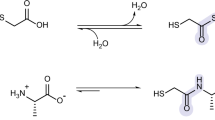
Abstract
Thioamide bonds are important intermediates in prebiotic chemistry. In cyanosulfidic prebiotic chemistry, they serve as crucial intermediates in the pathways that lead to the formation of many important biomolecules (e.g., amino acids). They can also serve as purine and pyrimidine precursors, the two classes of heterocycle employed in genetic molecules. Despite their importance, the formation of thioamide bonds from nitriles under prebiotic conditions has required large excesses of sulfide or compounds with unknown prebiotic sources. Here, we describe the thiol-catalyzed formation of thioamide bonds from nitriles. We show that the formation of the simplest of these compounds, thioformamide, forms readily in spark-discharge experiments from hydrogen cyanide, sulfide, and a methanethiol catalyst, suggesting potential accumulation on early Earth. Lastly, we demonstrate that thioformamide has a Gibbs energy of hydrolysis (\(\Delta G^{\circ }_r\)) comparable to other energy-currencies on early Earth such as pyrophosphate and thioester bonds. Overall, our findings imply that thioamides might have been abundant on early Earth and served a variety of functions during chemical evolution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Thioamide-containing compounds are important intermediates in the formation of biomolecules, such as nucleobases and amino acids, under prebiotic conditions. \(\mathrm {\alpha ,\beta -unsaturated}\) thioamides cyclize and subsequently react with a variety of nucleophiles to generate pyrimidines (e.g., uracil, cytosine, and 4-thiouracil) [1]. It has also been demonstrated that the simplest thioamide-containing molecule, thioformamide, might mediate the formation of purine precursors [2]. In cyanosulfidic prebiotic chemistry, the formation of \(\mathrm {\alpha }\)-hydroxythioamides is integral in the synthesis of amino acids and isoprenoid precursors from cyanohydrins [3].
However, the formation of thioamide bonds from nitriles and sulfide can be challenging. Often times these reactions require large excesses of sulfide (five to ten-fold) or long reaction times [1, 4, 5]. More recent work has demonstrated that thiophosphate (\(\hbox {PSO}_3^{3-}\)) can efficiently facilitate the thiolysis of nitriles to form thioamides [5] with only a two-fold excess of thiophosphate. However, a source of thiophosphates on early Earth remains uncertain. Some recent work has shown that meteoritic delivery of phosphorous-bearing minerals and subsequent photoredox reactions might offer a source [6]. Here, we demonstrate that alkyl-thiols can serve as catalysts for the thiolysis of nitriles to yield thioamides. Thiols are ubiquitous in biochemistry (e.g., cysteine residues in the active sites of many enzymes) and thiol-containing compounds, such as thioesters, are pivotal intermediates in many prebiotic networks [7,8,9,10]. Furthermore, thiols might have been incorporated into biochemistry before phosphate [11, 12].
We sought to test whether thiols can catalyze the thiolysis of nitrile bonds to form thioamides under prebiotic conditions. We began our inquiry by conducting spark discharge experiments and performed subsequent reactions with isolated nitriles to further explore thioamide bond formation. We also used ab initio modeling to characterize the thermochemical properties of the simplest thioamide, thioformamide. The results presented below illustrate the catalytic role thiols might have played in prebiotic chemistry, mirroring the role they play in biochemistry today.
Methods
Unless stated otherwise, all reactions were carried out in an \(\hbox {N}_{2}\)-sparged phosphate-buffer (0.1 M) with an \(\hbox {N}_{2}\)-headspace at room temperature. Degassed HCl or NaOH was used to to adjust the pH.
Spark discharge experiments
Spark discharge experiments were carried out under a mildly reducing atmosphere (\(\hbox {H}_{2}-\hbox {N}_{2}-^{13}\hbox {CO}_{2}\); 0.33 bar each). Gaseous \({}^{13}\hbox {CO}_{2}\) was generated from sodium bicarbonate (\(\hbox {NaH}^{13}\hbox {CO}_{3}\hbox {(s)}\), Cambridge Isotope Laboratories) and degassed hydrochloric acid (4 N; HCl). Excess HCl was added to \(\hbox {NaH}^{13}\hbox {CO}_{3}\) in a 1100-mL round-bottom flask under vacuum. The reaction was allowed to proceed for 30 min at room temperature, after which residual acid and water was removed before proceeding. The same procedure was used to generate a neutral atmosphere (\(\hbox {N}_{2}-^{13}\hbox {CO}_{2}\); 0.5 bar each).
Phosphate buffer (0.2 M; pH 8; VWR) and sodium sulfide nonahydrate (0.1 M; \(\hbox {Na}_{2}\hbox {S}\) \(\cdot\) \(9\hbox {H}_{2}\hbox {O}\); EMD Millipore) were added as the aqueous phase in a 1100-mL round-bottom flask. An Electrotechnics BD50E Tesla coil and two tungsten electrodes were used to generate a spark for 72 h at 42 kV. During this time, the flask was kept in a water bath at \(\mathrm {\sim 5^{\circ } C}\) to maintain a consistent temperature. After 72 h, the aqueous phase was incubated with methanethiol (20 mM; introduced as \(\hbox {CH}_{3}\hbox {SNa}\)) at room temperature for 48 h.
HCN reactions
Reactions with HCN were carried out with isotopically labeled potassium cyanide (20 mM, \(\hbox {K}^{13}\hbox {CN}\); Cambridge Isotope Laboratories) and sodium sulfide (20 mM, \(\hbox {Na}_{2}\hbox {S} \cdot 9 \hbox {H}_{2}\hbox {O}\)). Alkyl thiol was also added as either methanethiol (20 mM, \(\hbox {CH}_{3}\hbox {SNa}\); Sigma Aldrich) or ethanethiol (50 mM, \(\hbox {CH}_{3}\hbox {CH}_{2}\hbox {SH}\); Sigma Aldrich).Footnote 1 Each reactant was placed into a serum vial and dissolved in degassed phosphate buffer (0.1 M) and under an \(\hbox {N}_{2}\) headspace.
