Abstract
Background
Observational series suggest a mortality benefit from metformin in the heart failure (HF) population. However, the benefit of metformin in HF with preserved ejection fraction (HFpEF) has yet to be explored. We performed a systematic review and meta-analysis to identify whether variation in EF impacts mortality outcomes in HF patients treated with metformin.
Methods
MEDLINE and EMBASE were searched up to October 2019. Observational studies and randomised trials reporting mortality in HF patients and the proportion of patients with an EF > 50% at baseline were included. Other baseline variables were used to assess for heterogeneity in treatment outcomes between groups. Regression models were used to determine the interaction between metformin and subgroups on mortality.
Results
Four studies reported the proportion of patients with a preserved EF and were analysed. Metformin reduced mortality in both preserved or reduced EF after adjustment with HF therapies such as angiotensin converting enzyme inhibitors (ACEi) and beta-blockers (β = − 0.2 [95% CI − 0.3 to − 0.1], p = 0.02). Significantly greater protective effects were seen with EF > 50% (p = 0.003). Metformin treatment with insulin, ACEi and beta-blocker therapy were also shown to have a reduction in mortality (insulin p = 0.002; ACEi p < 0.001; beta-blocker p = 0.017), whereas female gender was associated with worse outcomes (p < 0.001).
Conclusions
Metformin treatment is associated with a reduction in mortality in patients with HFpEF.
Similar content being viewed by others
Background
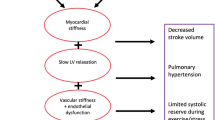
Heart failure (HF) with preserved ejection fraction (HFpEF) is a distinct phenotype hallmarked by clinical signs and symptoms of HF coupled with a normal ejection faction (EF ≥ 50%) and evidence of increased left ventricular (LV) pressures and impaired LV filling on echocardiography [1,2,3]. HFpEF accounts for almost half of the cases of HF and carries an equally poor prognosis to those with HF with reduced ejection fraction (HFrEF), with an estimated 4-year mortality rate of 32% [4]. In contrast to HFrEF, where several therapies have shown good long-term morbidity and mortality outcomes, despite multiple aetiologies leading to the same pathophysiological end-point [1,2,3, 5], effective therapy options for HFpEF have yet to be established. In part, this is because HFpEF is a heterogenous condition, with phenotypic clusters based on age, gender and comorbid illnesses such as obesity, type 2 diabetes mellitus (T2DM) and hypertension [6, 7]. This ultimately leads to dysfunctional metabolic pathways and mechanics within the myocardium resulting in the condition [6, 8]. Therefore, establishing therapy that targets these phenotypes may be the means by which HFpEF therapy evolves [6].
Metformin is a common anti-diabetic drug with both systemic and cardioprotective benefits in addition to its hypoglycaemic effect [9, 10]. At the cellular level metformin activates adenosine monophosphate-activated protein kinase (AMPK) an important regulator of several metabolic pathways resulting in enhanced glucose utilisation, reduction of protein synthesis and improvement of mitochondrial function [11,12,13]. Furthermore, metformin has been shown to reduce collagen accumulation and potentially reduce LV hypertrophy and improve diastolic function in the diabetic myocardium [14]. Several observational series have shown a reduction in mortality in the HF population [15, 16]. Its mortality benefit in the HFpEF population however has yet to be explored. We performed a systematic review and meta-regression analysis to identify whether variations in ejection fraction (EF) impact mortality outcomes in HF patients treated with metformin.
Methods
This systematic review was undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [17]. The review was registered with the PROSPERO International prospective register of systematic reviews (ID CRD42019133780) in September 2019.
Literature search
MEDLINE (1946 to October 2019) and EMBASE (1947 to October 2019) electronic databases using Ovid® were searched for randomised controlled trials and observational studies that assessed the impact of metformin therapy on mortality outcomes in adult HF patients (aged over 18 years). Common search terms included (‘heart failure’ or ‘cardiomyopathies’), (‘diastolic heart failure’ or ‘preserved ejection fraction’), (‘metformin’ or ‘biguanide’) and (‘mortality’ or ‘death’). The full MEDLINE and EMBASE search strategies are detailed in the Additional file 1: Appendix S1. Reference mining of articles in the full-text review was undertaken as well as grey literature searching. Searches were restricted to human studies and those reported in the English language.
