Abstract
Background
COPD is associated with the development of lung cancer. A protective effect of inhaled corticosteroids (ICS) on lung cancer is still controversial. Hence, this study investigated the development of lung cancer according to inhaler prescription and comorbidties in COPD.
Methods
A retrospective cohort study was conducted based on the Korean Health Insurance Review and Assessment Service database. The development of lung cancer was investigated from the index date to December 31, 2020. This cohort included COPD patients (≥ 40 years) with new prescription of inhalers. Patients with a previous history of any cancer during screening period or a switch of inhaler after the index date were excluded.
Results
Of the 63,442 eligible patients, 39,588 patients (62.4%) were in the long-acting muscarinic antagonist (LAMA) and long-acting β2-agonist (LABA) group, 22,718 (35.8%) in the ICS/LABA group, and 1,136 (1.8%) in the LABA group. Multivariate analysis showed no significant difference in the development of lung cancer according to inhaler prescription. Multivariate analysis, adjusted for age, sex, and significant factors in the univariate analysis, demonstrated that diffuse interstitial lung disease (DILD) (HR = 2.68; 95%CI = 1.86–3.85), a higher Charlson Comorbidity Index score (HR = 1.05; 95%CI = 1.01–1.08), and two or more hospitalizations during screening period (HR = 1.19; 95%CI = 1.01–1.39), along with older age and male sex, were independently associated with the development of lung cancer.
Conclusion
Our data suggest that the development of lung cancer is not independently associated with inhaler prescription, but with coexisting DILD, a higher Charlson Comorbidity Index score, and frequent hospitalization.
Similar content being viewed by others
Introduction
Several epidemiologic studies suggest a close association between chronic obstructive pulmonary disease (COPD) and lung cancer [1,2,3,4]. COPD even in never smokers is associated with lung cancer, and the presence of COPD in smokers is associated with a two to six times higher risk for the development of lung cancer [1, 5, 6].
Pathogenic mechanisms for the association between COPD and lung cancer comprise cigarette smoking, the increased expression of growth factors in COPD, chronic inflammation, genetic predisposition, epigenetic mechanism, and premature aging [7, 8]. Furthermore, some comorbidities including diabetes mellitus and tuberculosis in COPD are reported to be risk factors of lung cancer [6, 9].
Recently, pharmacological treatment with inhaled corticosteroids (ICS) was suggested as a strategy to reduce the risk of lung cancer, since chronic inflammation in COPD promotes tumor growth and suppresses antitumor immune responses [10, 11]. Retrospective meta-analyses have shown that ICS lowers the risk of lung cancer in COPD, although the quality of the evidence is low [12, 13]. However, some studies failed to confirm the link between ICS and lung cancer [13, 14]. Time-related biases, including immortal time bias, latency time bias, and protopathic bias, were not fully accounted for in previous studies, leading to conflicting results. Moreover, the effects of other anti-inflammatory therapies including long-acting muscarinic antagonist (LAMA) and long-acting β2-agonist (LABA) therapy on the development of lung cancer in COPD remain to be determined.
We investigated the development of lung cancer in COPD according to inhaler prescription and comorbidities by analyzing the Korean Health Insurance Review and Assessment Service (HIRA) database. The study design used accounted for time-related biases, to provide further information regarding the risks of lung cancer development using a large sample size.
Materials and methods
Study design
This study analyzed the data from the HIRA database from January 1, 2015, to December 31, 2020. The HIRA database contains medical service claims records including all diagnoses and medications from all medical care settings for almost the entire Korean population under mandatory and universal national health insurance.
