Abstract
Background
Coronary atherosclerosis progresses faster in patients with diabetes mellitus (DM) and causes higher morbidity and mortality in such patients compared to non-diabetics ones (non-DM). We quantify changes in plaque volume and plaque phenotype during lipid-lowering therapy in DM versus non-DM patients using advanced intracoronary imaging.
Methods
We analyzed data from 61 patients with stable angina pectoris included to the PREDICT trial searching for prediction of plaque changes during intensive lipid-lowering therapy (40 mg rosuvastatin daily). Geometrically correct, fully 3-D representation of the vascular wall surfaces and intravascular ultrasound virtual histology (IVUS-VH) defined tissue characterization was obtained via fusion of two-plane angiography and IVUS-VH. Frame-based indices of plaque morphology and virtual histology analyses were computed and averaged in 5 mm long baseline/follow-up registered vessel segments covering the entire length of the two sequential pullbacks (baseline, 1-year). We analyzed 698 5-mm-long segments and calculated the Liverpool active plaque score (LAPS).
Results
Despite reaching similar levels of LDL cholesterol (DM 2.12 ± 0.91 mmol/l, non-DM 1.8 ± 0.66 mmol/l, p = 0.21), DM patients experienced, compared to non-DM ones, higher progression of mean plaque area (0.47 ± 1.15 mm2 vs. 0.21 ± 0.97, p = 0.001), percent atheroma volume (0.7 ± 2.8% vs. − 1.4 ± 2.5%, p = 0.007), increase of LAPS (0.23 ± 1.66 vs. 0.13 ± 1.79, p = 0.018), and exhibited more locations with TCFA (Thin-Cap Fibro-Atheroma) plaque phenotype in 5 mm vessel segments (20.3% vs. 12.5%, p = 0.01). However, only non-DM patients reached significant decrease of LDL cholesterol. Plaque changes were more pronounced in PIT (pathologic intimal thickening) compared to TCFA with increased plaque area in both phenotypes in DM patients.
Conclusion
Based on detailed 3D analysis, we found advanced plaque phenotype and further atherosclerosis progression in DM patients despite the same reached levels of LDLc as in non-DM patients.
Trial registration ClinicalTrials.gov identifier: NCT01773512
Similar content being viewed by others
Background
Studies with intravascular ultrasound (IVUS) have shown that atherosclerosis progression can be stopped [1, 2] or reversed [3,4,5] by using aggressive lipid-lowering therapy. However, these changes are less pronounced in diabetic patients compared to non-diabetic patients despite the same reduction of LDL cholesterol (LDLc) [6, 7]. Furthermore, poor glycemic control in diabetic patients is associated with plaque progression [8, 9] and the presence of diabetes is found as a predictor of plaque progression despite achieving very low levels of LDLc [10].
Atherosclerosis occurs earlier in diabetic patients [11] and shortens their life expectancy [12]. Impaired glycemic homeostasis has a direct influence on the formation and propagation of atherosclerotic plaque [13], and diabetic patients are at risk for a first myocardial infarction that is comparable to non-diabetic patients who have already experienced at least one myocardial infarction [14]. As a consequence, diabetic patients with coronary artery disease have a higher morbidity and mortality compared to non-diabetics [15].
Plaque composition is an important factor related to future clinical presentation [16]. Using IVUS-virtual histology (IVUS-VH), six plaque phenotypes can be distinguished corresponding to those described in the American Heart Association’s Committee on Vascular Lesions [17]. These phenotypes are as follows:
-
no lesion—NL (plaque burden less than 40%)
-
pathologic intimal thickening—PIT
-
fibrous plaque—FP
-
fibro-calcified plaque—FcP
-
thick cap fibro-atheroma—ThCFA
-
thin cap fibro-atheroma—TCFA.
Fibro-atheromas (ThCFA and TCFA) are risk factors for future cardiac events (TCFA more so than ThCFA) [18, 19]. The aim of this study was to compare changes in plaque phenotype during lipid-lowering therapy using 3D reconstruction of coronary arteries based on fusion of IVUS-VH with coronary angiography in diabetic patients (DM group) versus patients without diabetes mellitus (non-DM group). Image data were obtained at baseline and at 1-year follow-up, covering the entire length of IVUS-VH pullback and allowing us to follow changes of plaque phenotypes in a systematic, representative fashion. Unlike the frame-based analysis that is the most common approach in similar trials [1,2,3,4,5], we divided the imaged vessels into 5 mm segments after geometrically correct, 3-D vessel reconstruction via fusion of two-plane angiography and IVUS-VH.
Methods
Study population, catheterization and IVUS imaging
Patient data were taken from the database of the PREDICT trial assessing the ability to predict plaque behavior during intensive lipid-lowering therapy (rosuvastatin 40 mg daily) (ClinicalTrials.gov identifier: NCT01773512). DM-patients were identified as those receiving treatment with either oral antidiabetics or insulin and also based on patient’s history of DM treated by diet. There were no patients with DM type 1 in the study. All patients signed an informed consent, and the study was approved by the local ethics committee.
In all cases only one coronary artery was examined per patient. From the acquired data, only those patients who fulfilled all the following criteria were enrolled:
-
1.
IVUS-VH of a native coronary artery with stenosis ≤ 50% of lumen diameter determined by angiography with no indication for either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG).
-
2.
Good-quality baseline and follow up IVUS-VH pullbacks (i.e., without noticeable pullback speed discontinuity).
-
3.
Imaged vessels free of severe calcification to avoid inconsistency of IVUS-VH plaque type determination in areas of acoustic shadowing.
-
4.
Baseline and follow up pullbacks that were least 30 mm long and that had at least 25 mm long segments that were imaged both at baseline and at follow-up.
One segment from each patient was chosen for the study. The lesion located in the proximal coronary segment or located in a non-angulated segment was selected in cases when several similar stenoses were present in the imaged vessel.