Study selection
Two reviewers (A.H. and J.S.) independently undertook abstract screening and included studies reporting mortality outcomes in HF patients treated with metformin. Studies were divided as preserved or reduced EF; with the preserved group further subdivided into those that reported proportion of patients with EF ≥ 50% or EF ≥ 40%. and reported the proportion of patients with an EF ≥ 50%. Studies were excluded if [1] diagnosis of HF was based purely on hospital discharge codes and no information specifically pertaining to EF and mortality outcomes were recorded, and [2] quality of methodology was not able to be critically appraised, for example in conference abstracts and unpublished studies. After exclusions based on title and abstract review, two investigators (A.H. and J.S.) independently undertook full text reviews for eligibility. Reference searching of review articles was undertaken to search for additional studies, however review articles were not formally included in the systematic review. Covidence® (Melbourne, Australia) software was used to track articles in the systematic review process. Conflicts were resolved by a third reviewer (T.H.M.).
Data extraction
Data extraction from eligible studies was undertaken independently by two researchers (A.H. and J.S.). Data was extracted on study design and characteristics, including year of publication, number of subjects, gender, duration of follow-up, medical history (including history of coronary artery disease (CAD), hypertension and peripheral vascular disease (PVD)), baseline treatment with cardio-protective (angiotensin converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB), beta-blocker), and anti-diabetic medications (insulin and sulfonylurea therapy) and EF ≥ 50% on echocardiography. Hazard ratios (HR) with associated 95% confidence intervals (95% CI) on mortality outcomes stratified by the presence or absence of metformin treatment were extracted.
Quality and risk of bias assessment
Quality and risk of bias was assessed using the Newcastle–Ottawa quality assessment scale for cohort studies. This scale assesses the quality of a study based on patient selection, comparability and outcome. Included studies were ranked as good, fair or low quality as outlined in Additional file 1: Appendix S2.
Statistical analysis
Proportion of individuals within each subgroup are expressed as a percentage (%). The meta-analysis was performed using maximally adjusted HR and 95% CI to obtain an overall effect size using a random-effects model. Heterogeneity between studies was tested for mortality outcomes using the Chi square test with a p value < 0.05 being statistically significant. An I2 statistic was generated with > 20% heterogeneity considered significant. A sampling weight was assigned to adjust for differences in study size contributions between each study by calculating the proportion of patients within each sub-group based on the total number of individuals included in the final meta-analysis. Meta-regression models were then performed to determine the interaction between the presence or absence of metformin therapy on mortality outcomes in each sub-group. All statistical analyses were performed using STATA software (StataCorp LLC 2019, v.16.0, College Station, TX, USA).
Results
Study selection
There were 836 studies identified in the search strategy on mortality outcomes in HF patients treated with metformin, with 10 undergoing full-text review (Fig. 1). Of these, 4 reported the total proportion of patients at baseline with an EF ≥ 50% (Table 1) and were included in the final analysis [18,19,20,21]. Of the 6 studies excluded from the final analysis, 1 study excluded HF patients with an LVEF ≥ 40% [22] and 5 studies diagnosed HF at baseline based on hospital discharge diagnosis or International Classification of Diseases (ICD) or Medicare codes and did not report EF data [23,24,25,26,27] as outlined in the Additional file 1: Appendix S2.
Study characteristics
Study characteristics, baseline patient data and mortality outcomes are reported in Tables 1, 2 and 3. Populations studied were primarily in the United States of America and Spain. A total of 22, 469 individuals with HF were analysed with 7655 mortality events identified. Of the total number of individuals 10,168 (45%) had a preserved EF, however, one study reported the total proportion of participants with an EF ≥40% [18]. Follow-up ranged from 1 to 4.7 years. All studies used multiple covariates in adjustments of the effect size (Table 3) with two studies performing propensity matching in the analysis [18, 21].
Risk of bias and quality assessments
All studies included were observational, retrospective cohort studies (Table 1). Based on Newcastle–Ottawa quality assessment, all studies were deemed high quality (Additional file 1: Appendix S3).
Patient characteristics
Overall, patients in the metformin group tended to be younger than those in the non-metformin group (age 71.2 ± 4 years vs. 73.3 ± 3 years, respectively; Table 2). In the metformin group, 1796 (39%) of patients were female, 3499 (76%) were on an ACEi or ARB and 1351 (29%) were on insulin therapy. In contrast, in the non-metformin group 7890 (44%) were female, 11,546 (65%) were on an ACEi or ARB and 9762 (55%) were on insulin therapy.
