Abstract
Background
Strict glycaemic control in patients with type 2 diabetes has proven to have microvascular benefits while the effects on CVD and mortality are less clear, especially in high risk patients. Whether strict glycaemic control would reduce the risk of future CVD or mortality in patients with type 2 diabetes and pre-existing CVD, is unknown. This study aims to evaluate whether the relation between baseline HbA1c and new cardiovascular events or mortality in patients with type 2 diabetes and pre-existing cardiovascular disease (CVD) is modified by baseline vascular risk.
Methods
A cohort of 1096 patients with type 2 diabetes and CVD from the Second Manifestations of ARTerial Disease (SMART) study was followed. The relation between HbA1c at baseline and future vascular events (composite of myocardial infarction, stroke and vascular mortality) and all-cause mortality was evaluated with Cox proportional hazard analyses in a population that was stratified for baseline risk for vascular events as calculated with the SMART risk score. The mean follow-up duration was 6.9 years for all-cause mortality and 6.4 years for vascular events, in which period 243 and 223 cases were reported, respectively.
Results
A 1 % increase in HbA1c was associated with a higher risk for all-cause mortality (HR 1.18, 95 % CI 1.06–1.31). This association was also found in the highest SMART risk quartile (HR 1.33, 95 % CI 1.11–1.60). There was no relation between HbA1c and the occurrence of cardiovascular events during follow-up (HR 1.03, 95 % CI 0.91–1.16). The interaction term between HbA1c and SMART risk score was not significantly related to any of the outcomes.
Conclusion
In patients with type 2 diabetes and CVD, HbA1c is related to the risk of all-cause mortality, but not to the risk of cardiovascular events. The relation between HbA1c and all-cause mortality in patients with type 2 diabetes and vascular disease is not dependent on baseline vascular risk.
Similar content being viewed by others
Background
Cardiovascular disease (CVD) is a major healthcare problem [1], especially in high income countries [2, 3], and it remains the most common cause of death and disability in patients with type 2 diabetes [4]. As the number of patients with type 2 diabetes is expected to grow to 592 million worldwide by 2035 [5], it is relevant to expand the understanding of the role of type 2 diabetes in the development and progression of CVD.
In cohort studies with patients with type 2 diabetes, poor glycaemic control, as measured by HbA1c, is associated with an increased risk for cardiovascular disease [6–8]. The relationship between increasing plasma HbA1c levels and a higher risk for incident macrovascular and microvascular disease is most prominent above a HbA1c of 7.0 % (53 mmol/mol) for macrovascular and 6.5 % (48 mmol/mol) for microvascular disease [9].
In clinical trials, lowering HbA1c levels in patients with type 2 diabetes has beneficial effects on incident microvascular complications [10–12], while the effect on macrovascular complications is less clear [10–13]. Follow-up analyses of these trials display positive effects of strict glycaemic control [14–16] as well as the absence of such benefits [14, 17] or even adverse effects [16]. Recent meta-analyses seem to support the more negative results and state that strict glucose regimes might not be the optimal treatment to reduce vascular risk for patients with type 2 diabetes [18, 19].
It has been suggested that these different findings might be explained by differences in patient characteristics between the studies [20]. It seems that healthier patients (younger, shorter history of CVD and/or type 2 diabetes, lower HbA1c at baseline) benefit more from strict glycaemic control than their older counterparts with a longer history of disease (CVD and/or type 2 diabetes) and a higher baseline HbA1c [13, 15–17].
These findings suggest that strict glycaemic control might not be beneficial in high risk patients, including patients with type 2 diabetes and cardiovascular disease [20]. However, little is known about the relation between HbA1c and risk for new cardiovascular events in these high risk patients [21]. As patients with type 2 diabetes and pre-existing CVD are at very high risk for new cardiovascular events and death, it is relevant to investigate whether glycaemic control remains an important amendable risk factor in these patients.
The recently published SMART (Second Manifestations of ARTerial Disease) risk score predicts 10-year risk of recurrent major vascular events and vascular mortality in patients with cardiovascular disease [22]. Their work showed that patients with a history of cardiovascular disease do not, as was previously assumed, always classify as high-risk patients (defined as having a 10-year risk of above 20 %), as the 10-year risk for recurrent events in their cohort ranged from 6 to 44 % [22]. The SMART risk score enables clinicians to quantify risk in individual patients and to identify those at the highest risk. We therefore investigated the relation between HbA1c and new cardiovascular events and mortality in patients with type 2 diabetes and CVD, stratified by their baseline risk for new cardiovascular events and mortality as calculated by the SMART risk score.