Reactions with a headspace containing \(\hbox {H}_{2}\hbox {S}\) (g) were carried out in a manner similar to those above, with equimolar amounts of \(\hbox {K}^{13}\hbox {CN}\) and \(\hbox {CH}_{3}\hbox {SNa}\) (20 mM). Gaseous \(\hbox {H}_{2}\hbox {S}\) was generated by placing \(\hbox {Na}_{2}\)S ⋅ 9 \(\hbox {H}_{2}\)O (s) under vacuum and adding degassed 4 N HCl. This reaction was allowed to proceed for 24 h at room temperature. After 24 h, 7.5 mL of \(\hbox {H}_{2}\)S (g) (1.5 bar) was added to a reaction of \(\hbox {K}^{13}\)CN and \(\hbox {CH}_3\)SNa in degassed phosphate buffer with an \(\hbox {N}_{2}\)-headspace.
Non-alkyl thiols were screened for their ability to catalyze the formation of thioformamide. Reactions containing cysteamine (2-aminoethanethiol hydrochloride) and N-acetyl cysteamine were carried out in the same manner as the thioformamide-forming reactions above.
To confirm the identity of thioformamide, reactions were also carried out to form thioformamide from HCN and sodium thiophosphate (\({\hbox {Na}_{2}\hbox {PO}_{3}S}\); Sigma-Aldrich) following the methods of Ritson et al. [5].
Further reactions were conducted to determine if compounds other than HCN present in the spark discharge mixture could serve as precursors to thioformamide. Reactions with formamide (20 mM, \(\hbox {HCONH}_{2}\), Acros Organics) and sodium thiocyanate (20 mM, NaSCN, Acros Organics) were carried out in a manner similar to those described above, “HCN reactions” replacing \(\hbox {K}^{13}\)CN with either formamide or sodium thiocyanate. To determine whether or not alkyl thiols were serving as catalysts themselves via the mechanism proposed below or just acting as an acid, we reacted imidazole (20 mM, Thermo Scientific) with \(\hbox {K}^{13}\)CN and \(\hbox {Na}_{2}\)S \(\cdot\) 9\(\hbox {H}_{2}\)O and did not detect the production of thioformamide.
3-cyanopyridine reactions
Thionicotinamide-forming reactions were carried out with 3-cyanopyridine (25 mM, Sigma-Aldrich), sodium sulfide (50 mM), and methanethiol (50 mM).
Nuclear magnetic resonance spectroscopy
Samples were prepared for nuclear magnetic resonance spectroscopy (NMR) by adding 0.1 mL of deuterium oxide (\(\hbox {D}_2\)O, 99.8 atom \(\%\) D; Acros Organics) to 0.5 mL of the reaction mixture. NMR spectra were collected on either a Bruker Avance-III-HD-500 or a Bruker NEO-400 spectrometer. Spectra for \({}^{1}\)H (500 MHz, 400 MHz) were collected using solvent suppression (zgesgp). Proton-decoupled spectra for \({}^{13}\)C (125 MHz, 100 MHz) were collected using the pulse program zgpg30. Yield determinations for HCN reactions were carried out by increasing the recycle delay (\(\mathrm {d_1\,=\,60\,s}\); zgig). The optimal recycle delay was determined using inversion recovery experiments. Spectra were also collected for Distortionless Enhancement by Polorization Transfer (DEPT 135; deptsp135) and J modulation (jmod) in initial experiments when determining the structure of thioformamide. All spectra were processed using the MestReNova software suite [13].
Thermodynamics and kinetics
Density functional theory calculations
To compute the thermochemical properties of the compounds involved in the hydrolysis of thioformamide, we employed Gaussian 16 [14] to perform ab initio density functional theory calculations. B3LYP level of theory (Becke-style 3-parameter density functional theory with the Lee-Yang-Parr correlation functional) was used with the basis set 6-311++g(3df,3pd), which have been previously used to study thioformamide [15]. Solvation effects were taken into account with a self-consistent reaction field (SCRF) and a polarizable continuum model (with integral equation formalism; IEFPCM).
Standard state enthalpy (\(\Delta H_r^{\circ }\)) and Gibbs energy (\(\Delta G_r^{\circ }\)) of reaction were calculated from thermochemical output using the equations below [16]:
where \(\varepsilon _0\) is the total electronic energy of a compound and \(H_{corr}\) and \(G_{corr}\) are the thermal corrections to enthalpy and free energies, respectively.
Kinetic rate constants
Kinetic rate constants for the formation of thioformamide were determined from reaction yields from quantitative \(\mathrm {^{13}C}\) NMR. The formation of thioformamide was assumed to follow a second-order rate law and the kinetic rate constant (k) was determined accordingly. Since the concentration of HCN was equal to the concentration of \(\hbox {H}_{2}\hbox {S}\),
The hydrolysis of thioformamide was determined in previous work to have a half-life (\(t_{1/2}\)) of 3 days [2]. Taken to be a pseudo-first order reaction (where the rate of reaction is only dependent on the concentration of thioformamide) the kinetic rate constant can be calculated from:
If \([thio] = \frac{[thio]_0}{2}\) at \(t = t_{1/2}\)
Results
The results of the reactions described below are described in Table 1.