Mortality outcomes
The pooled HR for mortality in all HF individuals treated with metformin therapy was 0.82 (95% CI 0.74, 0.90, p < 0.001; Fig. 2). The summary estimate demonstrated a moderate degree of heterogeneity between studies (I2 = 58.0%, p < 0.001).
In the subgroup analysis (Table 4), metformin reduced mortality in both reduced and preserved EF after adjusting for concurrent treatment with cardio-protective medications, such as ACEi/ARB and beta-blocker therapy (β = − 0.2 [95% CI − 0.3, − 0.1], p = 0.02). After adjusting for the benefit of metformin in the EF ≥50% group irrespective of cardio-protective therapies, there was a significantly greater reduction in mortality (β = − 2.3 [95% CI − 3.3, − 1.3], p = 0.003). In the reduced EF sub-analysis, metformin was not associated with a mortality benefit (β = 0.2 [95% CI − 0.3, 0.6], p = 0.28). Furthermore, treatment with cardio-protective medications or insulin alone was associated with mortality reduction (ACEi/ARB p < 0.001; beta-blocker p = 0.017; insulin p = 0.002). Interestingly, female gender was associated with worse outcomes (p < 0.001).
Discussion
This review demonstrates that metformin is associated with an 18% mortality reduction in all HF patients and that this benefit is observed in patients treated with concurrent cardio-protective medications, as seen in other clinical trials. However, this meta-analysis is the first to examine a mortality benefit of metformin therapy specifically in patients with a preserved EF.
Diabetic cardiomyopathy
T2DM is a complex metabolic disorder, with the initial hallmarks of insulin resistance and progressive impairment in insulin secretion from the pancreas [28]. Over time a pro-inflammatory state develops, potentiated by alterations in gut microbiota and excess adiposity [29]. Ultimately, end-organ failure ensues.
Diabetic cardiomyopathy is a major adverse outcome of the disease. Where the pathophysiology of DM and atherosclerotic CAD is well understood [30], there is an emergence of data on the cellular and metabolic mechanisms of non-ischaemic driven diabetic cardiomyopathy. Alterations in the AMPK pathway and mitochondrial dysfunction are major components in the development of myocardial impairment [10].
Metformin systemic and myocardial mechanism of action
Metformin is the most commonly prescribed anti-diabetic drug [31]. It has negligible hypoglycaemic risk, has beneficial effects on HbA1c and weight reduction and is relatively inexpensive [31]. In recent years its position in guideline-directed management of new-onset T2DM has somewhat changed. The American Diabetes Association still recommends metformin as first-line therapy in all newly diagnosed T2DM patients [31]; however, the European Society of Cardiology now recommends metformin as first-line therapy only in patients who are deemed not at high-risk or do not have established cardiovascular disease, instead recommending a sodium glucose cotransporter-2 inhibitor (SGLT-2i) or glucagon-like protein-1 receptor agonist (GLP-1 RA) for these patients [32].
In recent years our understanding of metformin’s mechanism of action has evolved. At the cellular level, metformin accumulates in the mitochondrial matrix ultimately causing a reduction in the synthesis of adenosine triphosphate (ATP) and an increase in the level of AMP resulting in the activation of the AMPK pathway [10]. In the liver, decreased ATP availability and inhibition of enzymes involved in lactate uptake results in inhibition of gluconeogenesis [9, 10, 33, 34]. Additionally, by activating AMPK metformin modifies lipid production and breakdown [10, 34]. In the intestinal tract, metformin inhibits glucose absorption and improves insulin production by the incretin affect [35]. In adipocyte tissue metformin reduces free-fatty acid release [36], further improving glucose uptake in other tissues such as skeletal muscle [37].
In the myocardium, metformin also activates AMPK resulting in increased glucose uptake, reduction in protein synthesis and improved mitochondrial function [10]. Furthermore, metformin decreases nitric oxide (NO) production via inhibition of inducible NO synthetase (iNOS) [12]. Finally, metformin has been shown to reduce collagen synthesis and fibrosis in myocardial tissue [14].
