and non-expanded CVD diseases, which were identified from the annual record linkage with the National Death Datasets provided by Taiwan Ministry of Health and Welfare by using basic information (personal identification number and date of birth). All patients were followed up from the index date to August 2021 or withdrawal from the DCMP or death. The index date was the entry date into the DCMP. The underlying cause of death was classified in accordance with the rules of ICD-9-CM from 2006 to 2008 and the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) from 2009 to 2021. Expanded CVD mortality was defined as death from CVD (ICD-9-CM codes 390–459, ICD-10-CM codes I00–I99), plus diabetes (ICD-9-CM code 250, ICD-10-CM codes E10–E14), and plus kidney diseases (ICD-9-CM 580–589; ICD-10-CM N00–N29), whereas non-expanded CVD mortality was defined as total deaths not from expanded CVD causes [35].
Statistical analysis
Simple descriptive analyses of proportion were performed for categorical variables and mean with standard deviation for continuous variables. Then, mortality cumulative incidence was estimated using the Kaplan–Meier (K–M) approach, presented as plots. Log-rank tests were applied to examine the differences in K–M mortality curves between subgroups of baseline sleep characteristics. Multivariate Cox proportional hazard models were used to estimate the hazard ratios (HRs) and their 95% confidence intervals (CIs). The assumption of proportionality was tested by entering an interaction (product) term for variables of sleep characteristics and person–time in the Cox models. The p for linear trend was reported for ordinal variables of sleep characteristics. Restricted cubic splines were used in the Cox models to examine the presence of a dose response or non-linear association of sleep duration as continuous variable with mortality. In addition, sensitivity analyses were performed to evaluate whether the findings of this study were similar under two conditions. One condition is that when patients with stroke, severe hypoglycemia, HHNK syndrome, and DKA were excluded for assessing the impact of comorbidities and the other condition is to include patients aged under 30 years as the proportion of this subgroup is increasing.
The interaction of sleep duration with age, diabetes-related factors, metabolic factors, and obesity in association with mortality was examined using three measures of additive interaction: RERI, AP, and S index [36]. In the absence of additive interaction, RERI and AP are equal to 0, and the S index is equal to 1. In the presence of a negative interaction, PERI or AP < 0 and S index < 1. In the presence of a positive interaction, PERI or AP > 0 and S index > 1. The analyses were performed with SAS version 9.4 (SAS, Cary, NC). Two-tailed p-values were considered, and a p-value < 0.05 indicated statistical significance.
Results
Of the 12,526 participants enrolled in the DCMP, 2918 died (including 1328 expanded CVD deaths and 1590 non-expanded CVD deaths) during the mean 10.82 years of follow-up (standard deviation of 3.90 years). Table 1 presents the associations between sleep duration categories (≤ 4, 5–6, 7, 8, 9–10, and > 10) and baseline sociodemographic factors, lifestyle behaviors, diabetes-related variables, and complications. The patients who slept for more than 10 h/day had high proportions in female gender, non-alcohol drinking, physical inactive, age at diabetes diagnosis > 45 years, combination of oral hypoglycemic drug and insulin use, hypertension, stroke, coronary artery disease, peripheral neuropathy, hypertension medication use, and cardiovascular medication use than patients who slept for 7 h/day. On the contrary, they had lower mean values in BMI and eGFR but higher mean values in age, diabetes duration, HbA1c, FPG level, TG, and TC than patients who slept for 7 h/day. In addition, those who slept for less than and equal to 4 h/day had higher proportions in sleep disorders and hypnotic-sedative medications than patients who slept 7 h/day.
The prevalence rates of self-reported sleep durations of ≤ 4, 5–6, 7, 8, 9–10, and > 10 h/day were 1.64%, 16.04%, 26.15%, 29.32%, 23.23%, and 3.62%, respectively. The associations of sleep duration categories with all-cause, expanded CVD, and non-expanded CVD mortalities are reported in Table 2. Multivariate Cox regression analysis showed that patients with sleep durations of ≤ 4, 9–10, and > 10 h/day had significantly higher mortality risks than those with a sleep duration of 7 h/day (HR = 1.41, 95% CI 1.06–1.86; 1.37, 1.23–1.52, and 1.82, 1.54–2.14, respectively). For expanded CVD mortality, patients who slept for ≤ 4, 9–10, and > 10 h/day had higher risks than those who slept for 7 h/day (HR 1.54, 95% CI 1.04–2.28; 1.34, 1.15–1.57; and 1.88, 1.49–2.37, respectively). For non-expanded CVD mortality, the excess risks were observed among patients with sleep durations of 8, 9–10, and > 10 h/day (HR 1.16, 95% CI 1.01–1.34; 1.39, 1.20–1.60; and 1.77, 1.41–2.23, respectively).
