Abstract
Background
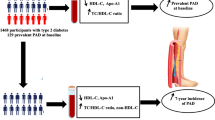
Peripheral artery disease (PAD) is recognized as a significant predictor of mortality and adverse cardiovascular outcomes in patients with coronary heart disease (CHD). In fact, coexisting PAD and CHD is strongly associated with a greater coronary event recurrence compared with either one of them alone. High-density lipoprotein (HDL)-mediated cholesterol efflux capacity (CEC) is found to be inversely associated with an increased risk of incident CHD. However, this association is not established in patients with PAD in the context of secondary prevention. In this sense, our main aim was to evaluate the association between CEC and PAD in patients with CHD and whether the concurrent presence of PAD and T2DM influences this association.
Methods
CHD patients (n = 1002) from the CORDIOPREV study were classified according to the presence or absence of PAD (ankle-brachial index, ABI ≤ 0.9 and ABI > 0.9 and < 1.4, respectively) and T2DM status. CEC was quantified by incubation of cholesterol-loaded THP-1 cells with the participants' apoB-depleted plasma was performed.
Results
The presence of PAD determined low CEC in non-T2DM and newly-diagnosed T2DM patients. Coexisting PAD and newly-diagnosed T2DM provided and additive effect providing an impaired CEC compared to non-T2DM patients with PAD. In established T2DM patients, the presence of PAD did not determine differences in CEC, compared to those without PAD, which may be restored by glucose-lowering treatment.
Conclusions
Our findings suggest an inverse relationship between CEC and PAD in CHD patients. These results support the importance of identifying underlying mechanisms of PAD, in the context of secondary prevention, that provide potential therapeutic targets, that is the case of CEC, and establishing strategies to prevent or reduce the high risk of cardiovascular events of these patients.
Trial registration https://clinicaltrials.gov/ct2/show/NCT00924937. Unique Identifier: NCT00924937

Similar content being viewed by others
Background
Peripheral artery disease (PAD) is characterized by the development of atherosclerotic occlusion of arteries in the lower extremities. PAD is recognized as one of the more significant predictors of mortality and adverse cardiovascular outcomes in patients with coronary heart disease (CHD) [1]. In fact, coexisting PAD and CHD is strongly associated with a greater coronary event recurrence compared with either one of them alone [2]. PAD risk factors, similar to those for CHD (such as age, hypertension, dyslipidemia or type 2 diabetes mellitus, T2DM), are also involved in its progression and are associated with a worsening of arterial perfusion of the lower extremities [3, 4].
The ankle-brachial index (ABI; the systolic blood pressure obtained at the ankle divided by the systolic blood pressure obtained at the brachial artery) is a simple, non-invasive and inexpensive tool accepted as a diagnostic test in the evaluation of PAD [5]. A low ABI value is a strong and independent predictor of fatal and nonfatal cardiovascular events and ischemic stroke [6, 7].
Reduced high-density lipoprotein (HDL)-cholesterol levels have been associated with increased risk of cardiovascular complications and mortality in PAD patients [8]. Several randomized controlled trials have failed to show a relationship between the pharmacologic improvement of HDL-cholesterol levels and a decreased risk of cardiovascular events [9, 10]. Accordingly, some clinical approaches have moved away from HDL-cholesterol levels towards HDL function as a causal risk factor for cardiovascular disease (CVD) [11]. HDL particles not only protect against atherosclerosis exerting vasodilatory effects and decreasing inflammation and oxidative stress but also by its action through the reverse cholesterol transport (RCT), considered the key cardioprotective property of HDL [12, 13]. RCT is the physiological process by which cholesterol in peripheral tissues is transported by HDL to the liver for excretion. In the initial step of RCT, through a process termed cholesterol efflux, HDLs accept cholesterol from cells, including artery-wall macrophages [14, 15]. Several studies suggest that cholesterol efflux capacity (CEC) may be a potential biomarker of atherosclerosis development, being inversely associated with the incidence of CHD, independent of HDL-cholesterol concentrations [16,17,18]. It is recently demonstrated that, in patients with chronic renal disease, aberrations in delivery of cholesterol effluxed from macrophages to liver may underlie the increased risk of coronary disease found in these patients [19].
However, the association between CEC and PAD is not well established. Only a recent prospective study, the MESA—Multi-Ethnic Study of Atherosclerosis, has analyzed the relationship between CEC and risk of PAD in a multi-ethnic cohort, free of baseline CVD, finding no association between CEC and risk of either clinical or incident subclinical PAD [20]. To the best of our knowledge, there are no studies evaluating the association between CEC and PAD in the context of secondary prevention.