Metformin in T2DM and cardiovascular disease
In the UK Prospective Diabetes Study, obese DM-individuals treated with metformin had a 42% (p = 0.017) risk reduction in diabetes-related deaths and a 36% (p = 0.011) reduction in all-cause mortality [38]. Furthermore, there was a 30% (p = 0.020) reduction of all macrovascular complications (MI, sudden death, angina, stroke and PVD) with metformin treatment [38].
The benefit of metformin therapy has been observed across multiple cardiovascular subgroups. In patients with established CAD, metformin was associated with a 29% reduction in cardiovascular mortality (HR 0.81 [95% CI 0.79, 0.84], p < 0.001) and a 33% reduction in all-cause mortality (HR 0.67 [95% CI 0.60, 0.75], p < 0.001) [39]. Furthermore, in a propensity-matched study of patients followed up after an acute coronary syndrome (ACS), metformin was associated with a 50% reduction (HR 0.50 [95% CI 0.26, 0.95], p = 0.035) in all-cause mortality [40]. However, in an analysis of patients treated with metformin presenting with their first ACS, metformin was associated with increased MACE (HR 1.13 [95% CI 1.03, 1.23], p = 0.006) but in those patients who survived beyond 30-days after the index event, metformin was not associated with MACE (HR 1.06 [95% CI 0.95, 1.17, p = 0.305) [41].
In heart transplant recipients, cardiac allograft vasculopathy (CAV) is a major cause of morbidity and mortality with limited treatment options [42]. However, in heart transplant patients treated with metformin prior to the development of CAV, metformin therapy was associated a 90% risk reduction in the development of CAV over a 20-year follow-up period (HR 0.1 [0.02, 0.46], p = 0.003) [42]. Furthermore, metformin was independently associated with a 91% reduction (p = 0.003) in the combined end-point of CAV and cardiovascular mortality in these patients [42].
Metformin in HF
Historically, the use of metformin has been restricted in HF owing to concerns regarding the development of life-threatening lactic acidosis [43]. This adverse side-effect was largely extrapolated from data regarding phenformin, a biguanide that was ultimately withdrawn from the market [43]. However, the development of metformin-associated lactic acidosis in HF patients has since been refuted. In a large observational study spanning over 10 years, 27% of HF patients were on metformin therapy and there were no observed hospitalisations or deaths due to lactic acidosis [23].
Nonetheless, several observational studies have shown both morbidity and mortality benefits of HF patients treated with metformin. In a previous meta-analysis, metformin in HF patients was associated with a 7% reduction in HF hospitalisations (adjusted RR 0.93 [95% CI 0.86, 0.98], p = 0.01) and a 20% reduction in all-cause mortality (RR 0.80 [95% CI 0.74, 0.87], p < 0.001) [16]. Furthermore, this benefit extends to subgroups of HF such that in patients with CAD, all-cause mortality was reduced by 16% (HR 0.84 [0.81, 0.87], p = 0.03) [39]. In patients with hypertension, long-term metformin treatment was associated with a reduction in LV filling pressures and LV mass over-time [44]. In these patients the incidence of symptomatic HFpEF was also reduced with metformin therapy compared to non-metformin therapy (4.6% vs. 11.9% respectively, p = 0.020) [44]. The results of the current meta-analysis supporting these findings in the HF population with an 18% reduction in mortality associated with metformin therapy. Furthermore, this meta-analysis has shown a mortality benefit associated with metformin in patients with HFpEF. This is of clinical relevance as there are limited therapeutic options with mortality benefits in this population of HF patients.
Limitations
Despite a thorough literature search, there is a potential risk of not identifying all studies that have evaluated metformin use in HF patients, particularly those that were performed as a sub-group analysis. However, our use of reference mining and grey-literature searches are likely to have minimised this. Furthermore, due to the nature of observational studies, unaccounted confounding variables may have influenced individual study results. No randomised control trial of the use of metformin in HF patients exists and so this issue cannot be mitigated. The use of aggregate data rather than individual patient data may have limited the analysis.
Finally, our analysis was limited to observational studies of metformin in all HF patients, rather than HFpEF as a single entity. Due to the lack of reporting of outcomes in HFpEF patients our analysis was limited to a meta-regression in this sub-group. Furthermore, due to the low number of studies included we encountered a significant amount of heterogeneity, ultimately reflecting the paucity of evidence in this area and the requirement for further research.
Conclusions
This meta-analysis is the first to highlight a mortality benefit for metformin therapy in HF patients with a preserved EF.