Thioformamide-forming reactions
Spark-discharge experiments
Carbon-13 NMR spectra for the mildly reducing (\(\hbox {H}_{2}-\hbox {N}_{2}-{}^{13}\hbox {CO}_{2}\)) spark discharge mixture indicates that a variety of organics were produced in these experiments (Fig. 1). When this solution was incubated with methanethiol (as described in Sect. "Spark discharge experiments"), a peak corresponding to a carbonyl carbon was detected (\(\mathrm {\delta ^{13}C = 193.6\, ppm}\)). Spectra collected using the DEPT135 and JMOD pulse programs indicated that this corresponded to a carbon with one proton attached. A lower diversity of organic compounds was detected in the neutral spark discharge mixture but after incubation with methanethiol, the same peak (\(\mathrm {\delta ^{13}C = 193.6 \, ppm}\)) was again detected Figs. 2, 3 with \(\mathrm {^{13}C}\) NMR.
Carbon-13 NMR spectra for reducing spark discharge experiment (\({\hbox {H}_{2}-\hbox {N}_{2}-^{13}\hbox {CO}_{2}}\)). Spectrum A shows the spark discharge mixture. Spectrum B shows the spark discharge mixture after 48 h of incubation with methanethiol at room temperature. Identifiable carbon compounds are labeled; the omitted region did not contain peaks. Peaks further downfield represent carbon nuclei with a poorer electron density (e.g. carbonyl and nitrile carbons), while those further upfield correspond to carbon nuclei with a greater electron density (e.g. alkyl and alkenyl carbons). The peak corresponding to the carbonyl carbon of thioformamide is highlighted in blue
Carbon-13 NMR spectra for neutral spark discharge experiment (\({\hbox {N}_{2}-^{13}\hbox {CO}_{2}}\)). Spectrum A shows the spark discharge mixture. Spectrum B shows the spark discharge mixture after 48 h of incubation with methanethiol. Identifiable carbon compounds are labeled; the omitted region did not contain peaks. Peaks further downfield represent carbon nuclei with a poorer electron density (e.g. carbonyl and nitrile carbons), while those further upfield correspond to carbon nuclei with a greater electron density (e.g. alkyl and alkenyl carbons). The peak corresponding to the carbonyl carbon of thioformamide is highlighted in blue
Isolated HCN reactions
We tried several reactions to reproduce the compound detected in the spark discharge incubations. Reactions of cyanide, methanethiol, and sulfide produced thioformamide at 4\(^\circ\)C and room temperature, as well as at \(\mathrm {pH \sim 7}\) and \(\mathrm {pH \sim 11}\). Quantitative \(\mathrm {^{13}C}\) NMR indicated a \(\mathrm {\sim 33 \%}\) yield of thioformamide from HCN at \(\mathrm {pH \sim 7}\). We determined that the phosphate buffer was not having any influence on the reaction by conducting the same reaction in carbonate buffer as well as in water. Both of these reactions produced thioformamide. (see Supplemental data). Reactions of formamide and sodium thiocyanate in place of cyanide did not produce any detectable thioformamide (see Supplemental Data).
Carbon-13 NMR spectra for reactions of cyanide and sulfide with methane- and ethanethiol. Spectrum A shows the methanethiol-catalyzed formation of thioformamide at pH 7; spectrum B corresponds to this reaction at pH 11. Spectrum C shows the ethanethiol-catalyzed formation of thioformamide at pH 7; spectrum D shows the reaction at pH 11. The peak corresponding to the carbonyl carbon of thioformamide is highlighted in blue
We reacted hydrogen cyanide with sodium thiophosphate (a known way to produce thioformamide [5]) to confirm the production of thioformamide. Carbon-13 NMR spectra from this reaction showed an identical chemical shift (\(\mathrm {\delta ^{13}C = 193.6 \, ppm}\)), confirming the production of thioformamide in our experiments (Fig. 4, Supplementary Data).
Notably, reactions of cyanide and sulfide alone did not lead to the production of thioformamide indicating that methanethiol is acting as a catalyst in the formation of thioformamide. Indeed, reactions of cyanide and sulfide with ethanethiol also lead to the production of thioformamide, signifying that these alkyl thiols are likely acting to catalyze the formation of thioamide bonds in the presence of sulfide.
Additionally, a few reactions reactions where non-alkyl thiols were used in place of methane- or ethanethiol failed to produce thioformamide. These reactions seem to have preferentially formed other products instead of thioformamide. For example, cysteamine, a thiol-containing precursor to coenzyme A [17] reacted with cyanide to form a thiazole (a nitrogen- and sulfur-containing five-membered heterocycle).Footnote 2. Reactions using cysteine, a thiol-containing \(\alpha\)-amino acid, also did not catalyze the formation of thioformamide. We suspect this is due to the formation of cyanohydrins, a favorable product of the reaction of amino acids and cyanide.
Thionicotinamide-forming reactions
To test whether thiols can catalyze the formation of other thioamides, we examined the reaction of 3-cyanopyridine and sulfide with methanethiol as a catalyst. This reaction resulted in the near complete conversion of 3-cyanopyridine to thionicotinamide at \(\mathrm {pH \sim 7}\) (Fig. 5). In these experiments, visual precipitation of thionicotinamide was observed in the reaction vial owing to the poor solubility of thionicotinamide relative to 3-cyanopyridine in water at 25\(^\circ\)C (\(\mathrm {0.02 ~ g \,L^{-1}}\) compared to \(\mathrm {140 ~g\, L^{-1}}\) for thionicotinamide and 3-cyanopyridine, respectively). This reaction did not produce thionicotinamide at higher pH values. Reactions of 3-cyanopyridine with sulfide in the absence of a thiol catalyst still produced thionicotinamide, but not in the amounts produced in the presence of a thiol catalyst.