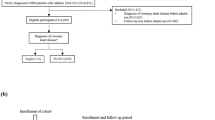
The COPD cohort out of the HIRA database was constructed by the following criteria: (1) patients aged ≥ 40 years, (2) at least three separate outpatient visits, (3) COPD (J43⎼J44 of International Classification of Diseases tenth revision (ICD-10) codes) as the primary diagnosis from January 1, 2015, to December 31, 2020, with the prescription of one of the following respiratory medications ; LAMA, LABA, combination of LAMA/LABA, ICS, combination of ICS/LABA, triple therapy (LAMA + LABA + ICS), phosphodiesterase-4 inhibitors, theophylline, and mucolytics (Fig. 1). Three or more prescriptions of an inhaler during the exposure period were required for being enlisted into each inhaler group. The oral corticosteroid (OCS) usage was identified as the prescription of prednisolone 420 mg (15 mg/day for four weeks) or more for COPD during the exposure period.
Each patient had one-year screening period without any inhaler medication before the index date. The index date was defined as the date of the first prescription for inhaler medication. A latency period before lung cancer diagnosis was set to allow sufficient time for inhaler exposure with regard to cancer development, as in other studies [14, 15] (Fig. 2).
During the screening period, subjects who had any cancer history or who had been prescribed an inhaler were excluded. Patients who had prescription switched between inhaler medications after the index date were also excluded.
This cohort consisted of three groups: 1) LAMA/LABA group as LAMA + LABA or LAMA/LABA fixed-dose combination, 2) ICS/LABA group as ICS + LABA or ICS/LABA fixed-dose combination, 3) LABA group using a LABA inhaler alone. The subjects were monitored for the diagnosis of lung cancer from January 1, 2016 to December 31, 2020 (Fig. 2).
Case identification
Cases of lung cancer (C33–C34) were identified by ICD-10 codes after the initial prescription of inhalers. Comorbidities were also identified based on following ICD-10 codes: asthma (J45-46), hypertension (I10-15), diabetes mellitus (E10–E14), diffuse interstitial lung disease (DILD) (J84), ischemic heart disease (I20–I25), heart failure (I50), cerebrovascular disease (I60–I69), and pulmonary thromboembolism (I26). The event date was the first date of cancer diagnosis based on the above ICD-10 codes. Patients in whom lung cancer was diagnosed in the latency period after the initial prescription were excluded.
Adjustment for covariates
Multivariate model analyses were performed including covariates affecting the risk of cancer development. Adjustment for the severity of COPD using the Charlson Comorbidity Index and the number of emergency room visits and hospitalizations was performed. The multivariate analyses included two models: Model 1 had all covariates and model 2 had covariates including age, sex, and significant factors in the univariate analysis.
Statistical analysis
Baseline characteristics and the prescription of medications were summarized by descriptive statistics including mean, standard deviation, and proportion. A chi-squared test was used for categorical variables, and a one-way analysis of variance (ANOVA) was used for continuous variables. The prevalence of lung cancer among the three groups according to inhaler therapy was tested by a chi-squared test and adjusted by a Bonferroni correction for multiple comparisons. Incidence rate of lung cancer per 10,000 person-years were computed with 95% confidence intervals (CIs) and compared with the Poisson regression analysis. The proportional hazard assumption was analyzed using Schoenfeld residuals for the Cox proportional hazards regression model. Univariate and multivariate Cox proportional hazards regression analyses were used to identify significant risk factors predicting the development of lung cancer.
Sensitivity analyses conducted by setting latency periods of 6 months, 12 months, and 24 months were performed to determine the effect of protopathic bias. When calculating the cancer risk, the inhaler medication used during the latency period was not considered. Hazard ratio (HR) with 95% CI was assessed for the risk of lung cancer. The analysis was performed only on cases with complete data. A threshold of p < 0.05 was deemed significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Ethics statement
The present study was approved by the Institutional Review Board of Ajou University Hospital (AJOUIRB-EXP-2021-582). The requirement for informed consent from the patients studied was waived by the ethical review board.