Availability of data and materials
Made on request to the authors.
Abbreviations
- ACEi:
-
Angiotensin converting enzyme inhibitors
- AMP:
-
Adenosine monophosphate
- AMPK:
-
Adenosine monophosphate-activated protein kinase
- ARB:
-
Angiotensin receptor blocker
- ATP:
-
Adenosine triphosphate
- CAD:
-
Coronary artery disease
- CAV:
-
Cardiac allograph vasculopathy
- EF:
-
Ejection fraction
- GLP-1 RA:
-
Glucagon like protein-1 receptor agonist
- HR:
-
Hazard ratios
- HF:
-
Heart failure
- HFpEF:
-
HF with preserved ejection fraction
- HFrEF:
-
HF with reduced ejection fraction
- ICD:
-
International Classification of Diseases
- iNOS:
-
Inducible nitric oxide synthetase
- LV:
-
left ventricular
- MACE:
-
Major adverse cardiovascular events
- NO:
-
Nitric oxide
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- PVD:
-
Peripheral vascular disease
- SGLT-2i:
-
Sodium glucose cotransporter-2 inhibitor
- T2DM:
-
Type 2 diabetes mellitus
References
Group NCHFGW, Atherton JJ, Sindone A, De Pasquale CG, Driscoll A, MacDonald PS, et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ. 2018;27(10):1123–208.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–52.
Somaratne JB, Berry C, McMurray JJ, Poppe KK, Doughty RN, Whalley GA. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail. 2009;11(9):855–62.
Bolam H, Morton G, Kalra PR. Drug therapies in chronic heart failure: a focus on reduced ejection fraction. Clin Med (Lond). 2018;18(2):138–45.
Shah SJ, Katz DH, Deo RC. Phenotypic spectrum of heart failure with preserved ejection fraction. Heart Fail Clin. 2014;10(3):407–18.
Xanthopoulos A, Triposkiadis F, Starling RC. Heart failure with preserved ejection fraction: classification based upon phenotype is essential for diagnosis and treatment. Trends Cardiovasc Med. 2018;28(6):392–400.
Gevaert AB, Boen JRA, Segers VF, Van Craenenbroeck EM. Heart failure with preserved ejection fraction: a review of cardiac and noncardiac pathophysiology. Front Physiol. 2019;10:638.
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–85.
Dziubak A, Wojcicka G, Wojtak A, Beltowski J. Metabolic effects of metformin in the failing heart. Int J Mol Sci. 2018;19(10):2869–91.
Bertero E, Maack C. Calcium signaling and reactive oxygen species in mitochondria. Circ Res. 2018;122(10):1460–78.
Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004;279(31):32771–9.
Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, et al. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119(19):2568–77.
Jyothirmayi GN, Soni BJ, Masurekar M, Lyons M, Regan TJ. Effects of metformin on collagen glycation and diastolic dysfunction in diabetic myocardium. J Cardiovasc Pharmacol Ther. 1998;3(4):319–26.
Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron CB, Stanifer JW, Mock CK, et al. Clinical outcomes of metformin use in populations with chronic kidney disease, congestive heart failure, or chronic liver disease: a systematic review. Ann Intern Med. 2017;166(3):191–200.
Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. 2013;6(3):395–402.
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9, W64.
Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A. Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ Heart Fail. 2011;4(1):53–8.
Facila L, Fabregat-Andres O, Bertomeu V, Navarro JP, Minana G, Garcia-Blas S, et al. Metformin and risk of long-term mortality following an admission for acute heart failure. J Cardiovasc Med (Hagerstown). 2017;18(2):69–73.
Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111(5):583–90.
Romero SP, Andrey JL, Garcia-Egido A, Escobar MA, Perez V, Corzo R, et al. Metformin therapy and prognosis of patients with heart failure and new-onset diabetes mellitus. A propensity-matched study in the community. Int J Cardiol. 2013;166(2):404–12.
Shah DD, Fonarow GC, Horwich TB. Metformin therapy and outcomes in patients with advanced systolic heart failure and diabetes. J Card Fail. 2010;16(3):200–6.
Andersson C, Olesen JB, Hansen PR, Weeke P, Norgaard ML, Jorgensen CH, et al. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: a retrospective nationwide cohort study. Diabetologia. 2010;53(12):2546–53.