T2DM is positively associated with macrovascular complications, being atherosclerotic CVD the leading cause of death in this population [21]. The combined presence of T2DM and CVD increases cardiovascular risk, as demonstrated by the high recurrence rate of major atherosclerotic complications (~ 6%/year) in the diabetic population [22]. Moreover, T2DM predisposes to PAD development [23]. Indeed, T2DM patients have a 2- to 4-fold greater risk of developing PAD than non-diabetic patients [24, 25]. We have recently found an inverse association between CEC and T2DM development in CHD patients, supporting the fact that cholesterol efflux could be an independent risk factor for the development of CHD and other chronic diseases, like T2DM [26].
Therefore, this study aimed to evaluate whether CEC was associated with PAD in CHD patients, with and without T2DM, for the purpose of identifying underlying mechanisms of the disease that could provide potential therapeutic target to prevent or reduce the high risk of cardiovascular events of these patients.
Methods
Design and study population
The current work was conducted within the framework of the CORDIOPREV (CORonary Diet Intervention with Olive oil and cardiovascular PREVention) study (Clinicaltrials.gov number NCT00924937). The CORDIOPREV study is an ongoing prospective, randomized, single-blind, controlled trial, including 1002 CHD patients who had their last coronary event more than six months before enrolment. Patients were recruited from November 2009 to February 2012, mostly at Reina Sofia University Hospital, Cordoba, Spain, and other hospitals in Cordoba and Jaen. Full details of the rationale, study methods, inclusion and exclusion criteria, cardiovascular risk factors and baseline characteristics of the patients have been described recently [27]. To summarize, patients were eligible if they were aged 20–75 years, with established CHD but without clinical events in the last six months, intending to follow a long-term monitoring study, with no other serious illnesses and a life expectancy of at least 5 years. All the patients gave their written informed consent to participate in the study. Following institutional and Good Clinical Practice guidelines, the Human Investigation Review Committee approved the study protocol at Reina Sofia University Hospital.
For the specific aims of this work, we performed a cross-sectional analysis of the CORDIOPREV population at baseline, in which the patients were classified according to 1) the presence or absence of PAD, based on their ABI value (patients with PAD, with an ABI ≤ 0.9; and patients with non-PAD, those with an ABI > 0.9 and < 1.4) [28] and 2) diabetes status (describe below). Those patients with and ABI ≥ 1.4 were excluded of the analysis.
Diabetes status criteria
The second classification of the study patients was carried out according to their diabetic status, at baseline: non-T2DM patients, those who did not meet the criteria for T2DM diagnosis proposed by the American Diabetes Association [29], newly diagnosed T2DM patients, those who had no previous history of T2DM, being diagnosed during the recruitment period of the study and thus, without diabetic treatment; established T2DM patients, those with a prior medical history of T2DM when entering the study and that were receiving diabetic treatment.
Laboratory tests
At 8.00 am, following a 12-h fast, the patients were admitted to the laboratory for anthropometric and biochemical tests [BMI, waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), HDL-cholesterol, LDL-cholesterol, triglycerides, cholesterol, high sensitive C-reactive protein (hsCRP), fasting glucose and insulin and hemoglobin A1c (HbA1c) as described previously [27]. Glomerular filtration rate (eGFR) was estimated with the Chronic Kidney Disease and Epidemiology equation (CKD-EPI) [30].
ABI measurement
Baseline evaluation of ABI was performed according to a standardized protocol by trained examiners. BP measurements were performed with a Doppler device (Minidop ES-100X, Hadeco/Hayashi Denki Co., Ltd.) after the participants rested supine for 10 min. Brachial SBP was measured in both arms by placing the Doppler transducer above the cubital segment of both brachial arteries. For systolic ankle pressure measurement, the posterior tibial and dorsalis pedis artery was measured for each leg. ABI was calculated per leg as the higher SBP in the posterior tibial or dorsalis pedis divided by the higher of the two-arm SBPs. The lower ABI of both legs was used as the participant-specific ABI. We imputed missing data (< 7%) with Multivariate Imputation by Chained Equations (MICE) package (3.8.0) in R. All ABI measurements were performed by a single independent examiner who was unaware of other clinical data.