In Table 3, patients with stroke, severe hypoglycemia, diabetic ketoacidosis, and hyperglycemic hyperosmolar nonketotic coma were excluded in the sensitivity analysis (n = 11,748). In the sensitivity analysis, similar results were observed for the HRs of all-cause mortality (1.41, 1.04–1.92 for ≤ 4 h/day; 1.12, 1.00–1.25 for 8 h/day; 1.41, 1.26–1.57 for 9–10 h/day; and 1.88, 1.58–2.24 for ≥ 10 h/day), expanded CVD mortality (1.90, 1.26–2.86 for ≤ 4 h/day; 1.40, 1.18–1.66 for 9–10 h/day; and 1.99, 1.54–2.58 for ≥ 10 h/day, respectively), and non-expanded CVD mortality (1.17, 1.01–1.35 for 8 h/day; 1.40, 1.21–1.63 for 9–10 h/day; and 1.79, 1.41–2.28 for ≥ 10 h/day, respectively). By including those whose aged under 30 years, patients with sleep duration ≤ 4, 9–10, and > 10 h/day exhibited a higher risk of all-cause mortality and expanded CVD mortality (adjusted HR: 1.41, 1.06–1.86; 1.37, 1.24–1.52; and 1.83, 1.56–2.15 for all-cause mortality, and 1.53, 1.04–2.27; 1.34, 1.15–1.57; and 1.90, 1.50–2.39 for expanded CVD mortality, respectively) than those with sleep duration of 7 h/day after multivariate adjustment (Additional file 2: Table S1).
The restricted multivariable cubic spline plots for all-cause, expanded CVD, and non-expanded CVD mortalities are presented in Fig. 1, demonstrating significant non-linear associations with the abovementioned mortalities. Significant interactions were found between sex and sleep duration (p for interaction = 0.03). Subgroup analysis showed different curves among women and men (Fig. 2). After adjustment for covariates, a negative association was found between sleep duration < 7 h and mortality in men but not in women.
The joint effects of age and diabetes-related and metabolic factors with sleep duration on all-cause, expanded CVD, and non-expanded CVD mortalities in the total sample were explored (Table 4). The adjusted HRs of sleep duration of more or less than 7 h with age ≥ 65 years, diabetes duration ≥ 5 years, age at diabetes diagnosis ≤ 45 years, insulin use, SBP/DBP > 130/85 mmHg, TG ≥ 150 mg/dL, HbA1c ≥ 7%, and BMI < 27 kg/m2 were all significant for all-cause, expanded CVD, and non-expanded CVD mortalities. Significant interactions were observed between sleep duration and obesity on all-cause mortality (p for interaction = 0.03), sleep duration and age on expanded CVD mortality (p for interaction = 0.04), and sleep duration and diabetes duration and obesity on non-expanded CVD mortality (p for interaction = 0.05 and 0.008, respectively).
Discussion
In this study, a J-shaped association was found with increased all-cause and expanded CVD mortalities for sleep durations in people with T2D. The patients with sleep duration of between 5 and 7 h/day had the lowest all-cause and expanded CVD mortalities. The risk of all-cause and expanded CVD mortalities was higher in patients who sleep for less or more than 7 h per night and with age ≥ 65 years, diabetes duration ≥ 5 years, diabetes diagnosis ≤ 45 years, insulin use, SBP/DBP > 130/85 mmHg, TG ≥ 150 mg/dL, and HbA1c ≥ 7% than in those with 7 h of sleep and their counterparts. This study suggested that sleep duration exerts synergistic interaction effects across subgroups of diabetes duration for non-expanded CVD mortality and antagonistic interactions across age subgroups for expanded CVD mortality, and across obesity subgroups for all-cause and non-expanded CVD mortalities.