Carbon-13 NMR spectrum for the formation of thionicotinamide from 3-cyanopyridine and sulfide with a methanethiol catalyst. Reactions were carried out for 72 h at room temperature. Peaks corresponding to the carbons on the pyridine ring are labeled in both (A and B). Unlabelled peaks in spectrum B correspond to carbons on the 3-cyanopyridine ring. In the presence of the thiol-catalyst, a near complete conversion of 3-cyanopyridine was observed (spectrum A). In the absence of the catalyst, some conversion is still observed, but not to the same extent (spectrum B)
Proposed mechanism
We propose that the formation of thioamide bonds from nitriles proceeds through two subsequent nucleophilic substitution reactions (Fig. 6). The catalytic thiol initially attacks the nitrile carbon (reducing the triple bond) to form a thioimidate intermediate. Bisulfide then attacks the carbonyl carbon of the thioimidate to yield a tetrahedral intermediate. This intermediate collapses to reform the C=N double bond as the thiol catalyst leaves to form the thiolimine which subsequently tautomerizes to form the more thermodynamically stable thioformamide [15].
Alternatively, the thiol might be protonating the nitrile nitrogen, making it more electrophilic and thus susceptible to nucleophilic attack by sulfide. To examine this possibility, we reacted cyanide and sulfide with imidazole, which could act as an acid (pKa \(\sim\) 7). We did not observe the production of thioformamide in these reactions and conclude that the mechanism above is a likely explanation for the thiol-catalyzed formation of thioamide bonds.
Thermodynamic and kinetic results
Density functional theory calculations and Gibbs energy of reaction
Thioformamide can potentially hydrolyze to form either formamide or thioformic acid. To study the energetics of these reactions, we performed density functional theory (DFT) calculations in Gaussian. We used the thermochemical output to calculate the enthalpy of reaction (\(\Delta H_r^{\circ }\)) and Gibbs energy of reaction (\(\Delta G^{\circ }_r\)) for these reactions. For the formation of thioformic acid, we calculated \(\Delta H_r^{\circ } = \mathrm {27.728 ~ kJ \, mol^{-1}}\) and \(\Delta G_r^{\circ } = \mathrm {23.593 ~ kJ \, mol^{-1}}\). The hydrolysis of thioformamide to yield formamide has an enthalpy of reaction of \(\Delta H_r^{\circ } = \mathrm {-25.0158 ~ kJ \, mol^{-1}}\) and a Gibbs energy of reaction of \(\Delta G_r^{\circ } = \mathrm {-27.350 ~ kJ \, mol^{-1}}\). Thus, under standard state conditions, the hydrolysis of thioformamide to formamide is significantly more favorable than the hydrolysis to thioformic acid (Fig. 7).
Next, we calculated a Gibbs energy of reaction (\(\Delta G_r\)) for varying concentrations of thioformamide and formamide under plausible prebiotic conditions. Previous estimates of formamide steady-state concentration depend largely on the pH and temperature, and range from \(\mathrm {\sim 10^{-14} - 10^{-10}}\) M (pH 4 to pH 10 at 50\(^\circ\)C), assuming the main source of formamide is the hydrolysis of HCN [19]. While there is not enough information to estimate a steady-state concentration of thioformamide (Sect. "Kinetic rate constants"), we demonstrate that the hydrolysis of thioformamide proceeds spontaneously under a wide range of formamide and thioformamide concentrations (Fig. 8).
Gibbs energy of reaction (\(\Delta G_r\)) for the hydrolysis of thioformamide to yield formamide. Here, a value of \(\mathrm {10^{-7}}\) M is used as an estimate for the \(\hbox {H}_{2}\hbox {S}\) concentration on early abiotic Earth under a weakly reducing atmosphere [20]. Steady-state concentrations of formamide are a function of temperature and pH; values here represent a temperature of 50\(^\circ\)C and a pH range of 4 to 10 [19]
Kinetic rate constants
We initially suspected the rate of thioformamide formation to be quite high given that product was observed in experiments after just one hour at room temperature (data not shown). We used quantitative \(\mathrm {^{13}C}\) NMR and Eq. 3 to confirm our observations and determined the kinetic rate constant for the thiol-catalyzed formation of thioformamide to be \(k_f \approx \mathrm {5.8 \times 10^{-4} ~ L \, mol^{-1} \, s^{-1}} (\mathrm {at ~ 25^{\circ } C})\). We compared this rate of formation to the rate of hydrolysis measured in previous studies. The kinetic rate constant for the hydrolysis of thioformamide to formamide is \(k_{h} \, = \,{\text{2}}{\text{.7 }} \times \,10^{{ - 6\,}} \,{\text{s}}^{{ - 1}}\) (at 25◦C) [2]. Given that , kf >> kh it seems likely that, absent a significant sink, thioformamide would accumulate in a prebiotic environment. While this neglects any utility thioformamide might have in prebiotic reactions (see below), it illustrates that thioformamide could have accumulated in potentially quite large quantities on early Earth.
Discussion
The experiments we have conducted show that alkyl thiols can catalyze the formation of thioamide bonds from nitriles and sulfide under prebiotic conditions. Furthermore, the simplest thioamide molecule, thioformamide, could accumulate under prebiotic conditions. The hydrolysis of thioformamide to formamide yields free energy in quantities comparable other prebiotic energy carriers.
