Abstract
Background
Angiopoietin-like protein 8 (ANGPTL8), an important regulator of lipid metabolism, is increased in diabetes and is associated with insulin resistance. However, the role of ANGPTL8 in the outcomes of diabetic patients remains unclear. This study aimed to investigate circulating levels of ANGPTL8 in participants with and without diabetes and its potential associations with clinical outcomes in a 5 year cohort study.
Methods
Propensity-matched cohorts of subjects with and without diabetes from the Risk Evaluation of Cancers in Chinese Diabetic Individuals: A longitudinal (REACTION) study were generated on the basis of age, sex and body mass index at baseline. The primary outcome was all-cause mortality. The secondary outcomes were a composite of new-onset major adverse cardiovascular events, hospitalization for heart failure, and renal dysfunction (eGFR < 60/min/1.73 m2).
Results
We identified 769 matched pairs of diabetic patients and control subjects. Serum ANGPTL8 levels were elevated in patients with diabetes compared to control subjects (618.82 \(\pm\) 318.08 vs 581.20 \(\pm\) 299.54 pg/mL, p = 0.03). Binary logistic regression analysis showed that elevated ANGPTL8 levels were associated with greater risk ratios (RRs) of death (RR in quartile 4 vs. quartile 1, 3.54; 95% CI 1.32–9.50) and renal dysfunction (RR in quartile 4 vs. quartile 1, 12.43; 95% CI 1.48–104.81) only in diabetic patients. Multivariable-adjusted restricted cubic spline analyses revealed a significant, linear relationship between ANGPTL8 and all-cause mortality in diabetic patients (p for nonlinear trend = 0.99, p for linear trend = 0.01) but not in control subjects (p for nonlinear trend = 0.26, p for linear trend = 0.80). According to ROC curve analysis, the inclusion of ANGPTL8 in QFrailty score significantly improved its predictive performance for mortality in patients with diabetes.
Conclusion
Serum ANGPTL8 levels were associated with an increased risk of all-cause mortality and could be used as a potential biomarker for the prediction of death in patients with diabetes.
Similar content being viewed by others

Background
Diabetes is the fastest increasing disease worldwide and is a substantial threat to human health [1]. Among adults in China, diabetes was associated with twofold increased mortality compared with adults without diabetes [2]. Patients with diabetes are at high risk for adverse outcomes from cardiovascular disease (CVD) [3], heart failure (HF) [4] and renal disease [5]. Diabetes and its macrovascular and microvascular complications account for more than 2 million deaths every year [6] and constitute the seventh most common cause of disability worldwide [7]. The role of dyslipidaemia in the progression of the macrovascular and microvascular complications of diabetes has long been known [8, 9].
Angiopoietin-like protein 8 (ANGPTL8), also known as betatrophin, TD26, “refeeding induced in fat and liver” (RIFL), lipasin, and PRO1185, is a protein primarily produced in the liver and adipose tissue and it plays an important role in triglyceride (TG) metabolism [10]. ANGPTL8, together with ANGPTL3 and ANGPTL4, could regulate TG metabolism by inhibiting the activity of lipoprotein lipase (LPL) and subsequently elevating serum TG since LPL is an enzyme for TG hydrolysis and plasma TG clearance [11,12,13]. These studies indicated that ANGPTL8 is involved in lipid metabolism and the pathogenesis of atherosclerosis. ANGPTL8 levels were increased in diabetes [14,15,16], dyslipidaemia [17, 18], CVD [19], renal function [20,21,22], obesity [23], hypertension [24], and nonalcoholic fatty liver [25]. Furthermore, studies have revealed that ANGPTL8 levels also increased with age [26, 27], suggesting its potential association with aging and with age-related metabolic diseases.
Frailty, a state characterized by reduced physiological reserve and loss of resistance to stressors caused by accumulated age-related deficits, is a major health concern associated with aging [3, 4]. The importance of frailty is highlighted by its consistent association with all-cause mortality and adverse outcomes, such as institutionalization, physical limitations, disability, recurrent hospitalizations, falls and fractures [28, 29]. The frailty index (FI) is a well-established predictor of all-cause mortality [30] and other adverse health outcomes in older adults [31]. The FI has been used in many health services to assess hospital-acquired complications, worsening health, and loss of independence [4,5,6,7,8,9], as well as conditions such as cognitive impairment [10], heart disease [11], osteoporosis [12], intellectual disability [13], and systemic sclerosis [14]. It is also used in epidemiological and clinical studies to grade the degree of risk of several adverse outcomes, including mortality [32]. Hippisley-Cox et al. developed the FI as the QFrailty score and included further key determinants of death [33]. In this work, we retrospectively investigated circulating levels of ANGPTL8 in participants with and without diabetes and its potential associations with death and cardiovascular and renal outcomes in a 5 year cohort study. Furthermore, we evaluated the predictive value of QFrailty score in conjunction with the ANGPTL8 in the prediction of death.