Assessment of HDL-mediated cholesterol efflux capacity
Blood samples were collected, at baseline, from 12-h fasting subjects in EDTA-containing tubes, placed on ice, centrifuged at 4 °C, and stored at -80 °C. Cholesterol efflux capacity was measured at baseline as previously described [26]. Efflux assays were performed using human THP-1 monocytes (ATCC TIB-202). For the assays, THP-1 cells were plated in 48 multi-well plates at a concentration of 125,000 cells/well and they were treated with phorbol 12-myristate 13-acetate (50 ng/mL) for 72 h to become fully differentiated macrophages. Then, THP-1 macrophages were labelled with 1.2 μCi/mL [1,2-3H(N)]-cholesterol (Perkin-Elmer) and cholesterol loaded with 50 µg/mL of acetylated-LDL (acLDL) in RPMI medium containing 10% lipoprotein deficient fetal bovine serum (density > 1.21 g/L). After 24 h, cells were washed 3 times with phosphate buffered saline containing 0.1% human serum albumin (HSA) to remove the excess of 3H-cholesterol and acLDL and they were equilibrated in serum-free medium overnight at 37 °C. The next day, plasma samples, from the study patients, were thawed and treated with polyethylene glycol to precipitate apolipoprotein B-containing lipoproteins. Briefly, 40 parts polyethylene glycol solution (20% polyethylene glycol 8000 molecular weight in 200 mM glycine buffer, pH 7.4) were added to 100 parts plasma and incubated at room temperature for 20 min before spinning in a microcentrifuge at 10,000 rpm for 30 min at 4 °C. The supernatant, which contained the HDL fraction, was recovered. Finally, efflux medium containing 2% apoB-depleted plasma was added to THP-1 macrophages. The efflux period was 4 h, at 37ºC, after which the medium was removed for quantifying the 3H − cholesterol present therein and in cells by scintillation counting. Each sample was run in triplicate, and within each plate were always included, besides the study subjects’ plasmas, serum-free medium containing 0.2% HSA and an inter-assay control (2%) consisting of pooled apoB-depleted plasmas from four healthy volunteers and stored at − 80 °C.
We calculated the percentage efflux to the medium by the formula: (disintegrations per minute (dpm) 3H − cholesterol in the medium × 100)/(dpm 3H − cholesterol in the medium + dpm 3H − cholesterol in cells). The efflux to serum-free medium value in each batch was subtracted from the corresponding plasma values. To standardize the percentage efflux obtained in the several analyses, we normalized values for the study patients to the inter-assay control in each batch as follows: (study patient cholesterol efflux × 100)/inter-assay control cholesterol efflux [31]. The inter-assay variability across plates was controlled by the inter-assay control. The inter-assay coefficient of variation was 6.4% and the intra-assay coefficient of variation was 4.5%.
From the total patients of the CORDIOPREV study (n = 1002), we included those whose cholesterol efflux assessment, and analytical and anthropometric data were available (n = 961). The reasons for the lack of data for the remaining 41 patients were as follows: 37 refused to undergo the ABI measurement and 4 exhibited an ABI ≥ 1.4. All participants were of European ancestry (Fig. 1).
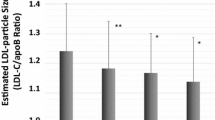
Cholesterol efflux capacity in patients with CHD. A) According to the presence or absence of PAD and B) According to the presence or absence of PAD and diabetes status * Significant differences between PAD and no PAD in each diabetes status s group. Different common letter superscripts denote significant differences among non-T2DM, newly-diagnosed T2DM and established T2DM. p1, effect of presence or absence of PAD; p2, effect of diabetes status; p3, interaction between presence of absence of PAD and diabetes status. CHD, coronary heart disease; PAD, peripheral artery disease; T2DM, type 2 diabetes mellitus Analyses were adjusted age, smoking habit, hypertension, eGFR, and medications—glucose-lowering treatment, lipid-lowering therapy, and anti-hypertensive drugs
Statistical analysis
The statistical analyses were carried out using SPSS version 19.0 for Windows (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov normality test was performed to evaluate the distribution of the quantitative variables, and continuous variables that deviated significantly from the assumption of normality were transformed. Categorical variables were compared using Chi-Square tests. Continuous data were compared using unpaired t-tests when comparing two groups or analysis of variance (ANOVA), adjusted for potential cofounders or potential effect modifiers (age, smoking habit, hypertension, eGFR, and medications—glucose-lowering treatment, lipid-lowering therapy, and anti-hypertensive drugs).
Backward multiple logistic analysis was carried out to estimate the independent contribution of diabetes status and parameters related to glucose metabolism (fasting glucose and HbA1c), cholesterol efflux capacity and different cardiovascular risk factors such age, HDL-cholesterol, triglycerides, hsCRP, eGFR, hypertension, glucose-lowering treatment and lipid-lowering therapy to the presence of PAD.
The differences were considered significant when p < 0.05. All the data presented in figures and tables are expressed as means ± standard error (SE).