Thioamides in prebiotic chemistry
Thioamides, especially \(\alpha\)-hydroxythioamides, have established importance in prebiotic chemistry, functioning as amino acid precursors in prominent theories of prebiotic metabolism [3]. However, much remains to be explored with regards to thioformamide’s possible roles in prebiotic chemistry. Thioformamide has been shown to readily convert aminomalononitrile (a cyanide trimer) into isochyrsean (a thiazole: a sulfur-containing heterocycle) [2]. It also plays a role in the conversion of diaminomalononitrile (a cyanide tetramer) into 4-aminoimidazole-5-carboxamide (AICA), an adenine precursor [2]. It is also possible that thioformamide stored chemical energy on a prebiotic Earth. The modeled Gibbs energy of hydrolysis for thioformamide to yield formamide (\(\Delta G_r^{\circ } \mathrm {\approx -27 ~ kJ \, mol^{-1}}\)) is comparable to the standard state values for thioester bond hydrolysis (\(\Delta G_r^{\circ } \mathrm {\approx -31 ~ kJ \, mol^{-1}}\) for acetyl-CoA), pyrophosphate bond hydrolysis (\(\Delta G_r^{\circ } \mathrm {\approx -19 ~ kJ \, mol^{-1}}\)), and ATP hydrolysis to ADP and orthophosphate (\(\Delta G_r^{\circ } \mathrm {\approx -31 ~ kJ \, mol^{-1}}\)) [21, 22]. It is possible that the free energy afforded by the hydrolysis of thioformamide could have fueled some of the earliest proto-biochemical or biochemical reactions on Earth. It is clear that a mechanism for energy transfer (e.g., phosphorylation, thioesterification) would need to exist for this energy to be harnessed, and such a mechanism remains unknown. Thioformamide might have the potential to serve as a condensing agent for processes like amino acid polymerization via N-terminal activation in a mechanism similar to other sulfur-containing compounds (e.g., \(\hbox {CS}_{2}\) and COS) [23]. Preliminary experiments testing this hypothesis have yet produce detectable amino acid oligomers or activated intermediates similar to N-carboxyanhydrides or thiono-oxazolidones.
Thiols and nitriles on prebiotic Earth
The novel route for thioamide formation presented above obviously requires the presence of simple alkyl thiols. On Earth today, it seems that most alkyl thiols, at least in hydrothermal settings, are produced from the thermal degradation of microbial biomass [24]. There have only been a few studies on the formation of thiols under prebiotic conditions. In spark discharge experiments containing \(\hbox {CH}_{4}\) and \(\hbox {H}_{2}\hbox {S}\), methane- and ethanethiol (as well n-propanethiol and 2-propanethiol) were produced in relatively modest amounts (<1% yield) [25]. Heinen and Lauwer further demonstrated that alkyl thiols (C1-C5) can be readily produced from \(\hbox {H}_{2}\hbox {S}\), \(\hbox {H}_2\), and \(\hbox {CO}_{2}\) via a carbonyl sulfide intermediate [26, 27]. Non-alkyl thiols such as the \({\alpha }\)-amino acid cysteine and other thiols (e.g., cysteamine) are produced in spark discharge experiments in the presence of gaseous \(\hbox {H}_{2}\)S [28]. Cysteine may have also been formed from Michael addition of \(\hbox {H}_{2}\hbox {S}\) to dehydroalanine [29], though the stability of free of dehydroaminoacids is questionable, as they convert rapidly to compounds like pyruvate. While we were not able to successfully form thioamide bonds using non-alkyl thiol catalysts (e.g. cysteine), we suspect that this is due to the formation of cyanohydrins, for example, from HCN and cysteine.
Despite the uncertainty in how simple alkyl thiols were synthesized on early Earth, they likely played a pivotal role in prebiotic chemistry by forming high-energy intermediates in many reaction pathways [7, 30, 31]. Thiol-based energy currencies are readily formed under prebiotic conditions [7, 32] and are thought to pre-date phosphate-based energy currencies [11, 33]. The work presented here demonstrates yet another role that thiols might have played on early Earth.
HCN is an important building block in many prebiotic chemical scenarios. Foundational experiments in prebiotic chemistry showed that HCN produced in spark discharge reacts with aldehydes and ketones to yield an \({\alpha }\)-aminonitriles which subsequently form amino acids [34,35,36]. Later work demonstrated that HCN polymerizes at higher concentrations (\(\mathrm {>\sim 10 \, mM}\)) to form the pentamer adenine, a nucelobase employed in RNA, DNA, and coenzymes such as coenzyme A [37, 38]. The stable tetramer of HCN, diaminomalononitrile can also serve as a precursor to other purines as well (e.g. gaunine and xanthine) [38].
HCN is also the fulcrum in the modern theory of cynaosulfidic protometabolism- e.g. [3, 39]. In this scheme, HCN, \(\hbox {H}_{2}\hbox {S}\), Cu ions, and phosphate serve as the feedstock molecules that result in the production of an array of vital biomolecules [3]. Numerous additional studies have refined and expanded the original theory [40,41,42,43], but HCN remains pivotal to this scenario of prebiotic metabolism.
HCN can be produced in several ways and was likely abundant on early Earth. Photolysis of methane produces methyl (\(\hbox {CH}_{3}\)) or methylene (\(\hbox {CH}_2\)) radicals, which can recombine with atomic nitrogen (N, itself a product atmospheric chemistry) to form HCN [44]. Before the origin of life on Earth (and thus a biological source of methane), photochemical production rates of HCN from methane photolysis are estimated to have been \(\sim 10^7 \; \mathrm {cm^{-2}\; s^{-1}}\) [45].
HCN is also produced from electrical discharges (e.g. spark and corona) under simulated prebiotic atmospheres. Using corona discharge, Raulin et al demonstrated that HCN is produced in the presence of \(\hbox {CH}_{4}\) and either \(\hbox {N}_{2}\) or \(\hbox {NH}_{3}\) [25]. Combinations of \(\hbox {H}_{2}\), \(\hbox {N}_{2}\), and \(\hbox {CH}_{4}\), CO, or \(\hbox {CO}_{2}\) were all shown to produce HCN under spark discharge conditions [25]. HCN can also be produced from spark discharge in neutral atmospheres (\(\hbox {N}_{2}\), \(\hbox {CO}_{2}\), and \(\hbox {H}_{2}\)O) [46]. Electrical discharge on CO, NH\({_3}\), and water have also recently been shown to yield HCN [47]. Many of these experiments rely on gas mixtures that are more reducing than some recent estimates of the composition of the early atmosphere, which is currently thought to have been mildly reducing or neutral [48]. While HCN production under neutral conditions is certainly possible, as shown here and in previous studies [46], it might not have been enough to reach millimolar levels required in some prebiotic chemical schemes.