Materials and methods
Study design and population
The present study participants were recruited from Hubei Province, China, from 2011 to 2012 as part of the Risk Evaluation of Cancer in Chinese Diabetic Individuals: a longitudinal (REACTION) study, which was conducted among 259,657 adults aged 40 years old and older in 25 communities across mainland China during 2011–2012 (baseline) and which invited participants to attend follow-up visits during 2014–2016 [34,35,36,37]. At baseline, a comprehensive set of questionnaires, clinical measurements, oral glucose tolerance tests (OGTTs), and laboratory examinations were conducted in all of the participants following standardized protocols. The diagnosis of diabetes was based on the American Diabetes Association 2014 criteria [38]. Diabetes was defined as fasting plasma glucose (FPG) concentration of 7.0 mmol/L or more, 2 h plasma glucose concentration (2 h PG) of 11.1 mmol/L or more, glycated haemoglobin A1c (HbA1c) of 6.5% or more, or a self-reported previous diagnosis of diabetes by health-care professionals. The duration of pre-existing diabetes was counted from the date of diagnosis, and the duration of newly diagnosed diabetes was counted as 0. Propensity scores were used to match subjects in the diabetic and control group (in a 1:1 ratio) with callipers of 0.05 using prespecified clinical variables, including age, sex and body mass index (BMI). Participants were excluded from the analysis if they had missing data for measures of glucose tolerance status at baseline or follow-up visits. The Committee on Human Research at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, approved the study protocol, and all of the participants provided written informed consent. All of the methods were performed in accordance with the relevant guidelines and regulations.
Study outcomes
The primary outcome was death from all causes. The secondary outcomes were a composite of new-onset major adverse cardiovascular events (MACE), hospitalization for HF and renal dysfunction. MACE were defined as cardiovascular death, myocardial infarction, or ischaemic stroke [39]. Renal dysfunction was defined as a glomerular filtration rate (eGFR) less than 60 mL per minute per 1.73 m2 of body-surface area and new end-stage renal disease. All of these outcomes were confirmed by death certificates and hospital records.
Clinical and biochemical evaluation
Data were collected from local community clinics at baseline and follow-up visits. As previously described in the REACTION study [37], information on sociodemographic characteristics, lifestyle factors, medical history and family history was collected by trained staff using a standard questionnaire. All of the participants were asked to fast for at least 10 h to undergo the OGTT and blood sampling for the analysis of various biochemical parameters. \(\beta\) cell function was assessed by homeostasis model assessment of \(\beta\) cell function (HOMA-\(\beta\)) [40]. Insulin resistance was estimated by the index of homeostasis model assessment of insulin resistance (HOMA-IR) [40].
Laboratory tests of ANGPTL8
Blood samples were collected after overnight fasting. Serum was obtained after centrifugation and was aliquoted and then stored at − 80 °C. Fasting serum ANGPTL8 levels were assessed using ELISA kits (Eiaab Science, Wuhan, China; Catalogue No. E11644h) with an intra-assay coefficient of variation (CV) of \(\le\) 6.5% and an interassay CV of \(\le\) 9.2% (provided by the manufacturer). The procedures were performed in accordance with the manufacturer’s instructions. All of the samples were analysed in duplicate.