Results
Study population characteristics based on the presence or absence of PAD
Of the total population, 188 CHD patients (19.6%) exhibited PAD, with an ABI ≤ 0.9 (n = 185). Table 1 shows the clinical and metabolic characteristics, lipid profiles and treatment regimens of the study patients classified according to the presence or absence of PAD. Patients with PAD were older (p < 0.001) and more frequently current or past smokers (p = 0.041) with higher levels of hsCRP (p < 0.001), compared to those without PAD. Patients with PAD also had lower levels of HDL-cholesterol (p = 0.030), higher levels of triglycerides, and greater SBP and prevalence of hypertension compared with their counterparts (all p < 0.01). Regarding glycemic control, PAD patients showed higher levels of fasting glucose and HbA1c, a higher prevalence of T2DM with more oral antidiabetic medication, and lower eGFR compared to non-PAD patients (all p < 0.05).
T2DM status and PAD
When we classified the patients according to diabetes status, established T2DM patients showed the highest PAD prevalence than non-T2DM and newly-diagnosed T2DM patients. Non-T2DM patients exhibited the lowest prevalence of PAD (p < 0.001) (data not shown). Moreover, the concomitant PAD and T2DM (in established T2DM patients but not in those newly-diagnosed T2DM) determine higher levels of total cholesterol and LDL-cholesterol compared with their counterparts without PAD (all p < 0.05) (Table 2).
Cholesterol efflux capacity according to the presence or absence of PAD and diabetes status
In the total CHD patients, we did not find differences in CEC according to the presence or absence of PAD (Fig. 2a). However, when diabetes status was considered, both non-T2DM and newly-diagnosed T2DM patients with PAD showed lower CEC than their counterparts without PAD (p = 0.039 and p = 0.021, respectively). Moreover, in the presence of PAD, CEC was lower in newly-diagnosed T2DM compared to non-T2DM patients (p = 0.011). The presence of PAD, in established T2DM patients, did not determine differences in CEC compared to those without PAD (Fig. 2b).
Multiple logistic regression analysis for the presence of PAD in patients with CHD. Squares denote hazard ratios; horizontal lines represent 95% confidence intervals. R2 = 0.201, constant = − 4.493 (p = 0.000). Predictive variables tested by backward (conditional) method: age (years), diabetes status (non-T2DM, newly-diagnosed T2DM and established T2DM), HbA1c (%), Fasting glucose (mg/dL), hypertension, HDL-cholesterol (mg/dL), Triglycerides (mg/dL), Cholesterol efflux (%), hsCRP (mg/mL), eGFR (mL/min/1.73 m2), oral antidiabetics use (%) and lipid-lowering therapy (%). HbA1c (%), Fasting glucose (mg/dL), HDL-cholesterol (mg/dL), eGFR (mL/min/1.73 m2), oral antidiabetics use (%), lipid-lowering therapy (%) have been eliminated from the model (p > 0.05). CHD, cardiovascular heart disease; T2DM, type2 diabetes mellitus; hsCRP, high sensitive C-reactive protein; eGFR, estimated glomerular filtration rate. HDL, high-density lipoprotein; HbA1c, glycated haemoglobin
Multiple logistic regression model for predicting the presence of PAD
To determine the contribution of CEC to PAD, we performed a multiple logistic regression analysis (Fig. 2). Diabetes status, parameters related to glucose metabolism, and different cardiovascular risk factors were also included in the analysis. In our model, an increase of an SD of cholesterol efflux determined a decrease of 0.984-fold (95% CI 0.973–0.995) the probability of having PAD. Moreover, the presence of established T2DM (but not newly-diagnosed T2DM) had a 2.171-fold (95% CI 1.411–3.340) more likelihood of having PAD than non-T2DM (referent). Presence of hypertension (odds ratio [OR], 2.155; 95% CI 1.385–3.355), age (OR, 1.041; 95% CI 1.018–1.065), triglycerides levels (OR, 1.003; 95% CI 1.001–1.006) and hsCRP levels (OR, 1.084; 95% CI 1.037–1.134) increased the probability of having PAD.
Discussion
Our findings are the first to evaluate CEC and PAD in patients with CHD to the best of our knowledge. In this cross-sectional study, we found an inverse relationship between CEC and PAD. The presence of PAD determined low CEC in non-T2DM and newly-diagnosed T2DM patients. Moreover, coexisting PAD and newly-diagnosed T2DM provided and additive effect providing an impaired CEC compared to non-T2DM patients with PAD. In established T2DM patients, the presence of PAD did not determine differences in CEC compared to those without PAD.