IVUS was performed according to the standard protocol using a phased-array IVUS probe (Eagle Eye 20 MHz 2,9F monorail, Volcano Corporation, Rancho Cordova, California), with automatic pullback at 0.5 mm/s (research pullback, model R-l00, Volcano Corporation, Rancho Cordova, California). After administration of 200 μg of intracoronary nitroglycerin, the IVUS catheter was inserted into the target vessel beyond a distal fiduciary point, and then pulled back to the aorto-ostial junction. The proximal fiduciary point was either the left main bifurcation in the left coronary artery or the first branch or well-defined calcification in the right coronary artery. The distal fiduciary point was determined by the presence of a reproducible side branch.
Original B-mode IVUS pullback image data were acquired at the Charles University Hospital in Prague, Czech Republic, archived onto DVDs, and transferred to the Iowa Institute for Biomedical Imaging, The University of Iowa, Iowa City, Iowa, USA for quantitative analysis. For each IVUS frame, luminal and external elastic membrane (EEM) surfaces were automatically segmented using fully three-dimensional LOGISMOS graph-based approach [20, 21]. This system has been developed at The University of Iowa for simultaneous, optimality-guaranteeing segmentation of multiple mutually-interacting surfaces and 3D/4D analysis of serial IVUS images of coronary atherosclerosis.
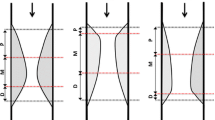
Automatically determined surfaces were reviewed and algorithmically refined by one expert cardiologist (TK) using an operator-guided computer-aided interface [22]. EEM and lumen surfaces/contours served as the input for off-line IVUS-VH computation using Volcano’s research software that is identical to that available on the IVUS console, but allows VH computations based on user-supplied segmentation (Volcano Corp.). Employing our previously reported approach [23], a geometrically correct, full 3-D representation of the vascular wall surfaces and IVUS-VH-defined tissue characterization was obtained via fusion of two-plane angiography and IVUS-VH. This geometrically correct 3-D model served as a basis for quantitative morphologic analyses and quantitative assessment of plaque composition in every frame of the imaged vessel [24]. Using this approach, vessel models were obtained for both the baseline and follow-up pullbacks. After identification of corresponding vascular landmarks in the two 3-D vessel models, the patient-specific model pairs were co-registered. Frame-based indices of plaque morphology and IVUS-VH analyses were computed and averaged in 5 mm long baseline/follow-up registered vessel segments (Fig. 1).
Vessel and plaque measurement morphologic indices included:
-
external elastic membrane (EEM) cross-sectional area (CSA),
-
lumen CSA,
-
percent atheroma volume (PAV) calculated as: PAV = 100 × Σ (EEMarea − Lumenarea)/Σ EEMarea, where EEMarea is the cross-sectional area of the external elastic membrane and Lumenarea is the cross-sectional area of the lumen.
Phenotype definitions
IVUS-VH data classifies plaque into four components: fibrous (F), fibro-fatty (FF), dense calcification (DC), and necrotic core (NC). Phenotypes of all 5 mm-long vessel segments were classified into six categories (NL, FcP, FP, PIT, ThCFA, and TCFA) according to the flowchart given in Fig. 2 and as previously published [25]. Each vessel segment was labeled according to the most advanced plaque phenotype found in each frame (in the following ascending order from least to most advanced phenotype: NL, PIT, FP, FcP, ThCFA, TCFA).
In addition, we calculated the Liverpool Active Plaque Score (LAPS) as adapted from Murray et al. [26]: − 2.149 + 0.68 × NC/DC + 3.39 × MLA + 5.1 (if remodeling index was > 1.05) + 3.7 × VH-TCFA. LAPS was calculated for each frame. LAPS for 5 mm vessel segments was the highest LAPS found in this segment, and LAPS for baseline/follow-up examination was the mean risk score from all analyzed 5 mm vessel segments.
Statistical analysis
Mean values ± standard deviations (or percentages) were calculated for all numerical variables. Differences of two numerical datasets were examined by Student’s t test. Mann–Whitney U test was used instead if the sample could not be assumed to be normally distributed. For categorical variables like diabetes status, contingency tables were used to display frequency distributions. Statistical significance was subsequently calculated by Fisher’s exact test. To investigate segmental plaque morphological changes, mixed-effect analysis with “patient” as random effect is used to correct the clustering of multiple segments within patients. The R statistical-computing environment was employed for analysis. A p-value of 0.05 denoted statistical significance.
Results
The analysis was performed in 698 5-mm-long vessel segments from 61 patients. Total number of DM patients was 17 and they were treated as follows: 2 patients with insulin, 3 patients with diet only, 12 patients with metformin. Patient demographic information is presented in Table 1. Analyzed vessel sections were 70.5 ± 15.8 mm long on average in the DM group and 72.9 ± 18.6 mm long in the non-DM group (p = 0.63). Changes of plaque volumes are summarized in Table 2, and changes in plaque composition in Table 3. Liverpool active risk score and its changes are summarized in Table 4.
Despite reaching similar levels of LDL cholesterol compared to non-DM patients (DM 2.12 ± 0.91 mmol/l, non-DM 1.8 ± 0.66 mmol/l, p = 0.21), DM patients experienced progression of both plaque area (0.21 ± 0.97 vs. − 0.47 ± 1.15, p = 0.001) and percent atheroma volume (0.7% ± 2.8% vs. − 1.4% ± 2.5%, p = 0.007). While the change of LDLc from baseline to follow-up was significant in the non-DM group (p < 0.001), it was non-significant in the DM group (p = 0.15). Distribution of LDLc changes is shown in Fig. 3. We found a significant correlation between the change of LDLc and the change of mean plaque area for non-DM patients (R = 0.47, p = 0.002). No significant correlation was found in DM patients.
The only significant difference in plaque composition was an increase of fibrous tissue in DM patients (0.21 ± 0.75 mm2) compare to a decrease in non-DM patients (− 0.12 ± 0.60 mm2, p = 0.001). Interestingly, both NC tissue and DC decreased in both group (differences between DM and non-DM groups were not significant). One of the most indicative markers for plaque composition risk—NC abutting lumen—increased in DM patients (1.27° ± 50.23°) and decreased in non-DM patients (− 3.27° ± 41.79°). However, this difference was not significant (p = 0.47). Plaque components responsible for increase or decrease of plaque area are summarized in Fig. 4. No change of plaque composition was significant when the DM and non-DM groups were compared. Plaque progression in DM patients was caused by an increase of fibrous and fibro-fatty tissue mean area. Progression in non-DM patients was negligible. Plaque regression was caused by decrease of necrotic tissue and calcified tissue mean area in both groups.