Statistical analysis
Baseline characteristics of the participants are presented as the means ± standard deviation (SDs) or medians (interquartile ranges, IQRs) for continuous variables and numbers (proportions) for categorical variables. The highest and lowest 0.5% for ANGPTL8 was trimmed [41]. We obtained p values using the Kruskal–Wallis test for continuous variables after passing Shapiro–Wilk normalization testing and the χ2 test for categorical variables. Correlations between variables were assessed using Pearson’s correlation analysis by controlling for the covariates, including age, sex, BMI and lipid profiles. Binary logistic regression analysis was conducted to calculate risk ratios (RRs) and 95% confidence intervals (CIs) for outcomes in quartiles of ANGPTL8. Potential nonlinear relationships between the levels of ANGPTL8 and the incidence of clinical outcomes were examined with restricted cubic splines [42]. A knot was located at the 25th, 50th, and 75th percentiles for ANGPTL8. Tests for nonlinearity were conducted using likelihood ratio tests. If a test for nonlinearity was not significant, we conducted a test for linearity. The QFrailty score (predictor variables are shown in Additional file 1. Appendix 1) was used as a standard criterion to quantify the absolute risk of death in the general population [33]. Receiver-operator characteristic (ROC) curves were drawn, and the performance of the model was evaluated by the area under the curve (AUC). Comparison of p values was performed using Medcalc ROC analysis software. A 2-tailed p value < 0.05 was considered significant. SPSS software (version 20.0), and Stata software (version 12.0) were used for all of the analyses.
Results
Baseline characteristics
Among 10,999 study participants, 9221 (83.8%) were followed up during 2011–2016. Of these participants, 1058 patients were diagnosed with diabetes. We excluded 277 patients with one or more glycaemic measures missing at baseline. Additionally, the highest and lowest 0.5% for ANGPTL8 was trimmed, leaving 781 patients for analysis. After the propensity matching, we acquired 769 matched pairs of diabetic patients and control subjects (Additional file 1. Figure S1). In diabetic patients, 60.6% (N = 466) were newly diagnosed with diabetes, and 39.4% (N = 303) had pre-existing diabetes (Additional file 1. Table S1). Only 19.5% (N = 150) of patients were treated with oral antidiabetic drugs (OADs) or insulin. In patients with medications, 86.6% patients were treated with OADs, 4.7% patients with insulin and 10.7% with a combination of OADs and insulin.
We found that serum ANGPTL8 levels were elevated in diabetic patients compared to control subjects (618.82 \(\pm\) 318.08 vs 581.20 \(\pm\) 299.54 pg/mL, p = 0.03, Additional file 1. Table S2). Furthermore, levels of BMI, HbA1c, FPG, 2 h PG, fasting insulin, HOMA-IR, low density lipoprotein (LDL), TG, cholesterol, creatinine, and incident rates of hypertension and hyperlipidaemia in diabetic patients were higher than in the control subjects (all p < 0.05, Additional file 1. Table S2). During up to 5 years of follow-up, there were 19 participants (2.5%) who died and 44 (5.7%) incident cases for the secondary outcomes among 769 control subjects. The incidence of death (N = 56, 7.3%) and the secondary outcomes (N = 91, 11.8%) were increased in patients with diabetes (all p < 0.05, Additional file 1. Table S2).
Baseline characteristics of participants with and without diabetes according to quartiles of ANGPTL8 are presented in Table 1. Age, sex, aspartate aminotransferase (AST), creatinine, eGFR and smoking status changed with ANGPTL8 levels in both the diabetic patients and control subjects (all p values < 0.05). In control subjects, ANGPTL8 levels were also associated with waist-hip ratio (WHR), systolic blood pressure (SBP), HbA1c and incidence of hypertension (all p values < 0.05). ANGPTL8 levels were associated with BMI high-density lipoprotein (HDL) and incidence of hyperlipidaemia in the diabetic patients (all p values < 0.05).
Next, we studied correlations between ANGPTL8 levels and related variables in control subjects and diabetic patients using Pearson’s correlation analysis. After controlling for multiple variables, ANGPTL8 levels positively correlated with age (r = 0.42), BMI (r = 0.10), and creatinine (r = 0.11) but inversely correlated with eGFR (r = −0.12) in the control subjects (all p values < 0.05) (Additional file 1. Table S3, model 3). Moreover, ANGPTL8 levels positively correlated with age (r = 0.18), duration of diabetes (r = 0.08), 2 h PG (r = 0.08), alanine transaminase (ALT) (r = 0.07), AST (r = 0.13) and creatinine (r = 0.10) but inversely correlated with BMI (r = − 0.07), HDL (r = − 0.09) and eGFR (r = − 0.13) in diabetic patients (all p values < 0.05) (Additional file 1. Table S3, model 3). The positive correlation of ANGPTL8 with TG (p = 0.001, model 2) was also observed, although it diminished after adjusting for other lipid profiles (p = 0.41, model 3).