The atheroprotective properties of HDL, through its capacity to promote cellular cholesterol efflux, have been well evidenced [32, 33]. CEC of HDL is also necessary for nitric oxide activation, which enhances endothelial function (whose impairment is involved in the development of arteriosclerotic disease) by mediating anti-inflammatory and anti-oxidant activities [34]. In fact, recent findings found that reduced ABCA1 expression in the macrophages, by proprotein convertase subtilisin/kexin type 9 (PCSK9), could accelerates atherosclerosis, thereby inhibiting RCT [35, 36]. In this line, evidences have also supported that adiponectin may play a critical atheroprotective role in promoting ABCA1-dependent cholesterol efflux [37].
In this context, different studies have supported an inverse relationship between CEC and prevalence of incidence of CHD [17, 18, 33, 38]. A recent meta-analysis has described that low CEC was associated with a higher risk of CVD, even in individuals at high cardiovascular risk, irrespective of HDL cholesterol levels and other classic cardiovascular risk factors [33], suggesting that the evaluation of CEC could be a key element to understand the role of HDL in CVD.
CEC is inversely associated with the presence of PAD in patients with CHD
Patients with advanced atherosclerosis in several territories are at very high risk of cardiovascular morbidity and mortality [1]. Hence, the importance of identifying the mechanisms involved in developing PAD in CHD patients to stratify their cardiovascular risk and apply effective therapies, as is the case of CEC. Only a clinical study has evaluated the contribution of CEC (by quantification of cholesterol mass efflux capacity, CMEC) to the risk of incident PAD in a multi-ethnic cohort, free of baseline CVD, MESA study [20]. In this prospective study, no association was found between CMEC and risk of developing either a low ABI or clinical PAD. Here, we found that an increase in cholesterol efflux reduced the probability of having PAD, suggesting that an impairment of CEC could be a mechanism involved in developing PAD in the context of established CVD, as is the case of patients with CHD. In addition to the fact that our study is carried out in the context of secondary prevention, we used a different method that one used in the MESA study. Recently, a couple of methods to evaluated CEC (a new stable isotope method and a cell-free assay) has also been developed [39, 40], whose data showed an association between CEC and the risk of coronary disease recurrence. In this sense, we could suggest that the difference in the relevance of CEC to PAD development might be attributed to the difference of CEC method.
T2DM status is an independent contributor of the relationship between CEC and PAD
It has been reported that T2DM patients have reduced CEC [18, 41, 42], which is further decreased by CVD [42]. Our results are in line with these findings, showing that, in the presence of PAD, newly-diagnosed T2DM (without glucose-lowering treatment) exhibited lower CEC than non-T2DM patients, likely reflecting the alteration in the compositional properties of HDL. In fact, glycation of HDL, that is exacerbated by chronic hyperglycemia conditions, has been associated with loss of HDL functionality, blunting CEC in vitro [43, 44]. However, other studies have not found impaired CEC in T2DM, which may be due to oral anti-diabetic treatment [45]. In fact, in our study, we did not observe an impairment in CEC in established T2DM patients (who were under glucose-lowering treatment), regardless of PAD. In this sense, diabetes medication could act as a modulator, restoring impaired cholesterol efflux and RCT [46, 47]. It has been found that metformin increases FGF21 expression and subsequently promotes the expression of ABCA1 and ABCG1 in macrophages, promoting cholesterol efflux [48] and reducing foam cell formation [49]. Moreover, liraglutide is found to improves lipid metabolism by enhancing cholesterol efflux associated with ABCA1 and ERK1/2 pathway [50].
In our study, it should be highlighted the importance of evaluating a population of newly-diagnosed T2DM patients (without glucose-lowering therapy) since it allows to discriminate the influence of diabetes treatment and time of evolution of the disease in comparison with those patients with established T2DM.
Our study has a number of major strengths. Cholesterol efflux is the significant first step within the RCT, and the technology used is useful in several clinical and preclinical studies by us and others [26, 51, 52]. Moreover, our analysis could provide the basis for future approaches in longitudinal studies to clarify the influence of cholesterol efflux and adds insight into the molecular mechanisms involved in the development of PAD.
One of the limitations found in our study is that it is cross-sectional, which offers no evidence of causal effects. Moreover, the results are limited to CHD patients and may not be suitable for extrapolation to other populations.