The associations between deviations from optimal sleep duration and a wide range of health risks may reflect the consequence of autonomic dysfunction due to sleep fragmentation, deterioration in quality, and micro-awakenings [37]. The autonomic nervous system plays an important role in the coordination of many important physiologic functions during sleep [38, 39]. Sleep disturbances may induce autonomic dysfunction [40] and exacerbate the metabolic control and thus lead to obesity [41], hypertension [42], impaired glucose tolerance [43], and insulin resistance [42, 43]. The biological consequences of sleep disturbances, including endocrine disorders [44], impaired immune function [44], oxidative stress [45], increasing inflammatory reactions, and endothelial dysfunction [45, 46], along with impaired physiology/diseases, may be involved in causing further adverse clinical outcomes, such as atherosclerosis and mortality [47]. These potentially biological mechanisms and impaired physiology and diseases may explain the findings in the present study, that is, short and long sleep durations are significant predictors of all-cause, expanded CVD, and non-expanded CVD mortalities. The joint effects of abnormal sleep pattern (sleep duration greater or shorter than 7 h) with age ≥ 65 years, duration ≥ 5 years, insulin use, SBP/DBP > 130/85 mmHg, TG ≥ 150 mg/dL, and poor glycemic control (HbA1c ≥ 7%) were associated with an increased risk of all-cause (HR 1.24–4.00) and expanded CVD (HR 1.25–3.82) mortalities. In addition, significant synergistic interactions were found between sleep duration and DM duration for non-expanded CVD mortality. By contrast, significant antagonistic interactions were observed between sleep duration and age for expanded CVD mortality, and between sleep duration and obesity (BMI ≥ 27 kg/m2) for all-cause and non-expanded CVD mortalities [48, 49]. The possible explanation for the antagonistic interaction between sleep duration and obesity is that obese patients with diabetes may receive better care and management, possess greater metabolic reserve against poor prognosis or outcomes [49], or acquire better prognosis or lower risk for complications and comorbidity than those with low BMI. A difference in disease etiology has been reported, that is, T2D in obesity is due to metabolic stress, which is associated with lower risk for complications and comorbidity [50], whereas diabetes in low BMI is due to genetic susceptibility [51].
Patients with T2D had the lowest risk of death at 7 h/day of sleep duration, similar to those observed in the general population [9, 31] and diabetic population [30, 33]. In addition, insufficient or excessive sleep was associated with all-cause and CVD mortalities in patients with T2D in the present study, showing a J-shaped relationship. When sex-specific associations were examined, short sleep duration associated with high risks of all-cause, expanded CVD, and non-expanded CVD mortalities was observed only in men but not in women, consistent with those reported in a USA study using data from the National Health Interview Survey [33]. However, this finding was contrary to that reported by a JACC Study [30]. The present study further reported the joint effects, demonstrating that the magnitude of associations between sleep duration and death was greater in patients with older age, longer diabetes duration, diabetes diagnosis at a younger age, hypertension, high TG, poor glycemic control, and insulin use than in their counterparts. Some of these joint effects supported the findings of a USA study, which revealed that the effects are pronounced for individuals diagnosed with early-onset diabetes, reported living with diabetes for a longer duration, and used oral glucose-lowering medication and insulin [33]. The present study further considered the joint effects of sleep duration and metabolic factors, such as TG and BMI, which were not accounted for by prior studies [26, 29]. The findings of the present study also suggested that monitoring of sleep duration in patients with T2D is warranted, particularly in those taking insulin, with early-onset diabetes, longer diabetes duration, poor glycemic control, and older age. Previous studies have not evaluated the biological interactions of sleep duration with metabolic factors, such as TG and BMI. The present study further assessed their modification effects on the survival of patients with diabetes. The results indicated that patients who had BMI < 27 kg/m2 and a longer or shorter sleep duration had 32%, 22%, and 40% higher risks of all-cause, expanded CVD, and non-expanded CVD mortalities, respectively, compared with those who had a normal sleep duration (7 h) and BMI < 27 kg/m2. However, patients with BMI ≥ 27 kg/m2 and a longer or shorter sleep duration did not encounter an increased risk of mortality. Future research is needed to elucidate the antagonistic interactions between sleep duration and obesity and the complex interplay between sleep duration and metabolic dysfunction (such as glycemia, hypertension, obesity, and dyslipidemia) to discern the pathophysiologic mechanisms linking their associations to mortality.
Disease status directly affecting sleep duration or abnormal sleep duration may contribute to poor disease conditions [52]. To rule out this possibility, our sensitivity analysis was performed in patients without those comorbidities. Even after excluding or adjusting for individuals with stroke, coronary artery disease, severe hypoglycemia, diabetic ketoacidosis, and hyperglycemic hyperosmolar nonketotic coma, a significant increase in all-cause and expanded CVD mortalities was still observed for patients with shorter or longer sleep duration (less than or equal to 4 h and greater than 9 h) and in non-expanded CVD mortality with longer than 9 h of sleep. Sensitivity analysis yielded similar results, indicating that the study findings are robust.