Results
Baseline characteristics
This cohort comprised 63,442 patients with a mean age of 69.1 years (75.7% male; Table 1). A total of 39,588 patients (62.4%) were categorized in the LAMA/LABA group, 22,718 (35.8%) in the ICS/LABA group, and 1,136 (1.8%) in the LABA group (Table 1). The mean age of the ICS/LABA group was younger, and the proportion of women in the ICS/LABA group was higher (p < 0.001) (Table 1). Among the comorbidities during the screening period, asthma was significantly more co-existent in the ICS/LABA group. Diabetes, DILD, ischemic heart disease, heart failure, and cerebrovascular diseases were more frequently observed in the LAMA/LABA group (p < 0.001) (Table 1). Accordingly, the Charlson Comorbidity Index score was higher in the LAMA/LABA group (p < 0.001) (Table 1).
The LAMA/LABA group and ICS/LABA group had more frequent hospitalizations than the LABA group, along with a higher rate of hospitalization for respiratory disease in the ICS/LABA group, and a higher rate of hospitalization for cardiovascular disease in the LAMA/LABA group (p < 0.001) (Table 1).
Medications
During the exposure period, xanthine and mucolytics (54.8% and 75.0%, respectively) were dominantly prescribed in this cohort, whereas only 1.59% of the patients were prescribed roflumilast (Table 2). The OCS prescription for COPD was highest in the ICS/LABA group among the three groups (p < 0.001) (Table 2).
Prevalence and incidence of lung cancer
Among the three groups, the ICS/LABA group had the lowest five-year prevalence of lung cancer (p = 0.031) (Table 3). The incidence rate of lung cancer per 10,000 person-years was lower in the ICS/LABA group compared to the LAMA/LABA group and the LABA group (p < 0.001) (Table 3).
Risk factors for the development of lung cancer
In multivariate model 1 adjusting for all the covariates, the risk of lung cancer was not statistically different in the LAMA/LABA group (Hazard ratio HR = 0.92; 95% CI = 0.67–1.28) and the ICS/LABA group (HR = 0.90; 95% CI = 0.65–1.26) compared to the LABA group (Table 4). Similar results were obtained in sensitivity analyses with 6-month, 12-month, and 24-month latency periods (Table 5). Furthermore, an effect modification analysis was undertaken to ascertain potential disparities in the effects of significant risk factors identified through multivariate analysis. Nevertheless, no statistically significant interaction was observed (Supplement Table 1). In multivariate model 2 adjusted for age, sex, and significant factors in the univariate analysis, independent associations with the development of lung cancer were observed for DILD (HR, 2.68; 95% CI, 1.86–3.85), a higher Charlson Comorbidity Index score (HR, 1.05; 95% CI, 1.01–1.08), and two or more hospitalizations during screening period (HR, 1.19; 95% CI, 1.01–1.39), along with male sex and older age (Table 4).
Discussion
Our study was performed to identify the risk of lung cancer associated with inhaler prescription and comorbidities in COPD. The study was designed to minimize time-related biases. Although the incidence of lung cancer was lower in the ICS/LABA group, multivariate analyses showed that the development of lung cancer was not associated with inhaler therapy but with DILD, a higher Charlson Comorbidity Index score, and two or more hospitalizations during screening period.
Our study used the HIRA database to analyze the development of lung cancer in COPD using a design based on the new initiation of LAMA/LABA, ICS/LABA, and LABA. Our multivariate analyses showed no significant difference in the development of lung cancer according to inhaler prescription. Several studies in COPD patients reported an association between ICS and a lower incidence of lung cancer [15,16,17,18,19], possibly attributed to a preventative role against lung cancer through anti-inflammatory effects [20]. Furthermore, Parimon et al. reported a dose-dependent reduced risk of lung cancer associated with ICS [16]. A recent study using a population-based cohort of COPD suggested that ICS usage was associated with a 30% decrease in the risk of lung cancer and a 43% reduction of lung cancer per gram of ICS use [15].
In contrast, some studies found no association between ICS therapy and lung cancer risk, compatible with our results [21,22,23]. A recent large cohort study reported no reduction of lung cancer incidence associated with ICS use in COPD patients [14]. There was no relationship between the duration and dosage of ICS therapy and the risk of lung cancer [14]. The authors pointed out that time-related biases, including immortal time bias, latency time bias and protopathic bias, and the inclusion of asthmatics may have influenced the studies previously reporting that ICS was associated with a reduced incidence of lung cancer [14].