ANGPTL8 correlates with all-cause mortality and renal dysfunction
As shown in Table 2, increasing quartiles of ANGPTL8 were associated with elevated incidences of death and renal dysfunction in the diabetic patients (all p values < 0.05) but not in the control subjects. Furthermore, death due to CVD in diabetic patients also increased numerically in the highest quartile of ANGPTL8 levels (p = 0.06, Table 2). Binary logistic regression analyses showed that, compared with the first quartile, non-adjusted RRs (96% CIs) (model 1, Table 3) for the primary outcome (all-cause mortality) were 4.67 (1.00–21.92) and 4.64 (1.86–11.59) for the fourth ANGPTL8 quartile in the control subjects and diabetic patients, respectively. The non-adjusted RRs (96% CIs) (model 1, Table 3) for the secondary outcomes were 2.57 (1.04–6.34) and 1.72 (0.95–3.12), respectively, for the fourth ANGPTL8 quartile compared with the first quartile in the control subjects and diabetic patients. However, the associations of ANGPTL8 with all-cause mortality only persisted in the diabetic patients, although they were slightly attenuated after additional adjustment for covariables, including age, sex and BMI (RR, 3.59; 95% CI 1.36–9.51; model 2) and further adjustment for lipid profiles and duration and treatment of diabetes (RR, 3.54; 95% CI 1.32–9.50; model 3). Then, we further analysed the association of ANGPTL8 with any single component of the secondary outcomes and found that elevated ANGPTL8 was associated with an increased risk for renal dysfunction in the diabetic patients (RR in quartile 4 vs. quartile 1, 12.43; 95% CI 1.48–104.81; Table 3, model 3) after adjusting for covariables.
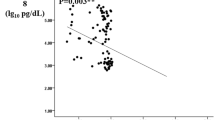
Multivariable-adjusted restricted cubic spline analyses suggested a linear relationship of ANGPTL8 with all-cause mortality in all of the participants (p for nonlinear trend = 0.16, p for linear trend = 0.01; Fig. 1a). Further analysis indicated a significant linear relationship between ANGPTL8 and all-cause mortality in diabetic patients (p for nonlinear trend = 0.99, p for linear trend = 0.01; Fig. 1b) but not in control subjects (p for nonlinear trend = 0.26, p for linear trend = 0.80; Fig. 1c) after adjusting for age, sex, BMI and lipid profiles.
Multivariable-adjusted risk ratios for all-cause mortality in all participants (a), diabetic patients (b) and controls (c). The solid lines indicate multivariate-adjusted risk ratios, and the shaded area indicate the 95% CIs derived from restricted cubic spline regression. A knot is located at the 25, 50, and 75th percentiles for ANGPTL8, and the highest and lowest 0.5% of ANGPTL8 was trimmed. The logistic regression was adjusted for age, sex, and BMI
Predictive values of ANGPTL8 for death
We observed that, when ANGPTL8 levels were combined with QFrailty score, there was improvement for death prediction compared with the QFrailty score alone (Fig. 2a–c, Additional file 1. Table S4). The AUC for the ANGPTL8 + QFrailty model was 0.71 vs 0.59 for the QFrailty score alone (p < 0.001; Fig. 2a) in all of the participants. Consistently, the inclusion of ANGPTL8 improved the predictive performance of QFrailty score in diabetic patients (AUC 0.70 vs 0.59; p < 0.001; Fig. 2b) and control subjects (AUC 0.71 vs 0.57; p = 0.01; Fig. 2c).
Discussion
This retrospective cohort study found that ANGPTL8 levels were elevated and independently associated with all-cause mortality and renal dysfunction in patients with diabetes. The linear relationship of ANGPTL8 with mortality was only observed in the patients with diabetes. The elevated levels of ANGPTL8 were correlated with risk factors related to mortality, such as sex, age, BMI, smoking, eGFR, creatinine, and hyperlipidaemia. Furthermore, the inclusion of ANGPTL8 in QFrailty score significantly improved its predictive performance for mortality in patients with diabetes.