Methods
Study population
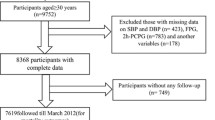
For this study we used data from patients with type 2 diabetes and cardiovascular disease enrolled in the Second Manifestations of ARTerial Disease (SMART) cohort. The design and rationale of the SMART study have been described previously [23]. To shortly summarise, patients aged 18–79 who are referred to the University Medical Center Utrecht with atherosclerotic vascular disease or for treatment of cardiovascular risk factors are included in the database. Physical and laboratory examinations are performed in the hospital after an at least 8-h fast. Information about a history of CVD, vascular risk factors and a detailed medical history are obtained via a questionnaire. Follow-up information is obtained via questionnaires sent to patients every 6 months or via the general practitioner (GP) if the patient is unwilling to fill out questionnaires and gives consent for GP consulting. The ethics committee of the University Medical Center Utrecht approved this study, all eligible patients received written and oral information about the study and all included participants gave informed consent [23].
For the current study, patients with type 2 diabetes and clinically manifest vascular disease at baseline were selected (n = 1205). Patients with a missing high-sensitivity C-reactive protein (hsCRP) value (n = 22) or a hsCRP value above 15 mg/L (n = 83) were excluded. The latter were excluded because we think such a high hsCRP value is more indicative of infection or a different cause of inflammation than the low-grade inflammation seen in patients with atherosclerosis [24]. Finally, we recalculated missing data regarding LDL using the Friedewald formula. Data that remained missing after this procedure (n = 6) was imputed. The participants for whom the SMART risk score could not be calculated (n = 4) were also excluded, bringing the final study population to 1096 participants.
Study definitions
Diabetes mellitus was defined as a referral diagnosis of type 2 diabetes, self-reported type 2 diabetes, a fasting serum glucose ≥7.0 mmol/l at inclusion with initiation of glucose lowering treatment within 1 year or the use of oral anti-hyperglycemic agents or insulin at baseline. Participants with known type 1 diabetes were excluded from our analyses. History of clinically manifest vascular disease is defined as ever having had clinical manifest vascular disease [23]. HbA1c was studied as a continuous variable.
The cardiovascular outcomes defined are myocardial infarction (MI), ischemic stroke, the composite of major cardiovascular events, cardiovascular mortality, and all-cause mortality. Their definitions have been described elsewhere [23]. Each notification of a possible event is thoroughly checked by collecting all available information about the patient regarding the event. This includes all correspondence and documented investigations concerned with the particular event. All events were audited by three independent committee members of the Outcome Committee.
Data analyses
Data are presented as mean ± standard deviation (SD) or median with interquartile range for variables with a skewed distribution. For all 1096 participants, the SMART risk score was calculated using the equation described by Dorresteijn et al. [22]. This equation predicts the 10-year risk of recurrent major vascular events (myocardial infarction, stroke and vascular death) in patients with CVD [22]. All analyses were performed for the whole cohort and in quartiles (Q1-Q4) of SMART risk. Non-HDL cholesterol was calculated for all patients by subtracting the measured HDL cholesterol from the measured total cholesterol value. To assess whether baseline risk as defined by the SMART risk score has a modifying effect on the relation between HbA1c and new outcomes, the interaction term between HbA1c and SMART risk score was used. We considered the effect modification by the SMART risk score significant when the p value of the interaction term was <0.05. Cox regression modelling was used to assess the relation between HbA1c and cardiovascular outcomes and mortality. Cubic spline analysis was rejected based on the inability of this technique to measure effect modification. Results are given as hazard ratios with 95 % confidence interval, and denote the increase in risk for a cardiovascular outcome or mortality related to a 1 % increase in HbA1c. Three models were constructed. The first model was a univariate model which only included HbA1c. The second model was adjusted for sex and age, and the third model was additionally adjusted for the known confounders current smoking, non-HDL cholesterol, diabetes duration, systolic blood pressure and eGFR (MDRD). We performed sensitivity analyses including the earlier excluded patients with a hsCRP value above 15 mg/L (n = 83) and additional analyses adjusted for all components of the SMART risk score. All analyses were performed in an imputed dataset, as missing data is seldom missing at random. We imputed missing data on eGRF(1, 0.09 %), LDL cholesterol (6, 0.5 %) and HbA1c (100, 9.1 %). All analyses were performed using SPSS 21 (IBM, New York). Graphs were created using R 3.1.1, (The R Foundation for Statistical Computing, Austria).