The current analysis attempted to overcome the methodological problems of previous studies [24,25,26]. In the current study, the date of the first drug administration was established as the index date for all patients to avoid immortal time bias. A substantial observation period is necessary to assess the development of cancer resulting from medication exposure, because an error can occur in the evaluation of drug-related cancer if the elapsing time after the initial drug exposure is relatively short. Therefore, our study established a latency period of one year before a lung cancer diagnosis for each patient to exclude cancer diagnosis within a short time after the first prescription, to minimize latency time bias as in other studies [14, 15]. To minimize protopathic bias, this study had one-year washout period before the start of an inhaler medication along with a latency period before lung cancer diagnosis.
This study found that the development of lung cancer in COPD was independently associated with a higher Charlson Comorbidity Index and two or more hospitalizations during screening period. Several studies have reported that a high Charlson Comorbidity Index score is an appropriate prognosticator in lung cancer, because of this index’s association with worse survival [27, 28]. However, the explanation for the causal link between Charlson Comorbidity Index and the risk for the development of lung cancer remains unclear.
This study assessed the association between the risk of lung cancer and the severity of COPD by various approaches including a Charlson Comorbidity Index and the number of emergency room visits and hospitalizations. Previous studies reported that emphysema and severe airflow obstruction increased the risk of lung cancer, irrespective of smoking exposure [5, 29, 30]. Frequent hospitalization is also a marker for the severity of COPD [31]. Therefore, our finding that frequent hospitalization was independently associated with the development of lung cancer can be explained by the link between the severity of COPD and frequent hospitalization.
This study found that the development of lung cancer in COPD was independently associated with the presence of DILD. Idiopathic pulmonary fibrosis is an independent risk factor for lung cancer, beyond the effect of smoking [32]. A recent meta-analysis reported the prevalence of lung cancer was 13.74% and incidence rate was 2.07 per 100 person-years in idiopathic pulmonary fibrosis [32]. An even higher prevalence of lung cancer is reported in combined pulmonary fibrosis and emphysema [33]. One study reported that abnormal CT findings of ILD including low attenuation area, fibrosis, and ground glass attenuation and spirometric parameter of FEV1/FVC < 70% suggestive of COPD were risk factors for lung cancer, even after adjusting for age, sex, and smoking status [34].
Lung tumorigenesis and fibrosis share common environmental risk factors (i.e., smoking, occupational and environmental exposures) and biological pathways including chronic inflammation, senescence, genetic susceptibility, and epithelial-mesenchymal transition [35, 36]. However, since our finding on the contribution of coexisting DILD to the development of lung cancer in a large COPD cohort has not been previously reported, further investigation is required.
This study has several limitations. First, this was not a prospective study, although the observational design reflects real world clinical practice. Second, because pulmonary function data were not available in the HIRA database, the diagnosis of COPD was based on ICD-10 codes and prescription profiles. Accordingly, the impact of airflow obstruction was not assessed. Third, despite our efforts to exclude asthma as the primary diagnosis, the cohort may still have included patients with asthma, and a lower incidence of lung cancer in asthma may be a potential confounder. Fourth, smoking status, family history of cancer, and the pathologic type of each cancer were not included in the analyses due to lack of information. Fifth, medication adherence was not measured. Sixth, air pollution and socioeconomic factors, such as occupation, were not included in our analysis. Seventh, one of the limitations is the relatively short length of follow-up for identifying a significant effect of inhaler therapy.
Conclusion
This observational study suggests that coexisting DILD, a higher Charlson Comorbidity Index score, and frequent hospitalization are independently associated with the development of lung cancer, whereas ICS therapy is not protective.
Availability of data and materials
HIRA is an open and public data to which any researcher can get access through the website (https://www.hira.or.kr).