Changes of the pro-inflammatory status were related to changes of plaque composition in the DM patients. The change of hs-CRP level correlated well with the increase of DC mean area (R = 0.57, p = 0.03), decrease of fibrous tissue mean area (R = − 0.69, p = 0.01) and there was a trend for increase of NC mean area (R = 0.46, p = 0.09). All these changes were not significant in non-DM patients: correlation coefficients were much smaller (0.22 for NC, 0.21 for DC and − 0.27 for fibrous tissue, with non-significant “p” values). However, the change of hs-CRP level correlated better with change of the TCFA number in non-DM patients (R = 0.36, p = 0.02).
Glycemia levels correlated well in both the baseline (R = 0.55, p = 0.02) and the follow-up plaque burden (R = 0.57, p = 0.02) in DM patients. Glycemia changes correlated well with changes of the necrotic tissue (R = 0.41, p = 0.01) and the fibro-fatty tissue (R = − 0.33, p = 0.04), but only in the non-DM patients. Baseline glycemia or change of glycemia did not correlate with number of TCFA locations during baseline examination or change of TCFA number during the study.
Figures 5 and 6 show the direction of changes in plaque phenotypes between baseline and follow-up in DM and non-DM patients. It can be clearly seen that changes of plaque phenotypes from early lesions (NL, FP, PIT) into advanced plaque phenotypes (ThCFA, TCFA) were more frequent in DM compared to non-DM patients. However, TCFA plaque phenotype was found in 94 of 192 (48.9%) vessel segments in DM patients and in 228 of 506 vessel segments (45.1%) in non-DM patients (p = 0.96), in part because we labeled each vessel segment according to the most advance identifiable plaque phenotype. In fact the total number of “TCFA frames” was lower, but still without a significant difference between the DM and non-DM groups (24.8% vs, 23.4%, p = 0.29). Figure 7 is showing and example of new TCFA in DM patient.
The TCFA plaque phenotype can experience two types of behavior. It can remain as TCFA (persistent TCFA), or it can change into another plaque phenotypes (suggesting a healed TCFA). An TCFA can also develop from another plaque phenotype during the follow-up period (new TCFA). DM patients experienced more new TCFA plaque phenotype compared to non-DM patients (20.3% vs. 12.6%, p = 0.01). Persistent TCFA plaque phenotype was more frequent in DM patients, but this trend was not statistically significant (82.1% vs. 71.3%, p = 0.12).
We also focused on differences between the DM and non-DM groups in morphological and plaque composition changes in early plaque phenotype (PIT) and advanced plaque phenotype (TCFA). Both PIT and TCFA types of plaques experienced plaque regression together with negative vessel remodeling in non-DM patients and plaque progression together with positive (TCFA) or neutral (PIT) vessel remodeling in DM patients.
We found interesting differences between PIT and TCFA plaque phenotypes according to change of plaque composition. Plaque progression in PIT plaque phenotype was caused by increases of NC and DC plaque composition, but plaque progression in TCFA plaque phenotype was caused by increases of fibrous and fibro-fatty tissue. Results are summarized in Figs. 8 and 9. Figure 10 shows a typical 3D analysis of coronary IVUS pullbacks in DM and non-DM patients. Note the visible differences in plaque area and plaque composition.
Discussion
The main findings of this study are:
-
1.
The coronary plaques in DM patients increased their plaque area and risk profile from baseline to 1-year follow up despite treatment with lipid lowering therapy and despite reaching a similar level of LDLc compared to non-DM patients. In comparison, plaques in non-DM patients experienced a decrease of the LAPS risk score.
-
2.
The plaque phenotype with the highest risk of future cardiac events (TCFA) developed more frequently during the study in DM patients compared to non-DM patients.
-
3.
Coronary plaques continue to progress in both early and advanced plaque in DM patients compared to plaque regression on these two plaque phenotypes in non-DM patients.
-
4.
Morphological and plaque composition changes were more pronounced in the early lesions type (PIT) than in the advanced lesion type (TCFA), and these changes transitioned toward higher risk plaque types (increased mean plaque area and necrotic core content) in DM patients.
The atherosclerotic process in diabetic patients seems to be different compared to non-diabetics. Larger plaques, with higher necrotic core content, were confirmed in DM patients during postmortem studies [27]. These findings were confirmed in vivo by studies using IVUS in plaques from both stable and acute patients [28,29,30]. Studies assessing plaque phenotypes describe more-developed lesions in DM patients [31, 32]. We found accelerated progression in DM patients of both plaque burden and plaque risk profiles in our study. These findings are supported by the study published by Bayturan et al. [10] with data from 7 clinical trials involving 3437 patients, where the presence of diabetes was found as one of the predictors of plaque progression despite the achievement of very low levels of LDLc. The impact of the presence of diabetes on clinical events was tested in a study done by Kennedy et al. Lesions not causing ischemia (with fraction flow reserve > 0.8) led to clinical events in DM patients in 18.1% compared to 7.5% in non-DM patients (p < 0.01), with hazard ratio for the presence of DM of 3.3 [33]. The same author published a provocative study where he suggests to routinely perform PCI of FFR negative lesions for poor outcome of such lesions for fast atherosclerosis progression in DM patients [34].
Factors that can play an important role in faster progression in DM patients are: inflammation, neovascularization, and intra-plaque hemorrhage [35]. Neovessels provide access for inflammatory cells, and thus correlate with plaque inflammation [36]. Cytokines coming from leucocytes decrease collagen production by vascular smooth muscle cells, and enhance production of matrix metalloproteinases, which further weaken the plaque stability through fibrous cap breakdown [37]. Metalloproteases are also important factor for development of positive vessel remodeling that is also known risk factor for plaque instability. Insulin resistance was shown as a factor related to positive vessel remodeling [38]. Neovessels are more fragile and therefore more prone to rupture, thus causing intra-plaque hemorrhage [39]. These processes are augmented in the diabetic plaques [35]. These studies give us the pathological background for our finding of the accelerated progression of plaque phenotype in DM patients, which now look more like expected results than merely accidental findings.