The findings of this study, including the independent effect of sleep duration and interaction effect with age; diabetes duration; and obesity on all-cause, expanded, and non-expanded CVD mortality, could provide information for health professionals to design sleep education intervention and identify persons at high risk, who could be targeted for sleep education intervention to reduce immature death. For future studies, the Mendelian randomization could be used to analyze the association of sleep duration with mortality to provide experimental evidence for causal inference.
Strengths and limitations
This study has several advantages and limitations. Strengths included large sample size; a 10-year longitudinal follow-up; exclusion of participants who died within 3 years from baseline; data collection using standardized procedures; and precise definition of death from the National Death Datasets, which increased the validity of the results. Furthermore, three measures (RERI, AP, and S index) were used to estimate the direction of interactions of sleep duration and diabetes-related and metabolic factors in patients with T2D. Two limitations are worth mentioning. One is about sleep measurement. Sleep was measured by a self-reported question about typical sleep duration, not an objective measure. It lacks sufficient information on sleep quality and depression. And sleep duration during weekends or holiday is not considered. The other limitation is that although a significant increase in all-cause and expanded CVD mortalities was observed in patients with shorter than or equal to 4 h of sleep. These results should be interpreted with caution, because only 1.6% (n = 205) of the study subjects had sleep duration of less 4 h.
In conclusion, sleep duration is a significant predictor of all-cause, expanded CVD, and non-expanded CVD mortalities in patients with T2D. Sleep duration of 5–7 h had the lowest mortality risk, and a J-shaped association was observed. Significant synergistic interactions were found between sleep duration and age as well as diabetes duration, and antagonistic interactions were observed between sleep duration and obesity. These findings could provide information for health professionals to design sleep education intervention as well as to identify persons at high risk who could be targeted for sleep education intervention for reducing immature death.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to the policy declared by National Health Insurance in Taiwan but are available from the corresponding author on reasonable request.
Abbreviations
- NSF:
-
National Sleep Foundation
- RRs:
-
Risk ratios
- CVD:
-
Cardiovascular disease
- T2D:
-
Type 2 diabetes
- RERI:
-
Relative excess risk due to interaction
- AP:
-
Proportion attributable to interaction
- S index:
-
Synergy index
- DCMP:
-
Diabetes Care Management Program
- CMUH:
-
China Medical University Hospital
- ICD-9-CM:
-
International Classification of Disease, 9th Revision, Clinical Modification
- FPG:
-
Fasting plasma glucose
- TG:
-
Triglyceride
- HDL-C:
-
High density lipoprotein-cholesterol
- LDL-C:
-
Low density lipoprotein-cholesterol
- TC:
-
Total cholesterol
- OSA:
-
Obstructive sleep apnea
- BZD:
-
Benzodiazepine
- BMI:
-
Body mass index
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- CVs:
-
Coefficient variations
- eGFR:
-
Estimated glomerular filtration rate
- HHNK:
-
Hyperglycemia hyperosmolar non-ketoacidosis
- DKA:
-
Diabetic ketoacidosis
- ICD-10-CM:
-
International Classification of Disease, Tenth Revision, Clinical Modification
- K–M:
-
Kaplan–Meier
- HRs:
-
Hazard ratios
- CIs:
-
Confidence intervals
References
Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–3.
Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15(1):42–50.
Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–26.
Larcher S, Benhamou PY, Pépin JL, Borel AL. Sleep habits and diabetes. Diabetes Metab. 2015;41(4):263–71.
Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren W. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN study. Sleep. 2011;34(11):1487–92.
Covassin N, Singh P. Sleep duration and cardiovascular disease risk: epidemiologic and experimental evidence. Sleep Med Clin. 2016;11(1):81–9.
Syauqy A, Hsu CY, Rau HH, Kurniawan AL, Chao JC. Association of sleep duration and insomnia symptoms with components of metabolic syndrome and inflammation in middle-aged and older adults with metabolic syndrome in Taiwan. Nutrients. 2019;11(8):1848.
Yin J, Jin X, Shan Z, Li S, Huang H, Li P, Peng X, Peng Z, Yu K, Bao W, et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: a systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(9): e005947.
Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33(5):585–92.
Strand LB, Tsai MK, Gunnell D, Janszky I, Wen CP, Chang S-S. Self-reported sleep duration and coronary heart disease mortality: a large cohort study of 400,000 Taiwanese adults. Int J Cardiol. 2016;207:246–51.
Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–56.
Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36.
Li M, Wang N, Dupre ME. Association between the self-reported duration and quality of sleep and cognitive function among middle-aged and older adults in China. J Affect Disord. 2022;304:20–7.
Chien KL, Chen PC, Hsu HC, Su TC, Sung FC, Chen MF, Lee YT. Habitual sleep duration and insomnia and the risk of cardiovascular events and all-cause death: report from a community-based cohort. Sleep. 2010;33(2):177–84.
Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp J, Kell DB. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20(1):172.
Barone MT, Menna-Barreto L. Diabetes and sleep: a complex cause-and-effect relationship. Diabetes Res Clin Pract. 2011;91(2):129–37.
Chawla A, Chawla R, Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: distinct or continuum? Indian J Endocrinol Metab. 2016;20(4):546.
Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, Nieto FJ. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165(8):863–7.
Knutson KL, Van Cauter E, Zee P, Liu K, Lauderdale DS. Cross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes: the Coronary Artery Risk Development in Young Adults (CARDIA) Sleep Study. Diabetes Care. 2011;34(5):1171–6.
Choi Y, Choi JW. Association of sleep disturbance with risk of cardiovascular disease and all-cause mortality in patients with new-onset type 2 diabetes: data from the Korean NHIS-HEALS. Cardiovasc Diabetol. 2020;19(1):61.
Gu KM, Min SH, Cho J. Sleep duration and mortality in patients with diabetes: results from the 2007–2015 Korea national health and nutrition examination survey. Diabetes Metab. 2021;48(3): 101312.
Brouwer A, van Raalte DH, Rutters F, Elders PJM, Snoek FJ, Beekman ATF, Bremmer MA. Sleep and HbA1c in patients with type 2 diabetes: which sleep characteristics matter most? Diabetes Care. 2020;43(1):235–43.
Grandner MA, Seixas A, Shetty S, Shenoy S. Sleep Duration and diabetes risk: population trends and potential mechanisms. Curr Diab Rep. 2016;16(11):106–106.
Lin CL, Tsai YH, Yeh MC. Associations between sleep duration and type 2 diabetes in Taiwanese adults: a population-based study. J Formos Med Assoc. 2016;115(9):779–85.
Lin CL, Chien WC, Chung CH, Wu FL. Risk of type 2 diabetes in patients with insomnia: a population-based historical cohort study. Diabetes Metab Res Rev. 2018;34(1):e2930.
Zeng Y, Wu J, Yin J, Chen J, Yang S, Fang Y. Association of the combination of sleep duration and sleep quality with quality of life in type 2 diabetes patients. Qual Life Res. 2018;27(12):3123–30.
Skomro RP, Ludwig S, Salamon E, Kryger MH. Sleep complaints and restless legs syndrome in adult type 2 diabetics. Sleep Med. 2001;2(5):417–22.
Aurora RN, Kim JS, Crainiceanu C, O’Hearn D, Punjabi NM. Habitual sleep duration and all-cause mortality in a general community sample. Sleep. 2016;39(11):1903–9.
Cai H, Shu XO, Xiang YB, Yang G, Li H, Ji BT, Gao J, Gao YT, Zheng W. Sleep duration and mortality: a prospective study of 113 138 middle-aged and elderly Chinese men and women. Sleep. 2015;38(4):529–36.
Kubota Y, Iso H, Ikehara S, Tamakoshi A. Relationship between sleep duration and cause-specific mortality in diabetic men and women based on self-reports. Metrics. 2015;13(1):85–93.
Kwok CS, Kontopantelis E, Kuligowski G, Gray M, Muhyaldeen A, Gale CP, Peat GM, Cleator J, Chew-Graham C, Loke YK, et al. Self-reported sleep duration and quality and cardiovascular disease and mortality: a dose-response meta-analysis. J Am Heart Assoc. 2018;7(15): e008552.
Wang C, Bangdiwala SI, Rangarajan S, Lear SA, AlHabib KF, Mohan V, Teo K, Poirier P, Tse LA, Liu Z, et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: a study of 116 632 people from 21 countries. Eur Heart J. 2019;40(20):1620–9.