Abbreviations
- ANOVA:
-
One-way analysis of variance
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- DILD:
-
Diffuse interstitial lung disease
- HIRA:
-
Health Insurance Review and Assessment Service
- HR:
-
Hazard ratio
- ICS:
-
Inhaled corticosteroids
- LABA:
-
Long-acting beta-2 agonist
- LAMA:
-
Long-acting muscarinic antagonist
- OCS:
-
Oral corticosteroid
References
Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34:380–6.
Young RP, Duan F, Chiles C, Hopkins RJ, Gamble GD, Greco EM, Gatsonis C, Aberle D. Airflow Limitation and Histology Shift in the National Lung Screening Trial. The NLST-ACRIN cohort Substudy. Am J Respir Crit Care Med. 2015;192:1060–7.
Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005;60:570–5.
Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, Sciurba FC. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–44.
Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data from the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med. 2003;163:1475–80.
Ahn SV, Lee E, Park B, Jung JH, Park JE, Sheen SS, Park KJ, Hwang SC, Park JB, Park HS, Park JH. Cancer development in patients with COPD: a retrospective analysis of the National Health Insurance Service-National Sample Cohort in Korea. BMC Pulm Med. 2020;20:170.
Barnes PJ, Adcock IM. Chronic obstructive pulmonary disease and lung cancer: a lethal association. Am J Respir Crit Care Med. 2011;184:866–7.
Adcock IM, Caramori G, Barnes PJ. Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respiration. 2011;81:265–84.
Park HY, Kang D, Shin SH, Choi H, Jang SH, Lee CH, Kim H, Kwon OJ, Rhee CK, Cho J. Pulmonary tuberculosis and the incidence of Lung Cancer among patients with chronic obstructive Pulmonary Disease. Ann Am Thorac Soc. 2022;19:640–8.
King PT. Inflammation in chronic obstructive pulmonary disease and its role in cardiovascular disease and lung cancer. Clin Transl Med. 2015;4:68.
Lee G, Walser TC, Dubinett SM. Chronic inflammation, chronic obstructive pulmonary disease, and lung cancer. Curr Opin Pulm Med. 2009;15:303–7.
Lee YM, Kim SJ, Lee JH, Ha E. Inhaled corticosteroids in COPD and the risk of lung cancer. Int J Cancer. 2018;143:2311–8.
Pitre T, Kiflen M, Ho T, Seijo LM, Zeraatkar D, de Torres JP. Inhaled corticosteroids, COPD, and the incidence of lung cancer: a systematic review and dose response meta-analysis. BMC Pulm Med. 2022;22:275.
Suissa S, Dell’Aniello S, Gonzalez AV, Ernst P. Inhaled corticosteroid use and the incidence of lung cancer in COPD. Eur Respir J. 2020;55:1901720.
Raymakers AJN, Sadatsafavi M, Sin DD, FitzGerald JM, Marra CA, Lynd LD. Inhaled corticosteroids and the risk of lung cancer in COPD: a population-based cohort study. Eur Respir J. 2019;53:1801257.
Parimon T, Chien JW, Bryson CL, McDonell MB, Udris EM, Au DH. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:712–9.
Liu SF, Kuo HC, Lin MC, Ho SC, Tu ML, Chen YM, Chen YC, Fang WF, Wang CC, Liu GH. Inhaled corticosteroids have a protective effect against lung cancer in female patients with chronic obstructive pulmonary disease: a nationwide population-based cohort study. Oncotarget. 2017;8:29711–21.
Kiri VA, Fabbri LM, Davis KJ, Soriano JB. Inhaled corticosteroids and risk of lung cancer among COPD patients who quit smoking. Respir Med. 2009;103:85–90.
Lee CH, Hyun MK, Jang EJ, Lee NR, Kim K, Yim JJ. Inhaled corticosteroid use and risks of lung cancer and laryngeal cancer. Respir Med. 2013;107:1222–33.
Barnes PJ. Inhaled corticosteroids. Pharmaceuticals (Basel). 2010;3:514–40.
Jian ZH, Huang JY, Lin FC, Nfor ON, Jhang KM, Ku WY, Ho CC, Lung CC, Pan HH, Liang YC, et al. The use of corticosteroids in patients with COPD or asthma does not decrease lung squamous cell carcinoma. BMC Pulm Med. 2015;15:154.