These data are in agreement with our findings of plaque area, remodeling index (marker of positive vessel remodeling) and LAPS score progression contributing to disease progression in DM patients. Interestingly, the amount of NC significantly decreased in both groups of patients. Only the amount of NC abutting lumen increased in DM patient and decreased in non-DM ones, but this difference did not reach statistical significance.
Plaque progression and increased necrotic core in DM patients was previously reported in the TRUTH study4. Inaba et al. [40] performed a serial IVUS study using integrated back scatter analysis (IB IVUS) for examination of plaque composition. They compared 20 mm of non-culprit coronary artery from DM and non-DM patients and found higher total plaque volume and lipid content in DM patients at baseline. Both plaque volume and lipid content continued to progress only in the DM patients. However, comparison of plaque composition changes is difficult using their approach, because IB-IVUS uses a different technique for analysis of plaque composition and cannot distinguish necrotic tissue [41].
The possible explanations for differences in plaque-type and plaque-composition transition frequencies in DM and non-DM patients during the 12-month follow-up period can be attributed to a lower efficacy of lipid-lowering treatment in DM patients, which was documented by observing a significant reduction of LDLc levels only in the non-DM patients. Another factor contributing to the unfavorable plaque changes is a higher inflammatory status biomarker in DM patients. Levels of hs-CRP during baseline and their changes during the study duration were significantly higher only in the non-DM group. However, the change of hs-CRP levels correlated with an increase of the necrotic core percentage and calcification, and the decrease of fibrous tissue more strongly in the DM patients. These findings are in a good agreement with a study performed by Kwon et al. [42], who found a decrease of necrotic tissue inside coronary plaque in statin-treated patients with decreased levels of hs-CRP. This can also be seen as an indirect marker of the more important role of inflammation in DM patients compared to the non DM ones. Surprisingly, the increase of TCFA plaque phenotype correlated with increase of hs-CRP level only in the non-DM patients, despite the fact that the DM patients developed more new TCFA plaque phenotypes.
Additional differences between the DM and non-DM patients included a higher glucose variability in DM patients leading to an increase of plaque burden and lipid content and a decrease of fibrous tissue in their atherosclerotic plaques [43]. Yoshida et al. [44] described an increase of necrotic core in plaque with DM patients with higher glucose fluctuations. We found a notable correlation of both baseline and follow-up glycemia with plaque burden in the DM patients. According to plaque composition, we found good correlation of he change of glycemia with the decrease of fibro-fatty tissue and the increase of necrotic tissue. Surprisingly these correlations were significant only in the non-DM patients (probably due to a negligible change of glycemia in the DM patients). These findings may explain the reported relationships between an increase of hs-CRP and an increase of TCFA plaques in the non-DM patients.
TCFA is a plaque phenotype, defined as a confluent NC with a thin fibrous cap (less than 65 µm). Because this distance cannot be measured by intravascular ultrasound, TCFA plaque phenotype is sometimes named VH-TCFA (TCFA based on virtual histology) or ID-TCFA (IVUS-derived TCFA) in studies using virtual histology, where the main criterion for this phenotype is the lack of a visible fibrous cap over the necrotic core. This type of plaque is known as a risk factor for future development of coronary events [45]. We find similar numbers of such plaque phenotypes in both patients’ groups. The same occurrence of TCFA in DM and non-DM patients is a surprising finding. But it is similar to a study done by Pundziute et al. [46], who did not find a higher occurrence of TCFA in DM patients. However, they found a substantially smaller number of TCFA type plaques (7% in DM and 10% in non-DM patients, p = 0.4), but they reported number of TCFAs per whole plaque, not per vessel segment or frame. Other trials [31, 47] found TCFA more frequent in DM patients (21.6% vs. 13.6% and 75% vs. 41%). Based on these numbers, it is obvious that TCFA definition based on IVUS-VH yields different values the absolute numbers of which are generally incomparable.
Because the determining part of the TCFA definition is fibrous cap thickness, studies using optical coherence tomography (OCT) seem to offer a better tool for its diagnosis. Kato et al. [48] published a study of OCT examinations of all three coronary arteries, and found only non-significantly higher numbers of TCFA plaque phenotype in DM patients than in non-DM ones (18.8% vs. 11%, p = 0.22). Niccoli [49] found the same number of TCFA in DM and non-DM patients (41% vs. 44%, p = 1). It seems that even OCT based TCFA definition is not ideal for discrimination of risk plaque phenotype in DM patients. The best approach may be a dual source of information composed of IVUS-VH for necrotic core detection and OCT for fibrous cap measurement [50] or even better near-infrared spectroscopy IVUS (for highly sensitive lipid pool detection) and OCT [51].
The behavior of TCFA over the period of 1 year has been studied by IVUS-VH in patients with stable coronary artery disease [52], and using non-culprit plaques in patients with acute myocardial infarction [53]. During these studies (over a period of 12 months in Kubo et al. [52] and 13 months in Zhiao et al. [53]), 75% of VH-TCFA healed, whereas 25% remained unchanged in stable patients. Completely different results were found in acute patients: 78% of VH-TCFA plaques remained unchanged and 22% healed. We found an increased number of new TCFA plaque phenotypes during our study. From all TCFA plaque types found during baseline examination, 82.1% remained as TCFA in DM patients and 71.3%, (p = 0.12) in non-DM ones, despite dealing with stable patients only. The main difference between our study and the aforementioned studies is lesion definition. Kubo [52] defined lesions as an area of plaque with at least three consecutive frames with plaque burden ≥ 40%, and analyzed all such frames as one lesion. The same definition was used in the study by Zhiao [53] (where new lesions were separated by at least a 5 mm long segment with plaque burden < 40%). Unlike these two studies, we analyzed vessel segments of coronary arteries of uniform size (5 mm). This method allows more precise spatial accuracy for comparison from baseline to follow up. Acute cardiac events are not caused by changes taking place inside the whole plaque: the development of plaque rupture is a very focal event, and only detailed plaque analysis can help us understand why some regions behave differently than others.