ANGPTL8 levels are increased in patients with diabetes and accompanied by elevated levels of factors associated with metabolic disorders
In our study, diabetic patients had higher ANGPTL8 levels, accompanied by increased incident rates of metabolic disorders, such as hyperlipidaemia, hyperglycaemia and hypertension, consistent with findings from previous observational studies [14,15,16]. Increased insulin levels in patients with diabetes, as shown in our study, are among the important factors for stimulating ANGPTL8 production [16]. The results from the present study that ANGPTL8 was positively correlated with TG and negatively correlated with HDL in diabetic patients implied that ANGPTL8 could make a partial contribution to dyslipidaemia by affecting lipid metabolism. This effect, combined with abnormal metabolism of glucose, high incident rates of hypertension and renal dysfunction, and increased BMI, might contribute together to atherosclerosis, CVD and death in diabetic patients. ANGPTL8 is related to HDL-C dysfunction and is involved in the association between dyslipidaemia and arteriosclerosis [27], regardless of glucose intolerance or diabetes mellitus [43, 44]. ANGPTL8 presents a negative effect on HDL-mediated cholesterol efflux capacity [17] and a strong link with subclinical atherosclerosis [27], and its levels are significantly increased in patients with coronary disease, proportional to disease severity [45]. However, underlying mechanisms for these pathophysiology associations await expounding. Regarding genetics and molecular pathways, miR-143-3p regulates expression of ANGPTL8 transcript and protein levels [46], the prevalence of T2DM and impaired glucose tolerance is greater in subjects with the R59W ANGPTL8 variant [47], and the concomitant presence of CETP B1, NOS3 T and ANGPTL8 T alleles augments the risk of CVD and T2DM [48]. Furthermore, it was reported that targeting ANGPTL8 with antisense oligonucleotide (ASO), a second-generation 2-O-methoxyethyl ASO against ANGPTL8, could prevent cardiovascular disorders [49]. Moreover, GLP-1R agonists stimulate ANGPTL8 production in human hepatocytes dose-dependently [50], and ANGPTL8 has been described as a novel vitamin D receptor target gene involved in nonalcoholic fatty liver pathogenesis [25]. In contradiction with the mainstream conceptions, it has been recently argued that a high ANGPTL8 level in coronary patients protects them from cardiovascular events [51]. ANGPTL8, therefore, has extensive involvement in clinical outcomes and plays critical roles in the progression of complications of cardiometabolic diseases.
All-cause mortality is increased with ANGTPL8 levels and the inclusion of ANGPTL8 in QFrailty score improves its performance for death prediction
We found that the incidence of death increased in participants with diabetes compared to control subjects, consistent with previous publications [2, 52]. Furthermore, a linear relationship between ANGPTL8 levels and risk for all-cause mortality in diabetic patients, but not in control subjects, clearly indicated that ANGPTL8 was a strong predictor of all-cause mortality in patients with diabetes. Further analysis revealed that inclusion of ANGPTL8 in QFrailty score improved the predictive performance for death compared with the QFrailty score alone in diabetic patients. Diabetes is associated with increased risk of death from a wide range of causes, including diabetes-related increased prevalence of microvascular and macrovascular diseases, cancers, infections, liver disease, obesity, and external causes [52]. Our study suggests that elevated levels of ANGPLT8 might actually be one of important risk factors for diabetic patients and might be used in combination with other factors to detect high-risk patients. In diabetic patients, hyperglycaemia and altered lipid profiles are associated with diabetic nephropathy development [20, 53, 54]. The present study showed that ANGPTL8 was inversely correlated with eGFR and independently associated with renal dysfunction in diabetic patients, consistent with previous publications [20], and might also contribute to all-cause mortality [55].
It is interesting to note that inclusion of ANGPTL8 in QFrailty score also improved its predictive performance for death compared with the QFrailty score alone in control subjects. However, no detection of an association of ANGPTL8 with death or a linear relationship between ANGPTL8 levels and risk of all-cause mortality in the control subjects might suggest that further studies are needed to explore the role of ANGPTL8 in death prediction in the general population.
Limitations
There are some limitations of this study. First, all of the participants in our study were Chinese, limiting the generalizability of the findings, which must be confirmed in other ethnic groups. Second, although we used propensity scores to match subjects in the diabetic and control subjects, the retrospective design might also have carried the risk of selection bias; therefore, prospective studies are needed to confirm the results. Regardless, this study is the first report showing that ANGPTL8 could be a potential biomarker for death prediction in patients with diabetes. Third, our study could not identify the causal mechanisms driving the observed associations between ANGPTL8 and detrimental outcomes. Future physiological studies are needed for the causal links and underlying mechanisms. Finally, the study participants were followed for only 5 years. This relatively short follow-up duration reduced the number of clinical events and the study’s statistical power, especially for determining CVD mortality and CVD events. Future studies should be conducted in larger sample sizes to verify the findings of our study.