Eurich DT, Majumdar SR, McAlister FA, Tsuyuki RT, Johnson JA. Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care. 2005;28(10):2345–51.
Evans JM, Doney AS, AlZadjali MA, Ogston SA, Petrie JR, Morris AD, et al. Effect of Metformin on mortality in patients with heart failure and type 2 diabetes mellitus. Am J Cardiol. 2010;106(7):1006–10.
MacDonald MR, Eurich DT, Majumdar SR, Lewsey JD, Bhagra S, Jhund PS, et al. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case-control study from the U.K. General Practice Research Database. Diabetes Care. 2010;33(6):1213–8.
Retwinski A, Kosmalski M, Crespo-Leiro M, Maggioni A, Opolski G, Ponikowski P, et al. The influence of metformin and the presence of type 2 diabetes mellitus on mortality and hospitalisation in patients with heart failure. Kardiol Pol. 2018;76(9):1336–43.
Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;340(8825):925–9.
Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14(1):50–9.
Chiha M, Njeim M, Chedrawy EG. Diabetes and coronary heart disease: a risk factor for the global epidemic. Int J Hypertens. 2012;2012:697240.
American Diabetes A. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90–102.
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–6.
Zheng J, Woo SL, Hu X, Botchlett R, Chen L, Huo Y, et al. Metformin and metabolic diseases: a focus on hepatic aspects. Front Med. 2015;9(2):173–86.
McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59(3):426–35.
Castro Cabezas M, van Wijk JP, Elte JW, Klop B. Effects of metformin on the regulation of free Fatty acids in insulin resistance: a double-blind, placebo-controlled study. J Nutr Metab. 2012;2012:394623.
Galuska D, Nolte LA, Zierath JR, Wallberg-Henriksson H. Effect of metformin on insulin-stimulated glucose transport in isolated skeletal muscle obtained from patients with NIDDM. Diabetologia. 1994;37(8):826–32.
UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–65.
Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol. 2019;18(1):96.
Jong CB, Chen KY, Hsieh MY, Su FY, Wu CC, Voon WC, et al. Metformin was associated with lower all-cause mortality in type 2 diabetes with acute coronary syndrome: a Nationwide registry with propensity score-matched analysis. Int J Cardiol. 2019;291:152–7.
Bromage DI, Godec TR, Pujades-Rodriguez M, Gonzalez-Izquierdo A, Denaxas S, Hemingway H, et al. Metformin use and cardiovascular outcomes after acute myocardial infarction in patients with type 2 diabetes: a cohort study. Cardiovasc Diabetol. 2019;18(1):168.
Ram E, Lavee J, Tenenbaum A, Klempfner R, Fisman EZ, Maor E, et al. Metformin therapy in patients with diabetes mellitus is associated with a reduced risk of vasculopathy and cardiovascular mortality after heart transplantation. Cardiovasc Diabetol. 2019;18(1):118.
Kuan W, Beavers CJ, Guglin ME. Still sour about lactic acidosis years later: role of metformin in heart failure. Heart Fail Rev. 2018;23(3):347–53.
Gu J, Yin ZF, Zhang JF, Wang CQ. Association between long-term prescription of metformin and the progression of heart failure with preserved ejection fraction in patients with type 2 diabetes mellitus and hypertension. Int J Cardiol. 2020;306:140–5.
Acknowledgements
The authors would like to thank the staff at the Ian Potter Library (Alfred Hospital, Melbourne, Australia) for their assistance with the systematic review. Professor Marwick is the guarantor for this paper and takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Author information
Authors and Affiliations
Contributions
AH performed the search, gathered the data and wrote the 1st draft, JS assisted with gathering data, QH assisted with the analysis and interpretation, THM supervised the project and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable (systematic review and meta-analysis).
Consent for publication
Not applicable (systematic review and meta-analysis).
Competing interests and funding
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Appendix S1.
Search terms. Appendix S2. Studies that underwent full-text review and were excluded from the final analysis. Appendix S3. Quality assessment using the Newcastle-Ottawa quality assessment scale. * indicates the study has met the criteria.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Halabi, A., Sen, J., Huynh, Q. et al. Metformin treatment in heart failure with preserved ejection fraction: a systematic review and meta-regression analysis. Cardiovasc Diabetol 19, 124 (2020). https://doi.org/10.1186/s12933-020-01100-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-020-01100-w