Exogenous sources of HCN could have also bolstered prebiotic inventories on early Earth. It has long been acknowledged that meteorites might have played an important role in delivering HCN, as well as other compounds of prebiotic importance, to an early Earth [37, 49]. HCN has also been detected in several meteorites ranging in abundance from 50 to 2500 \(\mathrm {nmol \, g^{-1}}\) [50]. Additionally, spectroscopic measurements have detected abundant HCN (1 % relative to water) in dozens of comets [51]. Other recent work has shown that the atmospheric reentry of impact ejecta could have been a significant source of HCN, even in an oxidized atmosphere [52].
Regardless of the source, HCN might have accumulated in local environments on early Earth such as lakes [53] or evaporative basins or in global oceans. This HCN feedstock could have provided abundant starting material for building molecules of prebiotic relevance [36, 37, 39]. HCN can add to other compounds to create longer nitriles and plays an important role in several proposed prebiotic reaction networks [3].
In the presence of sulfide and an alkyl thiol catalyst, this exogenously- or atmospherically-derived HCN feedstock would have been readily converted to thioformamide in any low-iron environments, such as surface waters, on early Earth. Given how rapidly the thiol-catalyzed formation of thioformamide proceeds, this process, if nothing else, might been a significant HCN-sink.
Thioamide abundance on early Earth
Given that the rate of formation of thioformamide is several orders of magnitude higher than the rate of hydrolysis [2], it seems likely that thioformamide could have accumulated to significant concentrations on early Earth absent significant sinks. Further roles for this compound in a prebiotic context are not well-established, but given the ease with which thioformamide can be formed from readily available prebiotic building blocks, the utility of the this simple compound merits further investigation Fig. 9.
We demonstrate in this work that thioformamide can be formed from HCN and \(\hbox {H}_{2}\hbox {S}\) with a thiol-catalyst (highlighted in blue). Thioformamide also reacts with the HCN trimer aminomalononitrile to form purine precursors [2]
The thiol-catalyzed formation of thioformamide from hydrogen cyanide represents an early form of proto-biochemical catalysis. Simple alkyl thiols such as methane- and ethanethiol can be produced under plausible early Earth conditions [25,26,27], and could have could have made nitrile-rich feedstocks more available to participate in early reaction networks.
Thiols play a vital role in biochemical reactions, such as activating carboxylic acids towards further carbon-carbon bond forming reactions. Thiol-containing cofactors such as coenzyme A facilitate crucial anabolic reactions in cells (e.g. in the citric acid cycle). Acetyl-CoA is a thiol-containing cofactor employed by many enzymes to activate carboxylic acids towards further reaction. While acetyl-CoA is structurally complex, it was likely preceded by much simpler molecules [54]. Thiol moieties on cysteine resides often activate compounds and catalyze reactions in the active sites of enzymes. An example of this can be found in the nitrilase superfamily of enzymes, which use cysteine in the active site to catalyze the hydrolysis of nitriles to carboxylic acids via a thioimidate intermediate [55].
Overall, our findings demonstrate a novel route for thioamide bond formation on early Earth. Thiols can activate nitriles to yield thioamide bonds in the presence of sulfide. Once formed from abundant nitrile feedstocks, these thioamides could have stored free energy or participated in prebiotic networks to further fuel the formation of other compounds of prebiotic importance.
Availability of data and materials
Data is available online at the Penn State Data Commons (doi:10.26208/HXJK-QF72) and upon request.
Notes
Initial reactions were carried out with a ratio of 2:5:5 HCN:sulfide:thiol, but subsequent reactions were carried out with equimolar amounts of each reactant.
We did not definitively identify the products of these experiments, but the formation of thiazolines from nitriles and cysteamine has been previously characterized [18].