Conclusion
In conclusion, serum ANGPTL8 levels were associated with an increased risk of all-cause mortality and renal dysfunction in patients with diabetes. Furthermore, inclusion of ANGPTL8 improved the performance of QFrailty scores in predicting all-cause mortality; therefore, serum ANGPTL8 levels might be used as an important biomarker for the prediction of death in patients with diabetes. These findings might suggest that ANGPTL8 is a novel regulator for metabolic diseases with adverse outcomes. Future studies are needed to evaluate ANGPTL8 as a biomarker for the risk of death in cardiometabolic diseases, including diabetes and to study the underlying mechanisms for its potential role in adverse health outcomes in these diseases.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate aminotransferase
- AUC:
-
Areas under the curve
- BMI:
-
Body-mass index
- CI:
-
Confidence intervals
- eGFR:
-
Glomerular filtration rate
- FPG:
-
Fasting plasma glucose
- 2 h PG:
-
2 h plasma glucose concentration
- HbA1c:
-
Glycated haemoglobin A1c
- HDL:
-
High density lipoprotein
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- HOMA-β :
-
Homeostasis model assessment of β cell function
- LDL:
-
Low density lipoprotein
- MACE:
-
Major adverse cardiovascular events
- OAD:
-
Oral antidiabetic drug
- ROC:
-
Receiver-operator characteristic
- RR:
-
Risk ratio
- SBP:
-
Systolic blood pressure
- TG:
-
Triglycerides
- WHR:
-
Waist hip rate
References
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 44 million participants. Lancet. 2016;387:1513–30.
Bragg F, Holmes MV, Iona A, Guo Y, Du H, Chen Y, et al. Association between diabetes and cause-specific mortality in rural and urban areas of China. JAMA. 2017;317:280–9.
Lorber D. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes Targets Ther. 2014;7:169–83.
Ahmad FS, Ning H, Rich JD, Yancy CW, Lloyd-Jones DM, Wilkins JT. Hypertension, Obesity, Diabetes, and Heart Failure-Free Survival. JACC Hear Fail. 2016;4:911–9.
In E, With A, Lesions R, Basis G. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999;341:1127–33.
Danaei G, Lu Y, Singh GM, Carnahan E, Stevens GA, Cowan MJ, et al. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: A comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634–47.
Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800.
Bardini G, Rotella CM, Giannini S. Dyslipidemia and diabetes: reciprocal impact of impaired lipid metabolism and beta-cell dysfunction on micro- and macrovascular complications. Rev Diabet Stud. 2012;9:82–93.
Valensi P, Picard S. Lipids, lipid-lowering therapy and diabetes complications. Diabetes Metab. 2011;37:15–24.
Zhang R, Abou-Samra AB. A dual role of lipasin (betatrophin) in lipid metabolism and glucose homeostasis: consensus and controversy. Cardiovasc Diabetol. 2014;13:133.
Chi X, Britt EC, Shows HW, Hjelmaas AJ, Shetty SK, Cushing EM, et al. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol Metab. 2017;6:1137–49.
Haller JF, Mintah IJ, Shihanian LM, Stevis P, Buckler D, Alexa-Braun CA, et al. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J Lipid Res. 2017;58:1166–73.
Kovrov O, Kristensen KK, Larsson E, Ploug M, Olivecrona G. On the mechanism of angiopoietin-like protein 8 for control of lipoprotein lipase activity. J Lipid Res. 2019;60:783–93.
Chen X, Lu P, He W, Zhang J, Liu L, Yang Y, et al. Circulating betatrophin levels are increased in patients with type 2 diabetes and associated with insulin resistance. J Clin Endocrinol Metab. 2015;100:E96–100.
Hu H, Sun W, Yu S, Hong X, Qian W, Tang B, et al. Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care. 2014;37:2718–22.
Lu P, Chen X, Zhang Z, Zhang J, Yang Y, Liu Z, et al. Insulin upregulates betatrophin expression via PI3K/Akt pathway. Sci Rep. 2017;7:5594.
Luo M, Zhang Z, Peng Y, Wang S, Peng D. The negative effect of ANGPTL8 on HDL-mediated cholesterol efflux capacity. Cardiovasc Diabetol. 2018;17:142.
Yi M, Chen RP, Yang R, Guo XF, Zhang JC, Chen H. Betatrophin acts as a diagnostic biomarker in type 2 diabetes mellitus and is negatively associated with HDL-cholesterol. Int J Endocrinol. 2015;2015:479157.