Vessel segments with new TCFA plaque phenotype were found in higher numbers in DM compared to non-DM patients in our study. This finding of higher incidence of new TCFA in DM patients despite lipid-lowering treatment correlates well with the study done by Lindsey et al. [54], who also reported correlation between prevalence of TCFA lesions and duration of diabetes.
This is important, because DM patients with TCFA have higher occurrence of MACE within 3 years, compared to DM ones without TCFA. Plaque progression in DM patients without TCFA led to similar occurrence of MACE like in non-DM patients [55].
An interesting finding is the different plaque behavior in early plaque phenotype (PIT) and advanced plaque phenotype (TCFA) in DM patients compared to non-DM patients. Changes in plaque volume and plaque composition are more pronounced in PIT than in TCFA. Plaque regression was found only in non-DM patients in both plaque phenotypes. The relative amount of necrotic core in plaque between baseline and follow-up increased in the PIT phenotype but decreased in the TCFA plaque phenotype. A similar behavior of NC was described by Hwang et al. [56], who found an increase of NC in non-TCFA plaques and a decrease in TCFA plaques during statin therapy in patients with acute coronary syndrome. Increase of NC closely correlated with changes of hs-CRP in that study. Unfortunately, we do not have data from our patients for this type of correlation. It seems that early lesion phenotype is much more active and it may be the main precursor of fibroatheromas despite statin therapy.
Conclusions
Atherosclerotic plaques in DM patients have more advanced risk profiles than plaques form non-DM patients, and these differences continue to progress despite lipid lowering therapy and despite reaching similar LDLc levels in both groups of patients. In contrary to the group of non-DM patients, the DM patients did not reach significant reduction of LDLc, which shows a decreased efficacy of the lipid lowering treatment in the DM patients. This finding can explain some differences that were observed in our study. The TCFA plaque phenotype detected by virtual histology is probably not the best discriminator for detection of high-risk plaques, because it was found in both groups of patients with the same frequency.
Changes in a plaque morphology and plaque composition are more pronounced in early types of lesions such as PIT. Our findings result from a novel method for detailed analysis of coronary arteries, which divides plaques into 5 mm vessel segments based on 3-D vessel reconstructions. This was done by fusion of IVUS-VH and angiography. This type of analysis allows us to compare corresponding vessel segments of coronary arteries between baseline and follow-up, and to focus on changes of plaque phenotypes, which play a critical role in the development of acute coronary events.
These findings should lead to further examination of plaque progression in DM patients and to development of new therapeutic strategies, which would increase the efficacy of lowering LDLc and slow the progression of atherosclerosis in DM patients. First recommendations come from the IMPROVE IT trial [57], where a combined lipid lowering therapy (statin + ezetimibe) was more efficient in preventing cardiac events in DM patients then in non-DM ones. The most potent lipid-lowering drug (inhibitors of the PCSK-9 protein) was shown as potentially causing a higher plaque regression in DM patients in GLAGOV trial. However this finding did not reach statistical significance (p = 0.39) in a trial with only 20% occurrence of DM patients [58].
Limitations
The main limitation of our study was the low number of patients, which was the result of strict inclusion criteria for high image quality of both baseline and follow-up IVUS examinations, with reliable and consistent pullbacks, and with unquestionably identified corresponding frames. On the other hand, the comparison of almost 600 corresponding 5 mm vessel segments of coronary arteries compensates for this disadvantage. A further limitation is the unequal number of patients with and without diabetes (17 vs. 44 patients). However, the roughly 38% presence of diabetes in our group corresponds well with the occurrence of diabetic patients among individuals with established coronary artery disease.
According to our results, the TCFA plaque phenotype was the most frequent plaque phenotype found in both DM and non-DM patients. This finding is a result of our TCFA definition. We divided all examined segment of each coronary artery into 5 mm segments that were labeled according to the worst plaque phenotype they contained. So, every time TCFA was part of 5 mm examined vessel this segment was labeled as TCFA. Moreover, it is necessary to find three consecutive frames with TCFA features to satisfy our TCFA definition. In situations where those three frames belonged into two different 5-mm vessel sections, both were labeled as “TCFA”. One of the most important parts of TCFA definition is the required presence of a thin fibrous cap. It means fibrous cap thinner than 65 μm. This value is below the resolution of intravascular ultrasound and therefore cannot be used in an IVUS-based trial. These are all facts, which may lead to an overestimation of the number of identified locations with the TCFA plaque phenotype. However, our main goal was to compare plaque phenotypes present in DM and non-DM patients using the same approach for both groups. Based on our findings, our fundamental results are in general agreement with similar studies, despite using slightly different methods and our detailed analysis brings new knowledge about local behavior of DM and non-DM plaque transitions.
Abbreviations
- ACEI:
-
angiotensin converting enzyme
- BL:
-
baseline
- CABG:
-
coronary artery bypass graft
- CAG:
-
coronary angiography
- CSA:
-
cross sectional area
- DC:
-
dense calcification
- DM:
-
diabetes mellitus
- EEM:
-
external elastic membrane
- F:
-
fibrous tissue
- FcP:
-
fibro-calcific plaque phenotype
- FF:
-
fibro-fatty tissue
- FP:
-
fibrous plaque phenotype
- FU:
-
follow up
- HDLc:
-
high-density lipoprotein cholesterol
- hs-CRP:
-
high sensitivity C reactive protein
- Ch:
-
cholesterol
- IB-IVUS:
-
integrated backscatter
- IVUS:
-
intravascular ultrasound
- IVUS-VH:
-
virtual histology
- MACE:
-
major cardiac adverse event
- MHz:
-
mega Hertz
- MI:
-
myocardial infarction
- MLA:
-
minimal lumen area
- NC:
-
necrotic core
- NIH:
-
National Institute of Health
- LAPS:
-
liverpool active plaque score
- LDLc:
-
low density lipoprotein cholesterol
- NL:
-
no lesion plaque phenotype
- Non-DM:
-
not diabetes mellitus
- OCT:
-
optical coherence tomography
- PCI:
-
percutaneous coronary intervention
- PIT:
-
pathological intimal thickening plaque phenotype
- TAG:
-
triacyl-glycerol
- TCFA:
-
thin cap fibroatheroma plaque phenotype
- ThCFA:
-
thick cap fibroatheroma plaque phenotype
- TK:
-
Tomas Kovarnik
- USA:
-
United States of America
References
Schartl M, Bocksch W, Koschyk DH, Voelker W, Karsch KR, Kreuzer J, Hausmann D, Backmann S, Gross M. Use of intravascular ultrasound to compare effect of different strategies of lipid-lowering therapy on plaque volume and composition in patients with coronary artery disease. Circulation. 2001;104:387–92.