References
Okamura H, Becker S, Tiede N, Wiedemann S, Feldmann J, Carell T (2019) A one-pot, water compatible synthesis of pyrimidine nucleobases under plausible prebiotic conditions. Chem Commun 55(13):1939–1942. https://doi.org/10.1039/c8cc09435g
Sanchez RA, Ferbis JP, Orgel LE (1967) Studies in Prebiotic Synthesis II. Synthesis of purine precursors and amino acids from aqueous hydrogen cyanide. J Mol Biol 30(2):223–253. https://doi.org/10.1016/s0022-2836(67)80037-8
Patel BH, Percivalle C, Ritson DJ, Duffy CD, Sutherland JD (2015) Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat Chem 7(4):301–307. https://doi.org/10.1038/nchem.2202
Tull R, Weinstock LM (1969) A new synthesis of thioformamide. Agnew. Chem. Int Edn 8(4):278–279
Ritson D, Xu J, Sutherland J (2016) Thiophosphate? A varsatile prebiotic reagent? Synlett 28(01):64–67. https://doi.org/10.1055/s-0036-1589414
Ritson DJ, Sutherland JD (2023) Thiophosphate photochemistry enables prebiotic access to sugars and terpenoid precursors. Nat Chem. https://doi.org/10.1038/s41557-023-01251-9
Huber C, Wächtershäuser G (1997) Activated acetic acid by carbon fixation on (Fe, Ni)S under primordial conditions. Science 276(5310):245–247. https://doi.org/10.1126/science.276.5310.245
Wächtershäuser G (1990) Evolution of the first metabolic cycles. Proc Natl Acad Sci 87(1):200–204. https://doi.org/10.1073/pnas.87.1.200
Sanden SA, Yi R, Hara M, McGlynn SE (2020) Simultaneous synthesis of thioesters and iron-sulfur clusters in water: two universal components of energy metabolism. Chem Commun 56(80):11989–11992. https://doi.org/10.1039/d0cc04078a
Weber AL, Orgel LE (1979) The formation of peptides from glycine thioesters. J Mol Evolut 13(3):193–202. https://doi.org/10.1007/bf01739479
Goldford JE, Hartman H, Smith TF, Segré D (2017) Remnants of an ancient metabolism without phosphate. Cell 168(6):1126–11349. https://doi.org/10.1016/j.cell.2017.02.001
Goldford JE, Hartman H, Marsland R, Segré D (2019) Environmental boundary conditions for the origin of life converge to an organo-sulfur metabolism. Nat Ecol Evolut 3(12):1715–1724. https://doi.org/10.1038/s41559-019-1018-8
Willcott MR (2009) MestRe Nova. J Am Chem Soc 131(36):13180–13180. https://doi.org/10.1021/ja906709t
Frisch M, Trucks G, Schlegel HB, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Petersson G, Nakatsuji H et al (2016) Gaussian 16. Gaussian Inc, Wallingford, CT
Bernhardt B, Dressler F, Eckhardt AK, Becker J, Schreiner PR (2021) Characterization of the simplest thiolimine: the higher energy tautomer of thioformamide. Chem Eur J 27(22):6732–6739. https://doi.org/10.1002/chem.202005188
Ochterski JW (2000) Thermochemistry in gaussian. Gaussian Inc 1(1)
Miller SL, Schlesinger G (1993) Prebiotic syntheses of vitamin coenzymes: I. Cysteamine and 2-mercaptoethanesulfonic acid (coenzyme M). J Mol Evolut 36(4):302–307. https://doi.org/10.1007/bf00182177
Trose M, Lazreg F, Lesieur M, Cazin CSJ (2015) A straightforward metal-free synthesis of 2-substituted thiazolines in air. Green Chem 17(5):3090–3092. https://doi.org/10.1039/c5gc00286a
Miyakawa S, James Cleaves H, Miller SL (2002) The cold origin of life: A. implications based on the hydrolytic stabilities of hydrogen cyanide and formamide. Origin Life Evolut Biosphere 32:195–208
Ranjan S, Todd ZR, Sutherland JD, Sasselov DD (2018) Sulfidic anion concentrations on early earth for surficial origins-of-life chemistry. Astrobiology 18(8):1023–1040
Jencks WP (2010) Handbook of biochemistry and molecular biology, 5th edn., pp. 579–584.https://doi.org/10.1201/b10501-64
Boiteau L, Pascal R (2011) Energy Sources, Self-organization, and the origin of life. Origin Life Evolut Biospheres 41(1):23–33. https://doi.org/10.1007/s11084-010-9209-y
Leman L, Orgel L, Ghadiri MR (2004) Carbonyl sulfide-mediated prebiotic formation of peptides. Science 306(5694):283–286
Reeves EP, McDermott JM, Seewald JS (2014) The origin of methanethiol in midocean ridge hydrothermal fluids. Proc Natl Acad Sci 111(15):5474–5479. https://doi.org/10.1073/pnas.1400643111
Raulin F, Toupance G (1975) Formation of prebiochemical compounds in models of the primitive earth’s atmosphere. Origin life 6(1–2):91–97. https://doi.org/10.1007/bf01372393
Heinen W, Lauwers AM (1996) Organic sulfur compounds resulting from the interaction of iron sulfide, hydrogen sulfide and carbon dioxide in an anaerobic aqueous environment. Origin life Evolut Biosphere 26(2):131–150. https://doi.org/10.1007/bf01809852
Heinen W, Lauwers A (1997) The iron-sulfur world and the origins of life: Abiotic thiol synthesis from metallic iron, H2S and C O2;a comparison of the thiol generating FeS/HCI(H2S)/C02-systemand its Fe\(^\circ\)/H2S/C02-counterpart. roceedings. Koninklijke Nederlandse Akademie van Wetenschappen 100:11–25
Parker ET, Cleaves HJ, Callahan MP, Dworkin JP, Glavin DP, Lazcano A, Bada JL (2011) Prebiotic Synthesis of Methionine and Other Sulfur-Containing Organic Compounds on the Primitive Earth: A Contemporary Reassessment Based on an Unpublished 1958 Stanley Miller Experiment. Origins of Life and Evolution of the Biosphere 41(3):201–212. https://doi.org/10.1007/s11084-010-9228-8
Schlesinger G, Miller SL (1983) Prebiotic synthesis in atmospheres containing CH4, CO, and CO2 I. amino acids. J Mol Evolut 19(5):376–382. https://doi.org/10.1007/bf02101642
Varma SJ, Muchowska KB, Chatelain P, Moran J (2018) Native iron reduces CO2 to intermediates and end-products of the acetyl-CoA pathway. Nat Ecol Evolut 2(6):1019–1024. https://doi.org/10.1038/s41559-018-0542-2