Huang Y, Fang C, Guo H, Hu J. Increased angiopoietin-like protein 8 levels in patients with type 2 diabetes and cardiovascular disease. Diabetes Res Clin Pract. 2016;120:229–31.
Chen CC, Susanto H, Chuang WH, Liu TY, Wang CH. Higher serum betatrophin level in type 2 diabetes subjects is associated with urinary albumin excretion and renal function. Cardiovasc Diabetol. 2016;15:3.
Yang L, Song J, Zhang X, Xiao L, Hu X, Pan H, et al. Association of serum angiopoietin-like protein 8 with albuminuria in type 2 diabetic patients: results from the GDMD Study in China. Front Endocrinol (Lausanne). 2018;9:414.
Maurer L, Schwarz F, Fischer-Rosinsky A, Schlueter N, Brachs S, Möhlig M, et al. Renal function is independently associated with circulating betatrophin. PLoS ONE. 2017;12:e0173197
Abu-Farha M, Al-Khairi I, Cherian P, Chandy B, Sriraman D, Alhubail A, et al. Increased ANGPTL3, 4 and ANGPTL8/betatrophin expression levels in obesity and T2D. Lipids Health Dis. 2016;15:181.
Abu-Farha M, Cherian P, Qaddoumi MG, AlKhairi I, Sriraman D, Alanbaei M, et al. Increased plasma and adipose tissue levels of ANGPTL8/Betatrophin and ANGPTL4 in people with hypertension. Lipids Health Dis. 2018;17:35.
García-Monzón C, Petrov PD, Rey E, Marañón P, del Pozo-Maroto E, Guzmán C, et al. Angiopoietin-like protein 8 is a novel vitamin D receptor target gene involved in nonalcoholic fatty liver pathogenesis. Am J Pathol. 2018;188:2800–10.
Pu D, Li L, Yin J, Liu R, Yang G, Liao Y, et al. Circulating ANGPTL8 is associated with the presence of metabolic syndrome and insulin resistance in polycystic ovary syndrome young women. Mediators Inflamm. 2019;2019:6321427.
Zheng T, Ge B, Liu H, Chen B, Qin L, Xiao L, et al. Triglyceride-mediated influence of serum angiopoietin-like protein 8 on subclinical atherosclerosis in type 2 diabetic patients: Results from the GDMD study in China. Cardiovasc Diabetol. 2018;17:84.
Vermeiren S, Vella-Azzopardi R, Beckwée D, Habbig AK, Scafoglieri A, Jansen B, et al. Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc. 2016;17(1163):e1–1163.e17.
Shamliyan T, Talley KMC, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12:719–36.
Li X, Ploner A, Karlsson IK, Liu X, Magnusson PKE, Pedersen NL, et al. The frailty index is a predictor of cause-specific mortality independent of familial effects from midlife onwards: a large cohort study. BMC Med. 2019;17:94.
Walston JD, Bandeen-Roche K. Frailty: a tale of two concepts. BMC Med. 2015;13:185.
Mitnitski A, Collerton J, Martin-Ruiz C, Jagger C, von Zglinicki T, Rockwood K, et al. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015;13:161.
Hippisley-Cox J, Coupland C. Development and validation of QMortality risk prediction algorithm to estimate short term risk of death and assess frailty: cohort study. BMJ. 2017;358:j4208.
Ning G, Guang N, Shanghai J, Jiajun Z, Yiming M, Chao L, et al. Risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes. 2012;4:172–3.
Bi Y, Lu J, Wang W, Mu Y, Zhao J, Liu C, et al. Cohort profile: Risk evaluation of cancers in Chinese diabetic individuals: a longitudinal (REACTION) study. J Diabetes. 2014;6:147–57.
Ning G, Bloomgarden Z. Diabetes and cancer: findings from the REACTION study. J Diabetes. 2015;7:143–4.
Lu J, Bi Y, Wang T, Wang W, Mu Y, Zhao J, et al. The relationship between insulin-sensitive obesity and cardiovascular diseases in a Chinese population: results of the REACTION study. Int J Cardiol. 2014;172:388–94.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81-90.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New Eengl JMed. 2019;380(4):347–57.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Lu J, He J, Li M, Tang X, Hu R, Shi L, et al. Predictive value of fasting glucose, postload glucose, and hemoglobin A1c on risk of diabetes and complications in Chinese adults. Diabetes Care. 2019;42:1539–48.
Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61.
Ida S, Kaneko R, Murata K. Efficacy and safety of pemafibrate administration in patients with dyslipidemia: a systematic review and meta-analysis. Cardiovasc Diabetol. 2019;18:38.
Gómez-Ambrosi J, Pascual-Corrales E, Catalán V, Rodríguez A, Ramírez B, Romero S, et al. Altered concentrations in dyslipidemia evidence a role for ANGPTL8/betatrophin in lipid metabolism in humans. J Clin Endocrinol Metab. 2016;101:3803–11.
Jiao X, He J, Yang Y, Yang S, Li J, Qin Y. Associations between circulating full-length angiopoietin-like protein 8 levels and severity of coronary artery disease in Chinese non-diabetic patients: a case-control study. Cardiovasc Diabetol. 2018;17:92.
DiStefano JK. Angiopoietin-like 8 (ANGPTL8) expression is regulated by miR-143-3p in human hepatocytes. Gene. 2019;681:1–6.
Liu J, Yagi K, Nohara A, Chujo D, Ohbatake A, Fujimoto A, et al. High frequency of type 2 diabetes and impaired glucose tolerance in Japanese subjects with the angiopoietin-like protein 8 R59W variant. J Clin Lipidol. 2018;12:331–7.
El-Lebedy D. Interaction between endothelial nitric oxide synthase rs1799983, cholesteryl ester-transfer protein rs708272 and angiopoietin-like protein 8 rs2278426 gene variants highly elevates the risk of type 2 diabetes mellitus and cardiovascular disease. Cardiovasc Diabetol. 2018;17:97.
Morelli MB, Chavez C, Santulli G. Angiopoietin-like proteins as therapeutic targets for cardiovascular disease: focus on lipid disorders. Expert Opin Ther Targets. 2020;24:79–88.
Liu J, Yang K, Xiao W, Le Y, Lang S, Zhang J, et al. GLP-1 receptor agonists stimulate ANGPTL8 production through the PI3K/Akt pathway in a GLP-1 receptor-dependent manner. Peptides. 2018;106:83–90.
Leiherer A, Ebner J, Muendlein A, Brandtner EM, Zach C, Geiger K, et al. High betatrophin in coronary patients protects from cardiovascular events. Atherosclerosis. 2020;293:62–8.
Gregg EW, Cheng YJ, Srinivasan M, Lin J, Geiss LS, Albright AL, et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet. 2018;391:2430–40.
McKay GJ, Savage DA, Patterson CC, Lewis G, McKnight AJ, Maxwell AP. Association analysis of dyslipidemia-related genes in diabetic nephropathy. PLoS ONE. 2013;8:e58472.
Thomas MC, Rosengård-Bärlund M, Mills V, Rönnback M, Thomas S, Forsblom C, et al. Serum lipids and the progression of nephropathy in type 1 diabetes. Diabetes Care. 2006;29:317–22.
Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–8.
Acknowledgements
We would like to thank Dr. Jianhua Zhang and Dr. Zhangping Li in the clinical laboratory of the Division of Endocrinology for their contributions in collecting and handling samples.
Funding
This study was supported by grants from the National Key R&D Program of China (2016YFC0901203) and the National Natural Science Foundation of China ( 81974109, 81570740).
Author information
Authors and Affiliations
Contributions
XY and HZ designed the study. HZ, YX, XC, WL, JX and SS collected the data. HZ and DL performed the serum ANGPTL8 measurements. HZ performed the statistical analysis and wrote the paper. PY and LL reviewed the paper and provided suggestions. XY revised the paper and contributed to the discussion. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Committee on Human Research at Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, approved the study protocol, and all of the participants provided written informed consent.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Appendix 1.
Predictor variables for QFrailty score. Table S1. Characteristics of patients with diabetes. Table S2. Metabolic parameters and outcomes for control subjects and diabetic patients. Table S3. Partial correlations between ANGPTL8 levels and clinical variables in control subjects and diabetic patients. Table S4. Predictive values for all-cause mortality in combination with ANGPTL8 in QFrailty score. Figure S1. Flow diagram for the study population selection.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zou, H., Xu, Y., Chen, X. et al. Predictive values of ANGPTL8 on risk of all-cause mortality in diabetic patients: results from the REACTION Study. Cardiovasc Diabetol 19, 121 (2020). https://doi.org/10.1186/s12933-020-01103-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-020-01103-7