Conclusions
Our findings support an inverse relationship between CEC and PAD in patients with CHD.The concomitant presence of PAD and newly-diagnosed T2DM provided and additive effect providing an impairment in CEC. CEC was not altered in established T2DM patients (with and without PAD), which may be due to the restorative effect of CEC by glucose-lowering therapies. These results support the importance of identifying underlying mechanisms of PAD, in the context of secondary prevention, that provide potential therapeutic targets, that is the case of CEC, and establishing strategies to prevent or reduce the high risk of cardiovascular events of these patients.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
17 April 2021
A Correction to this paper has been published: https://doi.org/10.1186/s12933-021-01269-8
Abbreviations
- ABI:
-
Ankle-brachial index
- CEC:
-
Cholesterol efflux capacity
- CHD:
-
Coronary heart disease
- CORDIOPREV:
-
CORonary Diet Intervention with Olive oil and cardiovascular PREVention
- IMT-CC:
-
Carotid intima-media thickness
- PAD:
-
Peripheral artery disease
- T2DM:
-
Type 2 diabetes mellitus
References
De Luca L, Di Pasquale G, Gonzini L, Chiarella F, Di Chiara A, Boccanelli A, Casella G, Olivari Z, De Servi S, Gulizia MM, et al. Trends in management and outcome of patients with non-ST elevation acute coronary syndromes and peripheral arterial disease. Eur J Intern Med. 2019;59:70–6.
Hussein AA, Uno K, Wolski K, Kapadia S, Schoenhagen P, Tuzcu EM, Nissen SE, Nicholls SJ. Peripheral arterial disease and progression of coronary atherosclerosis. J Am Coll Cardiol. 2011;57(10):1220–5.
Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–26.
Sozmen K, Unal B. Prevalence of low ankle brachial index and relationship with cardiovascular risk factors in a Western urban population in Turkey. Angiology. 2014;65(1):43–50.
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463-654.
Haine A, Kavanagh S, Berger JS, Hess CN, Norgren L, Fowkes FGR, Katona BG, Mahaffey KW, Blomster JI, Patel MR, et al. Sex-specific risks of major cardiovascular and limb events in patients with symptomatic peripheral artery disease. J Am Coll Cardiol. 2020;75(6):608–17.
Abboud H, Monteiro Tavares L, Labreuche J, Arauz A, Bryer A, Lavados PM, Massaro A, Munoz Collazos M, Steg PG, Yamout BI, et al. Impact of low ankle-brachial index on the risk of recurrent vascular events. Stroke. 2019;50(4):853–8.
Martinez-Aguilar E, Orbe J, Fernandez-Montero A, Fernandez-Alonso S, Rodriguez JA, Fernandez-Alonso L, Paramo JA, Roncal C. Reduced high-density lipoprotein cholesterol: a valuable, independent prognostic marker in peripheral arterial disease. J Vasc Surg. 2017;66(5):1527–33.
Group HTC, Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–12.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, Chaitman BR, Holme IM, Kallend D, Leiter LA, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–99.
Ronsein GE, Heinecke JW. Time to ditch HDL-C as a measure of HDL function? Curr Opin Lipidol. 2017;28(5):414–8.
Rosenson RS, Brewer HB Jr, Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 2011;57(3):392–410.
Schwertani A, Choi HY, Genest J. HDLs and the pathogenesis of atherosclerosis. Curr Opin Cardiol. 2018;33(3):311–6.
Favari E, Thomas MJ, Sorci-Thomas MG. High-density lipoprotein functionality as a new pharmacological target on cardiovascular disease: unifying mechanism that explains high-density lipoprotein protection toward the progression of atherosclerosis. J Cardiovasc Pharmacol. 2018;71(6):325–31.
Klancic T, Woodward L, Hofmann SM, Fisher EA. High density lipoprotein and metabolic disease: Potential benefits of restoring its functional properties. Mol Metab. 2016;5(5):321–7.
Qiu C, Zhao X, Zhou Q, Zhang Z. High-density lipoprotein cholesterol efflux capacity is inversely associated with cardiovascular risk: a systematic review and meta-analysis. Lipids Health Dis. 2017;16(1):212.
Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383–93.
Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol. 2015;3(7):507–13.
Gipson GT, Carbone S, Wang J, Dixon DL, Jovin IS, Carl DE, Gehr TW, Ghosh S. Impaired delivery of cholesterol effluxed from macrophages to hepatocytes by serum from CKD patients may underlie increased cardiovascular disease risk. Kidney Int Rep. 2020;5(2):199–210.
Garg PK, Jorgensen NW, McClelland RL, Allison M, Stein JH, Yvan-Chavret L, Tall AR, Shea S. Cholesterol mass efflux capacity and risk of peripheral artery disease: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2020;297:81–6.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410.
DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53(7):1270–87.
Signorelli SS, Katsiki N. Oxidative stress and inflammation: their role in the pathogenesis of peripheral artery disease with or without type 2 diabetes mellitus. Curr Vasc Pharmacol. 2018;16(6):547–54.
Wattanakit K, Folsom AR, Selvin E, Weatherley BD, Pankow JS, Brancati FL, Hirsch AT. Risk factors for peripheral arterial disease incidence in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2005;180(2):389–97.
Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287(19):2570–81.
Blanco-Rojo R, Perez-Martinez P, Lopez-Moreno J, Martinez-Botas J, Delgado-Lista J, van Ommen B, Yubero-Serrano E, Camargo A, Ordovas JM, Perez-Jimenez F, et al. HDL cholesterol efflux normalised to apoA-I is associated with future development of type 2 diabetes: from the CORDIOPREV trial. Sci Rep. 2017;7(1):12499.
Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Alcala-Diaz JF, Perez-Caballero AI, Gomez-Delgado F, Fuentes F, Quintana-Navarro G, Lopez-Segura F, Ortiz-Morales AM, et al. CORonary Diet Intervention with Olive oil and cardiovascular PREVention study (the CORDIOPREV study): Rationale, methods, and baseline characteristics: A clinical trial comparing the efficacy of a Mediterranean diet rich in olive oil versus a low-fat diet on cardiovascular disease in coronary patients. Am Heart J. 2016;177:42–50.
Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–909.
American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–S27.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Annema W, Dikkers A, de Boer JF, van Greevenbroek MMJ, van der Kallen CJH, Schalkwijk CG, Stehouwer CDA, Dullaart RPF, Tietge UJF. Impaired HDL cholesterol efflux in metabolic syndrome is unrelated to glucose tolerance status: the CODAM study. Sci Rep. 2016;6:27367.
Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384(9943):618–25.
Soria-Florido MT, Schroder H, Grau M, Fito M, Lassale C. High density lipoprotein functionality and cardiovascular events and mortality: a systematic review and meta-analysis. Atherosclerosis. 2020;302:36–42.
Mineo C, Shaul PW. Regulation of signal transduction by HDL. J Lipid Res. 2013;54(9):2315–24.
Tang Y, Li SL, Hu JH, Sun KJ, Liu LL, Xu DY. Research progress on alternative non-classical mechanisms of PCSK9 in atherosclerosis in patients with and without diabetes. Cardiovasc Diabetol. 2020;19(1):33.
Shi J, Zhang W, Niu Y, Lin N, Li X, Zhang H, Hu R, Ning G, Fan J, Qin L, et al. Association of circulating proprotein convertase subtilisin/kexin type 9 levels and the risk of incident type 2 diabetes in subjects with prediabetes: a population-based cohort study. Cardiovasc Diabetol. 2020;19(1):209.
Hafiane A, Gasbarrino K, Daskalopoulou SS. The role of adiponectin in cholesterol efflux and HDL biogenesis and metabolism. Metabolism. 2019;100:153953.
Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–35.
Shimizu T, Miyazaki O, Iwamoto T, Usui T, Sato R, Hiraishi C, Yoshida H. A new method for measuring cholesterol efflux capacity uses stable isotope-labeled, not radioactive-labeled, cholesterol. J Lipid Res. 2019;60(11):1959–67.
Harada A, Toh R, Murakami K, Kiriyama M, Yoshikawa K, Miwa K, Kubo T, Irino Y, Mori K, Tanaka N, et al. Cholesterol uptake capacity: a new measure of HDL functionality for coronary risk assessment. J Appl Lab Med. 2017;2(2):186–200.
Tsun JG, Shiu SW, Wong Y, Yung S, Chan TM, Tan KC. Impact of serum amyloid A on cellular cholesterol efflux to serum in type 2 diabetes mellitus. Atherosclerosis. 2013;231(2):405–10.
Syvanne M, Castro G, Dengremont C, De Geitere C, Jauhiainen M, Ehnholm C, Michelagnoli S, Franceschini G, Kahri J, Taskinen MR. Cholesterol efflux from Fu5AH hepatoma cells induced by plasma of subjects with or without coronary artery disease and non-insulin-dependent diabetes: importance of LpA-I:A-II particles and phospholipid transfer protein. Atherosclerosis. 1996;127(2):245–53.
Liu D, Ji L, Zhang D, Tong X, Pan B, Liu P, Zhang Y, Huang Y, Su J, Willard B, et al. Nonenzymatic glycation of high-density lipoprotein impairs its anti-inflammatory effects in innate immunity. Diabetes Metab Res Rev. 2012;28(2):186–95.