Results
Baseline
The mean age of the participants in the cohort was 62.6 ± 8.8 years and 76 % of the participants were male. The SMART risk score ranged from a predicted 5–100 % 10-year risk for recurrent vascular events. The creation of quartiles based on the SMART risk score resulted in four groups with a SMART risk score range of 5–16, 16–24, 24–37 and 37–100 % respectively (Table 1).
Mean age increased from 55.8 ± 7.1 years in the lowest risk quartile to 70.0 ± 6.5 years in the highest risk quartile, and the percentage of males increased from 72 to 76 %. In addition, the mean duration of diabetes increased from 4.0 to 6.0 years and time since first vascular event increased from 0 to 9 years.
The relation between HbA1c and risk for new cardiovascular events and mortality in the whole cohort
During a mean follow-up period of 6.9 years 243 patients’ deaths were reported. The mean follow-up period for vascular events was 6.4 years, in which period 223 cases were reported. The separate outcomes had follow-up periods of 5.8 years for MI and 6.7 years for stroke, in which period 84 and 48 events were reported, respectively.
In the whole cohort of patients with type 2 diabetes and vascular disease, a 1 % increase in HbA1c level was associated with a higher risk for all-cause mortality (HR 1.18, 95 % CI 1.06–1.31). There was no relation between HbA1c and risk for new cardiovascular events during follow-up (HR 1.03, 95 % CI 0.91–1.16) (Table 2).
The relation between HbA1c and new cardiovascular events and mortality according to baseline risk
In patients with the highest predicted 10 years risk for new cardiovascular disease or mortality (37–100 %), a 1 % increase in HbA1c was associated with an increased risk for cardiovascular mortality (HR 1.29, 95 % CI 1.03–1.61) and all-cause mortality (HR 1.33, 95 % CI 1.11–1.60) (Table 2). The results for the lowest SMART risk quartile were similar, albeit not significant (HR 1.36, 95 % CI 0.93–1.98). An inverse relation was suggested between HbA1c and risk for MI in all quartiles (Fig. 1). This was most apparent in the second risk quartile (HR 0.71, 95 % CI 0.46–1.10).
The relation between HbA1c and risk for new cardiovascular events or mortality in quartiles stratified by baseline risk. The relation between HbA1c and risk for each outcome under study is represented separately. The quartiles are denoted on the x axis with Q1–Q4 respectively. The SMART risk score range of each quartile is given between brackets. Hazard ratios are adjusted for sex, age, current smoking, non-HDL cholesterol, diabetes duration, systolic blood pressure and eGFR (MDRD). Figure 1 a–e show the association between the SMART risk score and risk for a specified cardiovascular event (b, c, d) or mortality (a, e). The SMART risk score calculates the 10-year risk for developing a new cardiovascular event in patients with a history of cardiovascular disease. For example, a person with a SMART risk score of 20 has a 20 % chance of experiencing a new cardiovascular event in the upcoming 10 years
However, the interaction term between HbA1c and the SMART risk score was not significant for the composite cardiovascular outcomes and for cardiovascular or all-cause mortality (p = 0.225, p = 0.259 and p = 0.179 respectively). Thus, baseline risk as estimated by the SMART risk score did not modify the relation between HbA1c and cardiovascular events or mortality in patients with type 2 diabetes and cardiovascular disease.
None of our sensitivity analyses changed the direction or magnitude of the modifying effect of the SMART risk score on the relation between HbA1c and vascular outcomes and mortality.
Discussion
In the present study we show that within a population of patients with type 2 diabetes and CVD at baseline, an increase in HbA1c is related to an increased risk for all-cause mortality, but not to an increased risk for cardiovascular events. The relation between HbA1c and risk for all-cause mortality is not significantly influenced by baseline vascular risk.
The Veterans Affairs Diabetes Trial (VADT) study also stratified their patients with type 2 diabetes, of which 38 % had CVD at baseline, by baseline risk, using coronary artery calcium (CAC) as a measure for atherosclerosis [25]. Strict glycaemic control was related to lower incidence of future cardiovascular events in patients with a CAC score below 100 at baseline, while this effect was not present in patients with higher CAC values [25]. Although not directly comparable to our study due to the interventional nature of the VADT, these findings do suggest that within a high risk population, the relation between glycaemic control and cardiovascular events differs according to risk for (new) cardiovascular events.
The absence of a relation between HbA1c and (new) vascular disease in our study population could be related to differences in the pathogenesis of vascular disease in patients with type 2 diabetes. In the later stages of type 2 diabetes other risk factors than glycaemic control may be more important in inducing vascular complications. These include hypertension [26, 27], lipids [28, 29], and the calcification of the intima media of blood vessels, called Mönckeberg’s sclerosis [30]. The latter condition is strongly associated with morbidity and mortality in diabetes and is independent of glycaemic control [30].