Wang Y, Huang W, O’Neil A, Lan Y, Aune D, Wang W, Yu C, Chen X. Association between sleep duration and mortality risk among adults with type 2 diabetes: a prospective cohort study. Diabetologia. 2020;63(11):2292–304.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371(9631):2173–82.
Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17(3):227–36.
Castro-Diehl C, Diez Roux AV, Redline S, Seeman T, McKinley P, Sloan R, Shea S. Sleep duration and quality in relation to autonomic nervous system measures: the multi-ethnic study of atherosclerosis (MESA). Sleep. 2016;39(11):1927–40.
Miglis M: Sleep and the autonomic nervous system. In: Sleep and neurologic disease. Elsevier; 2017: 227–244.
Cardinali DP. Autonomic nervous system: basic and clinical aspects. Berlin: Springer; 2017.
Tobaldini E, Costantino G, Solbiati M, Cogliati C, Kara T, Nobili L, Montano N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. 2017;74(Pt B):321–9.
Guarino D, Nannipieri M, Iervasi G, Taddei S, Bruno RM. The role of the autonomic nervous system in the pathophysiology of obesity. Front Physiol. 2017;8:665.
Frontoni S, Bracaglia D, Gigli F. Relationship between autonomic dysfunction, insulin resistance and hypertension, in diabetes. Nutr Metab Cardiovasc Dis. 2005;15(6):441–9.
Briançon-Marjollet A, Weiszenstein M, Henri M, Thomas A, Godin-Ribuot D, Polak J. The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms. Diabetol Metab Syndr. 2015;7:25–25.
Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 2011;5:31–43.
Atrooz F, Salim S: Sleep deprivation, oxidative stress and inflammation. In: Advances in Protein Chemistry and Structural Biology. vol. 119: Elsevier; 2020: 309–336.
Chung S, Yoon I-Y, Shin Y-K, Lee CH, Kim J-W, Ahn HJ. Endothelial dysfunction and inflammatory reactions of elderly and middle-aged men with obstructive sleep apnea syndrome. J Sleep Breath. 2009;13(1):11–7.
Wang YH, Wang J, Chen SH, Li JQ, Lu QD, Vitiello MV, Wang F, Tang XD, Shi J, Lu L, et al. Association of longitudinal patterns of habitual sleep duration with risk of cardiovascular events and all-cause mortality. JAMA Netw Open. 2020;3(5): e205246.
Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013;36(Supplement_2):S276–81.
Doehner W, Clark A, Anker SD. The obesity paradox: weighing the benefit. Eur Heart J. 2009;31(2):146–8.
Tobias DK, Pan A, Jackson CL, O’Reilly EJ, Ding EL, Willett WC, Manson JE, Hu FB. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370(3):233–44.
Perry JR, Voight BF, Yengo L, Amin N, Dupuis J, Ganser M, Grallert H, Navarro P, Li M, Qi L, et al. Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. PLoS Genet. 2012;8(5): e1002741.
Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–61.
Acknowledgements
This study was supported primarily by the Ministry of Science and Technology of Taiwan (MOST 104-2314-B-039-016 & MOST 105-2314-B-039-021-MY3 & MOST 105-2314-B-039-025-MY3 & MOST 107-2314-B-039-049 & MOST 108-2314-B-039-039 & MOST 108-2314-B-039-035-MY3 & MOST 108-2314-B-039-031-MY2 & MOST 109-2314-B-039 -031-MY2 & MOST 110-2314-B-039-021-) and China Medical University Hospital (DMR-110-137).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
TCL, CIL and CCL were responsible for drafting the article, the conception and design of the study. TCL, CIL and SSY acquired data and analysed data. CSL and CHL interpreted data. All authors revised the manuscript and approved the final version. TCL and CCL are responsible for the integrity of the work as a whole. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Review Board of China Medical University Hospital (CMUH109-REC1-197). Informed consent of the study participants was not required because the dataset used in this study consists of de-identified secondary data released for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Recruitment procedure.
Additional file 2: Table S1.
Hazard ratios of mortality for sleep duration in patients with type 2 diabetes for sensitivity analysis by including those aged younger than 30 years and having sleep and related information (n = 12,833).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, CI., Lin, CC., Liu, CS. et al. Sleep duration predicts subsequent long-term mortality in patients with type 2 diabetes: a large single-center cohort study. Cardiovasc Diabetol 21, 60 (2022). https://doi.org/10.1186/s12933-022-01500-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-022-01500-0