Nissen SE, Tuzcu EM, Shoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, De Maria AN, REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–80.
Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–65.
Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A, Nozato T, Miyake S, Takeyama Y, Morino Y, Yamauchi T, Muramatsu T, Hibi K, Terashima M, Michishita I, for the TRUTH Investigators. Impact of diabetes mellitus on coronary atherosclerosis and plaque composition under statin therapy—subanalysis of the truth study. Circ J. 2012;76:2188–96.
Kovarnik T, Mintz GS, Skalicka H, Kral A, Horak J, Skulec R, Uhrova J, Martasek P, Downe RW, Wahle A, Sonka M, Mrazek V, Aschermann M, Linhart A. Virtual histology evaluation of atherosclerosis regression during atorvastatin and ezetimibe administration: HEAVEN study. Circ J. 2012;76:176–83.
Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M, JAPAN-ACS Investigators. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol. 2009;54:293–302.
Daida H, Takayama T, Hiro T, Yamagishi M, Hirayama A, Saito S, Yamaguchi T, Matsuzaki M, COSMOS Investigators. High HbA1c levels correlate with reduced plaque regression during statin treatment in patients with stable coronary artery disease: results of the coronary atherosclerosis study measuring effects of rosuvastatin using intravascular ultrasound in Japanese subjects (COSMOS). Cardiovasc Diabetol. 2012;11:87.
Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M, JAPAN-ACS Investigators. Diabetes mellitus is a major negative determinant of coronary plaque regression during statin therapy in patients with acute coronary syndrome-serial intravascular ultrasound observations from the Japan Assessment of Pitavastatin and Atorvastatin in acute coronary syndrome trial (the JAPAN-ACS Trial). Circ J. 2010;74:1165–74.
Bayturan O, Tuzcu EM, Uno K, Lavoie AJ, Hu T, Shreevatsa A, Wolski K, Schoenhagen P, Kapadia S, Nissen SE, Nicholls SJ. Comparison of rates of progression of coronary atherosclerosis in patients with diabetes mellitus versus those with the metabolic syndrome. Am J Cardiol. 2010;105:1735–9.
Bayturan O, Kapadia S, Nicholls SJ, Tuzcu EM, Shao M, Uno K, Shreevatsa A, Lavoie AJ, Wolski K, Schoenhagen P, Nissen SE. Clinical predictors of plaque progression despite very low levels of low-density lipoprotein cholesterol. J Am Coll Cardiol. 2010;55:2736–42.
Hillier TA, Pedula KL. Complications in young adults with early-onset type 2 diabetes: losing the relative protection of youth. Diabetes Care. 2003;26:2999–3005.
Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368:29–36.
Boyle PJ. Diabetes mellitus and macrovascular disease: mechanisms and mediators. Am J Med. 2007;120:S12–7.
Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34.
Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–90.
Stone GW, Maehara A, Lansky A, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serryus PW. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35.
Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20:1177–8.
Dohi T, Mintz GS, McPherson JA, de Bruyne B, Farhat NZ, Lansky AJ, Mehran R, Weisz G, XU K, Stone GW, Maehara A. Non-fibroatheroma lesion phenotype and long-term clinical outcomes. a substudy analysis from the PROSPECT study. J Am Coll Cardiol Img. 2013;6:908–16.
Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D, European Association for Cardiovascular Prevention & Rehabilitation. ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–818.
Li K, Wu X, Chen DZ, Sonka M. Optimal surface segmentation in volumetric images-a graph-theoretic approach. IEEE Trans Pattern Anal Mach Intell. 2006;28:119–34.
Yin Y, Zhang X, Williams R, Wu X, Anderson DD, Sonka M. LOGISMOS–layered optimal graph image segmentation of multiple objects and surfaces: cartilage segmentation in the knee joint. IEEE Trans Med Imaging. 2010;29:2023–37.
Sun S, Sonka M, Beichel RR. Graph-based IVUS segmentation with efficient computer-aided refinement. IEEE Trans Med Imaging. 2013;32:1536–49.
Wahle A, Prause PM, DeJong SC, Sonka M. Geometrically correct 3D reconstruction of intravascular ultrasound images by fusion with biplane angiography—methods and validation. IEEE Trans Med Imaging. 1999;18:686–99.
Wahle A, Olszewski ME, Vigmostad SC, Medina R, Coskun AU, Feldman C, Stone PH, Braddy KC, Brennan TMH, Rossen JD, Chandran KB, Sonka M. Quantitative analysis of circumferential plaque distribution in human coronary arteries in relation to local vessel curvature. In: IEEE international symposium on biomedical imaging: nano to macro, 2004. New York: IEEE. p. 531–4
García-García HM, Mintz GS, Lerman A, Vince GD, Margolis PMD, van Es GA, More MA, Nair N, Virmani R, Burke AP, Stone GWMD, Serruys PW. Tissue characterization using intravascular radiofrequency data analysis: recommendations for acquisition, analysis, interpretation and reporting. EuroIntervention. 2009;5:177–89.
Murray SW, Stables RH, Garcia-Garcia HM, Grayson AD, Shaw MA, Perry RA, Serruys PW, Palmer ND. Construction and validation of a plaque discrimination score from the anatomical and histological differences in coronary atherosclerosis: the Liverpool IVUS-V-HEART (Intra Vascular UltraSound-Virtual-Histology Evaluation of Atherosclerosis Requiring Treatment) study. EuroIntervention. 2014;10:815–23.
Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–71.
Yang DJ, Lee MS, Kim WH, Park HW, Kim KH, Kwon TG, Kim SW, Rihal CS, Lerman A, Bae JH. The impact of glucose control on coronary plaque composition in patients with diabetes mellitus. J Invasive Cardiol. 2013;25:137–41.
García-García HM, Serruys PW, Mintz GS, Saito S, Klaus V, Margolis P, Carlier S, Goedhart D, Schwartz R. Synergistic effect of cardiovascular risk factors on necrotic core in coronary arteries: a report from the global intravascular radiofrequency data analysis registry. JACC Cardiovasc Imaging. 2009;2:629–36.
Hong YJ, Jeong MH, Choi YH, Ko JS, Lee MG, Kang WY, Lee SE, Kim SH, Park KH, Sim DS, Yoon NS, Yoon HJ, Kim KH, Park HW, Kim JH, Ahn Y, Cho JG, Park JC, Kang JC. Plaque characteristics in culprit lesions and inflammatory status in diabetic acute coronary syndrome patients. JACC Cardiovasc Imaging. 2009;2:339–49.
Marso SP, House JA, Klauss V, Lerman A, Margolis P, Leon MB, Global VH-IVUS. Diabetes mellitus is associated with plaque classified as thin cap fibroatheroma: an intravascular ultrasound study. Diab Vasc Dis Res. 2010;7:14–9.
Araki T, Nakamura M, Utsunomiya M, Sugi K. Visualization of coronary plaque in type 2 diabetes mellitus patients using a new 40 MHz intravascular ultrasound imaging system. J Cardiol. 2012;59:42–9.
Kennedy MW, Kaplan E, Hermanides RS, Fabris E, Hemradj V, Koopmans PC, Dambrink JH, Marcel Gosselink AT, Van’t Hof AW, Ottervanger JP, Roolvink V, Remkes WS, van der Sluis A, Suryapranata H, Kedhi E. Clinical outcomes of deferred revascularisation using fractional flow reserve in patients with and without diabetes mellitus. Cardiovasc Diabetol. 2016;19(15):100.
Kennedy MW, Hermanides RS, Kaplan E, Hemradj V, Fabris E, Koopmans PC, Dambrink JE, Gosselink AT, Van’t Hof AW, Ottervanger JP, Roolvink V, Remkes WS, van der Sluis A, Suryapranata H, Kedhi E. Fractional flow reserve-guided deferred versus complete revascularization in patients with diabetes mellitus. Am J Cardiol. 2016;118:1293–9.
Purushothaman KR, Purushothaman M, Muntner P, Lento PA, O’Connor WN, Sharma SK, Fuster V, Moreno PR. Inflammation, neovascularization and intra-plaque hemorrhage are associated with increased reparative collagencontent: implication for plaque progression in diabetic atherosclerosis. Vasc Med. 2011;16:103–8.
Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–54.
Nesto RW, Zarich S. Acute myocardial infarction in diabetes mellitus: lessons learned from ACE inhibition. Circulation. 1998;97:12–5.
Kim SH, Moon JY, Lim YM, Kim KH, Yang WI, Sung JH, Yoo SM, Kim IJ, Lim SW, Cha DH, Cho SY. Association of insulin resistance and coronary artery remodeling: an intravascular ultrasound study. Cardiovasc Diabetol. 2015;14:74.
Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–61.
Inaba S, Okayama H, Funada J, Higashi H, Saito M, Yoshii T, Hiasa G, Sumimoto T, Takata Y, Nishimura K, Inoue K, Ogimoto A, Higaki J. Impact of type 2 diabetes on serial changes in tissue characteristics of coronary plaques: an integrated backscatter intravascular ultrasound analysis. Eur Heart J Cardiovasc Imaging. 2012;13:717–23.
Kawasaki M, Sano K, Okubo M, Yokoyama H, Ito Y, Murata I, Tsuchiya K, Minatoguchi S, Zhou X, Fujita H, Fujiwara H. Volumetric quantitative analysis of tissue characteristics of coronary plaques after statin therapy using three-dimensional integrated backscatter intravascular ultrasound. J Am Coll Cardiol. 2005;45:1946–53.
Kwon O, Kang SJ, Kang SH, Lee PH, Yun SC, Ahn JM, Park DW, Lee SW, Kim YH, Lee CW, Han KH, Park SW, Park SJ. Relationship between serum inflammatory marker levels and the dynamic changes in coronary plaque characteristics after statin therapy. Circ Cardiovasc Imaging. 2017;10:e005934.
Okada K, Hibi K, Gohbara M, Kataoka S, Takano K, Akiyama E, Matsuzawa Y, Saka K, Maejima N, Endo M, Iwahashi N, Tsukahara K, Kosuge M, Ebina T, Fitzgerald PJ, Honda Y, Umemura S, Kimura K. Association between blood glucose variability and coronary plaque instability in patients with acute coronary syndromes. Cardiovasc Diabetol. 2015;14:111.
Yoshida N, Yamamoto H, Shinke T, Otake H, Kuroda M, Terashita D, Takahashi H, Sakaguchi K, Hirota Y, Emoto T, Amin HZ, Mizoguchi T, Hayashi T, Sasaki N, Yamashita T, Ogawa W, Hirata K. Impact of CD14++CD16+ monocytes on plaque vulnerability in diabetic and non-diabetic patients with asymptomatic coronary artery disease: a cross-sectional study. Cardiovasc Diabetol. 2017;16:96.
Calvert P, Obaid D, O’Sullivan M, Shapiro L, McNab D, Densem C, Schofield P, Braganza D, Clarke S, Ray K, West N, Bennett M. Association between IVUS findings and adverse outcomes in patients with coronary artery disease the VIVA (VH-IVUS in Vulnerable Atherosclerosis) study. J Am Coll Cardiol Img. 2011;4:894–901.