Following this line of reasoning, the significant relation between HbA1c and mortality in the whole cohort is an unexpected finding. This result may be powered by the patients in the highest risk quartile, who are older and have a longer history of vascular disease and diabetes. In this group of high risk patients, a high HbA1c may be a proxy of frailty. Indeed, higher HbA1c levels are related to a higher chance of being frail in women, and this relation is strongest above an HbA1c level of 9 % (75 mmol/mol) [31]. Frailty in patients with diabetes is associated with higher mortality and glucose dysregulation [32], and the prevalence of frailty increases with age.
We propose that in the highest risk quartile, HbA1c might be regarded as a proxy for overall health status or frailty. High HbA1c levels would then reflect a poor overall health status, which in itself can be regarded as a risk factor for mortality. Although the whole study population had a mean HbA1c of 51.9 mmol/mol, there was a significant distribution of HbA1c levels (range 29–116.4 mmol/mol) in the whole cohort. While it is quite possible that the relation between HbA1c and endpoints is different in very high HbA1c levels (for example >100 mmol/mol), our study lacks the statistical power to address this question. In that regard, our study population is an example of a cohort of patients with type 2 diabetes and cardiovascular disease which is comparable to patient population typically seen in an everyday out-patient clinic.
The main strengths of this study include the prospective design, the substantial follow-up period and large cohort size providing a relatively high number of events. Furthermore, the completeness of data reduced the risk of bias. The use of the SMART risk score, which was derived from the SMART cohort, ensured that the risk score used was well suited for the dataset. However, it should be noted that this score is not yet externally validated.
Several limitations of this study need to be addressed. The size of the study population limited the amount of analyses we could perform. As a result of the stratification we performed, some outcomes lost analytic power. This was specially the case for the lower risk quartiles, in which few events occurred during the study period. Validation of our findings in larger cohorts would aid to support our conclusions. As the current study was conducted in an observational cohort, no conclusions regarding treatment effects could be made. In addition, limited information about microvascular outcomes was available and these could therefore not be addressed in the current study. Our suggestion of a link with frailty could not be supported by our data due to the absence of data on frailty status. As precise information on non-cardiovascular causes of death was lacking, we could not evaluate whether the relation between HbA1c and all-cause mortality might still be powered by presumed diabetes-related non-cardiovascular cause of death like cirrhosis associated with non-alcoholic fatty liver disease.
Conclusions
In conclusion, in patients with type 2 diabetes and CVD, HbA1c is related to the risk of all-cause mortality, but not to the risk of cardiovascular events. The relation between HbA1c and all-cause mortality in patients with type 2 diabetes and vascular disease is not dependent on baseline vascular risk.
Abbreviations
- CAC:
-
coronary artery calcium
- CVD:
-
cardiovascular disease
- hs-CRP:
-
high-sensitivity C-reactive protein
- MI:
-
myocardial infarction
- SMART:
-
second manifestations of ARTerial disease
- VADT:
-
veterans affairs diabetes trial
References
World Health Organization. Cardiovascular disease 2014. Accessed 2014. http://www.who.int/cardiovascular_diseases/en/.
Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129(3):399–410.
Nichols M, Townsend N, Scarborough P, Rayner M, Leal J, Luengo-Fernandez R, et al. Mortality. European cardiovascular disease statistics. Sophia Antipolis: The European Heart Network, The European Society of Cardiology; 2012. p. 14–34.
Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44(2):S14–21.
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diab Res Clin Pract. 2014;103(2):137–49.
Zhang Y, Hu G, Yuan Z, Chen L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2012;7(8):e42551.
Freemantle N, Danchin N, Calvi-Gries F, Vincent M, Home PD. Relationship of glycaemic control and hypoglycaemic episodes to 4-year cardiovascular outcomes in people with type 2 diabetes starting insulin. Diab Obes Metab. 2016;18(2):152–8.
Cardoso CR, Leite NC, Ferreira MT, Salles GF. Prognostic importance of baseline and serial glycated hemoglobin levels in high-risk patients with type 2 diabetes: the Rio de Janeiro type 2 diabetes cohort study. Acta Diabetol. 2015;52(1):21–9.
Zoungas S, Chalmers J, Ninomiya T, Li Q, Cooper ME, Colagiuri S, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012;55(3):636–43.
Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39.
UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–53.