Ferry JG, House CH (2006) The stepwise evolution of early life driven by energy conservation. Mol Biol Evolut 23(6):1286–1292. https://doi.org/10.1093/molbev/msk014
Huber C, Wächtershäuser G (1998) Peptides by activation of amino acids with CO on (Ni, Fe)S surfaces: implications for the origin of life. Science 281(5377):670–672. https://doi.org/10.1126/science.281.5377.670
De Duve C (1998) Clues from present-day biology: the thioester world. The molecular origins of life, 219–236
Miller SL (1953) A production of amino acids under possible primitive earth conditions. Science 117(3046):528–529. https://doi.org/10.1126/science.117.3046.528
Miller SL (1955) Production of some organic compounds under possible primitive earth conditions 1. J Am Chem Soc 77(9):2351–2361. https://doi.org/10.1021/ja01614a001
Miller SL (1957) The mechanism of synthesis of amino acids by electric discharges. Biochimica et Biophysica Acta 23(3):480–489. https://doi.org/10.1016/0006-3002(57)90366-9
Oró J (1961) Mechanism of synthesis of adenine from hydrogen cyanide under possible primitive earth conditions. Nature 191(4794):1193–1194. https://doi.org/10.1038/1911193a0
Ferris JP, Hagan WJ (1984) HCN and chemical evolution: the possible role of cyano compounds in prebiotic synthesis. Tetrahedron 40(7):1093–1120. https://doi.org/10.1016/s0040-4020(01)99315-9
Sutherland JD (2017) Opinion: studies on the origin of life - the end of the beginning. Nat Rev Chem 1(2):0012. https://doi.org/10.1038/s41570-016-0012
Liu Z, Wu L-F, Kufner CL, Sasselov DD, Fischer WW, Sutherland JD (2021) Prebiotic photoredox synthesis from carbon dioxide and sulfite. Nat Chem. https://doi.org/10.1038/s41557-021-00789-w
Ritson D, Sutherland JD (2012) Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat Chem 4(11):895–899. https://doi.org/10.1038/nchem.1467
Ritson DJ, Battilocchio C, Ley SV, Sutherland JD (2018) Mimicking the surface and prebiotic chemistry of early earth using flow chemistry. Nat Commun 9(1):1821. https://doi.org/10.1038/s41467-018-04147-2
Xu J, Ritson DJ, Ranjan S, Todd ZR, Sasselov DD, Sutherland JD (2018) Photochemical reductive homologation of hydrogen cyanide using sulfite and ferrocyanide. Chem Commun 54(44):5566–5569. https://doi.org/10.1039/c8cc01499j
Zahnle KJ (1986) Photochemistry of methane and the formation of hydrocyanic acid (HCN) in the earth’s early atmosphere. J Geophys Res Atmos 91(D2):2819–2834. https://doi.org/10.1029/jd091id02p02819
Tian F, Kasting JF, Zahnle K (2011) Revisiting HCN formation in earth’s early atmosphere. Earth Planet Sci Lett 308(3–4):417–423. https://doi.org/10.1016/j.epsl.2011.06.011
Cleaves HJ, Chalmers JH, Lazcano A, Miller SL, Bada JL (2008) A reassessment of prebiotic organic synthesis in neutral planetary atmospheres. Origins Life Evolut Biospheres 38(2):105–115. https://doi.org/10.1007/s11084-007-9120-3
Ferus M, Kubelík P, Knížek A, Pastorek A, Sutherland J, Civiš S (2017) High energy radical chemistry formation of HCN-rich atmospheres on early earth. Sci Rep 7(1):6275. https://doi.org/10.1038/s41598-017-06489-1
Trail D, Watson EB, Tailby ND (2011) The oxidation state of Hadean magmas and implications for early earth’s atmosphere. Nature 480(7375):79–82. https://doi.org/10.1038/nature10655
Chyba CF, Thomas PJ, Brookshaw L, Sagan C (1990) Cometary delivery of organic molecules to the early earth. Science 249(4967):366–373. https://doi.org/10.1126/science.11538074
Smith KE, House CH, Arevalo RD, Dworkin JP, Callahan MP (2019) Organometallic compounds as carriers of extraterrestrial cyanide in primitive meteorites. Nat Commun 10(1):2777. https://doi.org/10.1038/s41467-019-10866-x
Mumma MJ, Charnley SB (2011) The chemical composition of comets-emerging taxonomies and natal heritage. Ann Rev Astron Astrophys 49(1):471–524. https://doi.org/10.1146/annurev-astro-081309-130811
Parkos D, Pikus A, Alexeenko A, Melosh HJ (2018) HCN production via impact ejecta reentry during the late heavy bombardment. J Geophys Res Planets 123(4):892–909. https://doi.org/10.1002/2017je005393
Toner JD, Catling DC (2019) Alkaline lake settings for concentrated prebiotic cyanide and the origin of life. Geochimica et Cosmochimica Acta 260:124–132. https://doi.org/10.1016/j.gca.2019.06.031
Smith H, Hyde AS, Simkus DN, Libby E, Maurer SE, Graham HV, Kempes CP, Lollar BS, Chou L, Ellington AD, Fricke GM, Girguis PR, Grefenstette NM, Pozarycki CI, House CH, Johnson SS (2021) The grayness of the origin of life. Life 11(6):498. https://doi.org/10.3390/life11060498
Gour-Salin BJ, Lachance P, Storer AC (1991) Inhibition of papain by peptide nitriles: conversion of the nitrile group into other functionalities via the papain:nitrile thioimidate ester adduct. Can J Chem 69(8):1288–1297. https://doi.org/10.1139/v91-192
Acknowledgements
The authors would like to thank Zhidan Zhang for her assisstance in the laboratory. They would also like to thank Dr. Jason Boettger for his assistance with Gaussian and Dr. Christy George for her assistance with NMR. This work was funded by NSF grant #EF-1724099 and NASA Pennsylvania Space Grant.
Author information
Authors and Affiliations
Contributions
CHH- Conceptualization, writing, funding acquisition. ASH- conceptualization, experimental design and execution, data analysis, and writing. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no Conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hyde, A.S., House, C.H. Prebiotic thiol-catalyzed thioamide bond formation. Geochem Trans 25, 5 (2024). https://doi.org/10.1186/s12932-024-00088-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12932-024-00088-6