Kok VC, Horng JT, Huang HK, Chao TM, Hong YF. Regular inhaled corticosteroids in adult-onset asthma and the risk for future cancer: a population-based cohort study with proper person-time analysis. Ther Clin Risk Manag. 2015;11:489–99.
Sørli K, Thorvaldsen SM, Hatlen P. Use of Inhaled corticosteroids and the risk of Lung Cancer, the HUNT study. Lung. 2018;196:179–84.
Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16:241–9.
Rivera DR, McGlynn KA, Freedman AN. Connections between pharmacoepidemiology and cancer biology: designing biologically relevant studies of cancer outcomes. Ann Epidemiol. 2016;26:741–5.
Horwitz RI, Feinstein AR. The problem of protopathic bias in case-control studies. Am J Med. 1980;68:255–8.
Yang CC, Fong Y, Lin LC, Que J, Ting WC, Chang CL, Wu HM, Ho CH, Wang JJ, Huang CI. The age-adjusted Charlson comorbidity index is a better predictor of survival in operated lung cancer patients than the Charlson and Elixhauser comorbidity indices. Eur J Cardiothorac Surg. 2018;53:235–40.
Marcus MW, Chen Y, Duffy SW, Field JK. Impact of comorbidity on lung cancer mortality - a report from the Liverpool Lung Project. Oncol Lett. 2015;9:1902–6.
Maldonado F, Bartholmai BJ, Swensen SJ, Midthun DE, Decker PA, Jett JR. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest. 2010;138:1295–302.
Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med. 2007;176:285–90.
Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–92.
Brown SW, Dobelle M, Padilla M, Agovino M, Wisnivesky JP, Hashim D, Boffetta P. Idiopathic pulmonary fibrosis and Lung Cancer. A systematic review and Meta-analysis. Ann Am Thorac Soc. 2019;16:1041–51.
Kitaguchi Y, Fujimoto K, Hanaoka M, Kawakami S, Honda T, Kubo K. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology. 2010;15:265–71.
Mizuno S, Takiguchi Y, Fujikawa A, Motoori K, Tada Y, Kurosu K, Sekine Y, Yanagawa N, Hiroshima K, Muraoka K, et al. Chronic obstructive pulmonary disease and interstitial lung disease in patients with lung cancer. Respirology. 2009;14:377–83.
Kinoshita T, Goto T. Molecular mechanisms of Pulmonary Fibrogenesis and its progression to Lung Cancer: a review. Int J Mol Sci. 2019;20:1461.
Tzouvelekis A, Gomatou G, Bouros E, Trigidou R, Tzilas V, Bouros D. Common pathogenic mechanisms between idiopathic pulmonary fibrosis and Lung Cancer. Chest. 2019;156:383–91.
Acknowledgements
Dr. Eun Kyung Kim passed away while dedicating herself to the care of the patients before the final publication. We appreciate her contribution and offer our condolences.
Data sharing statement
HIRA is an open and public data to which any researcher can get access through the website (https://www.hira.or.kr).
Funding
This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR16C0001).
Dave Singh is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC).
Author information
Authors and Affiliations
Contributions
Ji Eun Park, Eunyoung Lee, Dave Singh, and Eun Kyung Kim helped the preparation of this manuscript and equally contributed to this paper as a first author. Joo Hun Park coordinated and designed this study, helped the preparation of this manuscript, and is responsible for the integrity of this paper as a corresponding author. Eunyoung Lee and Bumhee Park contributed to the analysis of our data. Dave Singh and Eun Kyung Kim contributed to the design of this study and critically reviewed this study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Ajou University Hospital (AJOUIRB-EXP-2021-582) and was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Park, J.E., Lee, E., Singh, D. et al. The effect of inhaler prescription on the development of lung cancer in COPD: a nationwide population-based study. Respir Res 25, 229 (2024). https://doi.org/10.1186/s12931-024-02838-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-024-02838-7