Zoungas S, de Galan BE, Ninomiya T, Grobbee D, Hamet P, Heller S, et al. Combined effects of routine blood pressure lowering and intensive glucose control on macrovascular and microvascular outcomes in patients with type 2 diabetes: new results from the ADVANCE trial. Diab Care. 2009;32(11):2068–74.
The ACCORD Study Group. Effects of Intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372(23):2197–206.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–89.
The ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364(9):818–28.
Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15):1392–406.
Bejan-Angoulvant T, Cornu C, Archambault P, Tudrej B, Audier P, Brabant Y, et al. Is HbA1c a valid surrogate for macrovascular and microvascular complications in type 2 diabetes? Diab Metabol. 2015;41(3):195–201.
Wang P, Huang R, Lu S, Xia W, Sun H, Sun J, et al. HbA1c below 7 % as the goal of glucose control fails to maximize the cardiovascular benefits: a meta-analysis. Cardiovasc Diabetol. 2015;14:124.
Giorgino F, Leonardini A, Laviola L. Cardiovascular disease and glycemic control in type 2 diabetes: now that the dust is settling from large clinical trials. Ann NY Acad Sci. 2013;1281(1):36–50.
Kranenburg G, van der Graaf Y, van der Leeuw J, Nathoe HM, de Borst GJ, Kappelle LJ, et al. The relation between HbA1c and cardiovascular events in patients with type 2 diabetes with and without vascular disease. Diab Care. 2015;38(10):1930–6.
Dorresteijn JAN, Visseren FLJ, Wassink AMJ, Gondrie MJA, Steyerberg EW, Ridker PM, et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart. 2013;99(12):866–72.
Simons PCG, Algra A, Van de Laak MF, Grobbee DE, Van de Graaf Y, Group SS. Second manifestations of ARTerial disease (SMART) study: rationale and design. Eur J Epidemiol. 1999;15(9):773–81.
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511.
Reaven PD, Moritz TE, Schwenke DC, Anderson RJ, Criqui M, Detrano R, et al. Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes. 2009;58(11):2642–8.
UKPDS Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ Br Med J. 1998;317(7160):703–13.
The ACCORD Study Group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. New Engl J Med. 2010;362(17):1575–85.
Bansal S, Chawla D, Siddarth M, Banerjee BD, Madhu SV, Tripathi AK. A study on serum advanced glycation end products and its association with oxidative stress and paraoxonase activity in type 2 diabetic patients with vascular complications. Clin Biochem. 2013;46(1):109–14.
Mastorikou M, Mackness B, Liu Y, Mackness M. Glycation of paraoxonase-1 inhibits its activity and impairs the ability of high-density lipoprotein to metabolize membrane lipid hydroperoxides. Diabet Med. 2008;25(9):1049–55.
Chen N, Moe S. Arterial calcification in diabetes. Curr Diab Rep. 2003;3(1):28–32.
Blaum CS, Xue QL, Tian J, Semba RD, Fried LP, Walston J. Is hyperglycemia associated with frailty status in older women? J Am Geriatr Soc. 2009;57(5):840–7.
Morley JE, Malmstrom TK, Rodriguez-Mañas L, Sinclair AJ. Frailty, sarcopenia and diabetes. J Am Med Dir Assoc. 2014;15(12):853–9.
Authors’ contributions
SB and JW performed main research and wrote the manuscript. YVG, HN, GJDB, JK and FV reviewed and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We gratefully acknowledge the contribution of the SMART research nurses; R.van Petersen (data-manager); B.G.F. Dinther (vascular manager) and the participants of the SMART Study Group: A. Algra MD,PhD; Y. van der Graaf, MD, Ph.D.; DE Grobbee, MD, Ph.D.; GEHM Rutten, MD, Ph.D., Julius Center for Health Sciences and Primary care; F.L.J.Visseren, MD,PhD, Department of Internal Medicine; GJ de Borst, MD, Ph.D., Department of Vascular Surgery; LJ Kappelle, MD, Ph.D., Department of Neurology; T Leiner, MD, Ph.D., Department of Radiology; PA Doevendans, MD, Ph.D., Department of Cardiology.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All authors gave consent for publication.
Ethics approval and consent to participate
The ethics committee of the University Medical Center Utrecht approved this study, all eligible patients received written and oral information about the study and all included participants gave informed consent.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bots, S.H., van der Graaf, Y., Nathoe, H.M.W. et al. The influence of baseline risk on the relation between HbA1c and risk for new cardiovascular events and mortality in patients with type 2 diabetes and symptomatic cardiovascular disease. Cardiovasc Diabetol 15, 101 (2016). https://doi.org/10.1186/s12933-016-0418-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-016-0418-1