Pundziute G, Schuijf JD, Jukema JW, van Werkhoven JM, Nucifora G, Decramer I, Sarno G, Vanhoenacker PK, Reiber JH, Wijns W, Bax JJ. Type 2 diabetes is associated with more advanced coronary atherosclerosis on multislice computed tomography and virtual histology intravascular ultrasound. J Nucl Cardiol. 2009;16:376–83.
Nasu K, Tsuchikane E, Katoh O, Fujita H, Surmely JF, Ehara M, Kinoshita Y, Tanaka N, Matsubara T, Asakura Y, Asakura K, Terashima M, Suzuki T. Plaque characterisation by virtual histology intravascular ultrasound analysis in patients with type 2 diabetes. Heart. 2008;94:429–33.
Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, Yeh RW, Sakhuja R, Zhang S, Uemura S, Yu B, Mizuno K, Jang IK. Comparison of nonculprit coronary plaque characteristics between patients with and without diabetes: a 3-vessel optical coherence tomography study. JACC Cardiovasc Interv. 2012;5(11):1150–8.
Niccoli G, Giubilato S, Di Vito L, Leo A, Cosentino N, Pitocco D, Marco V, Ghirlanda G, Prati F, Crea F. Severity of coronary atherosclerosis in patients with a first acute coronary event: a diabetes paradox. Eur Heart J. 2013;34:729–41.
Goderie T, van Soest G, Garcia-Garcia H, Gonzalo N, Koljenovi S, van Leenders G, Mastik F, Regar E, Oosterhuis J, Serruys P, van der Steen A. Combined optical coherence tomography and intravascular ultrasound radio frequency data analysis for plaque characterization. Classification accuracy of human coronary plaques in vitro. Int J Cardiovasc Imaging. 2010;26:843–50.
Roleder T, Kovacic J, Ali Z, Sharma R, Cristea E, Moreno P, Sharma S, Narula J, Kini A. Combined NIRS and IVUS imaging detects vulnerable plaque using a single catheter system: a head-to-head comparison with OCT. EuroIntervention. 2014;10:303–11.
Kubo T, Maehara A, Mintz GS, Doi H, Tsujita K, Choi SY, Katoh O, Nasu K, Koenig A, Pieper M, Rogers JH, Wijns W, Bose D, Margolis MP, Moses JW, Stone GW, Leon MB. The dynamic nature of coronary artery lesion morphology assessed by serial virtual histology intravascular ultrasound tissue characterization. J Am Coll Cardiol. 2010;55:1590–7.
Zhao Z, Witzenbichler B, Mintz GS, Jaster M, Choi SY, Wu X, He Y, Margolis MP, Dressler O, Cristea E, Parise H, Mehran R, Stone GW, Maehara A. Dynamic nature of nonculprit coronary artery lesion morphology in STEMI: a serial IVUS analysis from the HORIZONS-AMI trial. JACC Cardiovasc Imaging. 2013;6:86–95.
Lindsey JB, House JA, Kennedy KF, Marso SP. Diabetes duration is associated with increased thin-cap fibroatheroma detected by intravascular ultrasound with virtual histology. Circ Cardiovasc Interv. 2009;2:543–8.
Kedhi E, Kennedy MW, Maehara A, Lansky AJ, McAndrew TC, Marso SP, De Bruyne B, Serruys PW, Stone GW. Impact of TCFA on unanticipated ischemic events in medically treated diabetes mellitus: insights from the PROSPECT study. JACC Cardiovasc Imaging. 2017;10:451–8.
Hwang DS, Shin ES, Kim SJ, Lee JH, Kim JM, Lee SG. Early differential changes in coronary plaque composition according to plaque stability following statin initiation in acute coronary syndrome: classification and analysis by intravascular ultrasound-virtual histology. Yonsei Med J. 2013;54:336–44.
Cannon Ch, Blazing M, Giugliano R, McCagg A, White J, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–97.
Nicholls S, Puri R, Anderson T, Ballantyne Ch, Cho L, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the glagov randomized clinical trial. JAMA. 2016;316:2373–84.
Authors’ contributions
TK: main author, idea of investigation, invasive coronary examination, IVUS data assessment, manuscript writing and editing. ZC: co-author of software for 3D reconstruction of coronary arteries based on fusion of IVUS-VH with coronary angiography, data assessment, statistical analysis. GSM: scientific mentor, supervision of manuscript writing. AW: co-author of software for 3D reconstruction of coronary arteries based on fusion of IVUS-VH with coronary angiography, data assessment. KB: collecting of patient’s data. AK: collecting of patient’s data, invasive coronary examinations. MC: statistical analysis. KK: collecting of patients’ data, building of database. JL: study design, manuscript writing and editing. MS: co-author of software for 3D reconstruction of coronary arteries based on fusion of IVUS-VH with coronary angiography, technivasl supervsision, manuscript writing and editing. AL: scientific supervision, manuscript writing and editing. All authors read and approved the final manuscript.
Acknowledgements
The material and software-enabling support from Volcano Corporation is gratefully acknowledged.
Competing interests
Tomas Kovarnik received non-restricted scientific support from Volcano Company.
Availability of data and materials
The data that support the findings of this study are available from The University of Iowa but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of The University of Iowa.
Consent to participate
All included human subjects signed informed consent with participation in the study.
Consent for publication
All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript in accordance with ICMJE criteria.
Submitted article is original, it has not already been published in other journal, and is not currently under consideration by another journal.
The authors agree to the terms of the BioMed Central Copyright and License Agreement. Dataset can be sent based on personal request to corresponding author email.
Ethics approval and consent to participate
All investigations were approved by local Ethical committee (Ethical committee of General University Hospital in Prague from 30th of June 2011) and were performed in accordance with the Declaration of Helsinki.
Funding
This study was supported, in part, by grants from the Ministry of Education, Youth and Sports of Czech Republic (LH12053), and the NIH (R01-HL63373 and R01-EB004640).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kovarnik, T., Chen, Z., Mintz, G.S. et al. Plaque volume and plaque risk profile in diabetic vs. non-diabetic patients undergoing lipid-lowering therapy: a study based on 3D intravascular ultrasound and virtual histology. Cardiovasc Diabetol 16, 156 (2017). https://doi.org/10.1186/s12933-017-0637-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-017-0637-0