Gomes Kjerulf D, Wang S, Omer M, Pathak A, Subramanian S, Han CY, Tang C, den Hartigh LJ, Shao B, Chait A. Glycation of HDL blunts its anti-inflammatory and cholesterol efflux capacities in vitro, but has no effect in poorly controlled type 1 diabetes subjects. J Diabetes Compl. 2020;34(12):107693.
de Vries R, Groen AK, Perton FG, Dallinga-Thie GM, van Wijland MJ, Dikkeschei LD, Wolffenbuttel BH, van Tol A, Dullaart RP. Increased cholesterol efflux from cultured fibroblasts to plasma from hypertriglyceridemic type 2 diabetic patients: roles of pre beta-HDL, phospholipid transfer protein and cholesterol esterification. Atherosclerosis. 2008;196(2):733–41.
Brownell N, Rohatgi A. Modulating cholesterol efflux capacity to improve cardiovascular disease. Curr Opin Lipidol. 2016;27(4):398–407.
Machado AP, Pinto RS, Moyses ZP, Nakandakare ER, Quintao EC, Passarelli M. Aminoguanidine and metformin prevent the reduced rate of HDL-mediated cell cholesterol efflux induced by formation of advanced glycation end products. Int J Biochem Cell Biol. 2006;38(3):392–403.
Luo F, Guo Y, Ruan G, Li X. Metformin promotes cholesterol efflux in macrophages by up-regulating FGF21 expression: a novel anti-atherosclerotic mechanism. Lipids Health Dis. 2016;15:109.
He X, Chen X, Wang L, Wang W, Liang Q, Yi L, Wang Y, Gao Q. Metformin ameliorates Ox-LDL-induced foam cell formation in raw264.7 cells by promoting ABCG-1 mediated cholesterol efflux. Life Sci. 2019;216:67–74.
Wu YR, Shi XY, Ma CY, Zhang Y, Xu RX, Li JJ. Liraglutide improves lipid metabolism by enhancing cholesterol efflux associated with ABCA1 and ERK1/2 pathway. Cardiovasc Diabetol. 2019;18(1):146.
Annema W, Dikkers A, de Boer JF, Dullaart RP, Sanders JS, Bakker SJ, Tietge UJ. HDL cholesterol efflux predicts graft failure in renal transplant recipients. J Am Soc Nephrol. 2016;27(2):595–603.
Fernandez-Suarez ME, Escola-Gil JC, Pastor O, Davalos A, Blanco-Vaca F, Lasuncion MA, Martinez-Botas J, Gomez-Coronado D. Clinically used selective estrogen receptor modulators affect different steps of macrophage-specific reverse cholesterol transport. Sci Rep. 2016;6:32105.
Acknowledgements
We would like to thank the EASP (Escuela Andaluza de Salud Publica), Granada (Spain), for carrying out the randomization process in this study. We would also like to thank to Verónica de Dios, Department of Biochemistry-Research, Hospital Universitario Ramón y Cajal, Madrid, for excellent technical assistance.
The CIBEROBN is an initiative of the Instituto de Salud Carlos III, Madrid, Spain.
Funding
The CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero (Cordioprev-CEAS, 1/2016 to Jose Lopez-Miranda). This study also received research grants from Consejería de Economía, Innovación, Ciencia y Empleo (CVI-7450) and Ministerio de Ciencia e Innovación y Universidades (AGL2012-39615 and PID2019-104362RB-I00 to Jose Lopez-Miranda; RTI2018-098113-B-I00 to DGC), integrated into the framework of the National Plan for Scientific Research, Technological Development and Innovation 2013–2016, co-financed by the Instituto de Salud Carlos III (ISCIII) of Spain and also by the Directorate General for Assessment and Promotion of Research and the EU’s European Regional Development Fund (FEDER). Elena M Yubero-Serrano was the recipient of the Nicolas Monardes Programme from the “Servicio Andaluz de Salud, Junta de Andalucia”, Spain (C1-0005–2019). The funding bodies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
JLM and DGC had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: JLM, PPM and JDL. Acquisition, analysis, or interpretation of data: EMYS, JFAD, FMGM, APAL, PPO, RBR. Drafting of the manuscript: EMYS and JFAD. Critical revision of the manuscript for important intellectual content: JLM, PMM, JDL, JMO, DGC. Statistical analysis: EMYS, JDL, FMGM. Obtained funding: JLM, JDL, DGC. Administrative, technical, or material support: JMB, JDTP. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All the patients gave their written informed consent to participate in the study. Following institutional and Good Clinical Practice guidelines, the Human Investigation Review Committee approved the study protocol at Reina Sofia University Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the error in the article note has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yubero-Serrano, E.M., Alcalá-Diaz, J.F., Gutierrez-Mariscal, F.M. et al. Association between cholesterol efflux capacity and peripheral artery disease in coronary heart disease patients with and without type 2 diabetes: from the CORDIOPREV study. Cardiovasc Diabetol 20, 72 (2021). https://doi.org/10.1186/s12933-021-01260-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-021-01260-3