Abstract
Background
Endobronchial ultrasound (EBUS) and cone-beam computed tomography-derived augmented fluoroscopy (CBCT-AF) are utilized for the diagnosis of peripheral pulmonary lesions (PPLs). Combining them with transbronchial cryobiopsy (TBC) can provide sufficient tissue for genetic analysis. However, cryoprobes of different sizes have varying degrees of flexibility, which can affect their ability to access the target bronchus and potentially impact the accuracy. The aim of this study was to compare the diagnostic efficacy of cryoprobes of varying sizes in CBCT-AF and EBUS for the diagnosis of PPLs.
Methods
Patients who underwent endobronchial ultrasound-guided transbronchial biopsy (EBUS-TBB) and TBC combined with CBCT-AF for PPLs diagnosis between January 2021 and May 2022 were included. Propensity score matching and competing-risks regression were utilized for data analysis. Primary outcome was the diagnostic accuracy of TBC.
Results
A total of 284 patients underwent TBC, with 172 using a 1.7-mm cryoprobe (1.7 group) and 112 using a 1.1-mm cryoprobe (1.1 group). Finally, we included 99 paired patients following propensity score matching. The diagnostic accuracy of TBC was higher in the 1.1 group (80.8% vs. 69.7%, P = 0.050), with a similar rate of complications. Subgroup analysis also revealed that the 1.1 group had better accuracy when PPLs were located in the upper lobe (85.2% vs. 66.1%, P = 0.020), when PPLs were smaller than 20 mm (78.8% vs. 48.8%, P = 0.008), and when intra-procedural CBCT was needed to be used (79.5% vs. 42.3%, P = 0.001). TBC obtained larger specimens than TBB in both groups. There is still a trend of larger sample size obtained in the 1.7 group, but there is no statistically different between our two study groups (40.8 mm2 vs. 22.0 mm2, P = 0.283).
Conclusions
The combination of TBC with CBCT-AF and EBUS is effective in diagnosing PPLs, and a thin cryoprobe is preferred when the PPLs located in difficult areas.
Similar content being viewed by others
Introduction
Accurate diagnosis of peripheral pulmonary lesions (PPLs) is an essential step in developing an appropriate treatment plan. Endobronchial ultrasound-guided transbronchial biopsy (EBUS-TBB) is widely used for the diagnosis of PPLs nowadays due to its long history of safety [1,2,3]. Cone-beam computed tomography-derived augmented fluoroscopy (CBCT-AF) is a technique that provides real-time 2-dimensional (2D) fluoroscopy and 3-dimensional (3D) CBCT scans, and has also been used in bronchoscopy procedures [4,5,6,7]. CBCT-AF can guide navigation for the bronchial route, and confirm the location of target PPLs, thereby improving diagnostic accuracy during EBUS-TBB [8, 9]. Traditionally, most TBB procedures employed standard biopsy forceps, which frequently obtained small, crushed tissue samples that could affect molecular analysis for further cancer management [10,11,12]. Therefore, a better biopsy device to collect larger and higher quality histologic specimens is required.
Cryobiopsy is performed using compressed gas, to create a cooling (Joule-Thomson) effect. This freezes the surrounding tissue, allowing for the extraction of a larger tissue specimen while preserving its internal structure. Transbronchial cryobiopsy (TBC) has become a commonly used method for diagnosing endobronchial tumors and interstitial pulmonary diseases [13,14,15,16,17], and has recently emerged as a diagnostic method for PPLs [18,19,20]. TBC provides sufficient tissue for gene analysis and histologic subtyping, which helps the physician determine further cancer treatment [11, 21]. Nevertheless, a usual-sized cryoprobe (≥ 1.7 mm) is often cumbersome to use and inflexible, making it difficult to advance to the target bronchus.
The thin cryoprobe (1.1 mm) is more flexible and appears to be easier to operate. However, few studies have reported on the differences between cryoprobes [22, 23]. Furthermore, there is limited evidence regarding the usefulness of CBCT-AF in guiding TBC for the diagnosis of PPLs. Therefore, the aim of this study was to investigate the effectiveness and safety of different-sized cryoprobes in CBCT-AF and EBUS for the diagnosis of PPLs.
Methods
Participants
This was a retrospective study of patients who underwent EBUS-TBB and TBC combined with CBCT-AF for the diagnosis of PPLs at the Department of Chest Medicine, National Taiwan University Cancer Center, from January 2021 to May 2022. Patient data, including age, gender, and final diagnosis were collected. To fully characterize the bronchoscopy procedure, the following data were also recorded: the indication (initial diagnosis or re-biopsy), lesion size, lesion pattern (solid or part-solid/ground glass opacity (GGO)), distance from the costal pleura, bronchus sign, location: upper lobes (right upper lobe, and left upper division) or non-upper lobes, visibility on radiograph, position of the EBUS probe (within, adjacent to and invisible the PPLs determined by EBUS image), procedure time, procedure-related major adverse events, radiation dose, intra-procedural CBCT use, TBC failure, and size of the cryoprobe.
Written informed consent was obtained from each patient prior to bronchoscopy. The study received approval from the Institutional Review Board (IRB #202305118RINA) of the National Taiwan University Cancer Center.
Procedures
All bronchoscopy procedures were performed by our experienced pulmonologists, each with more than eight years of experience in performing bronchoscopy procedures. These procedures were conducted in a hybrid bronchoscopy room equipped with a C-arm CBCT angiography system (Artis Zee Ceiling; Siemens Healthcare GmbH, Forchheim, Germany). The CBCT-AF image was created by the annotation software (syngo iGuide Toolbox; Siemens Healthcare GmbH, Forchheim, Germany) to highlight the area of the target lesion and target bronchus before the bronchoscopy exam.
The patients then underwent conscious sedation with intravenous midazolam, propofol, and fentanyl. A flexible bronchoscope (BF-Q290 or BF-P290; Olympus Co., Tokyo, Japan) was inserted through a supraglottic airway (i-gel®; Intersurgical Ltd, Berkshire, UK), and a 20 MHz radial-EBUS probe (UM-S20-17 S; Olympus Co., Tokyo, Japan) was inserted into the suspected target bronchus, guided by the CT image and navigated via the CBCT-AF image. Intra-procedural CBCT would be performed to confirm EBUS probe within the target lesion if EBUS was unable to identify it due to pure GGO, or atelectasis was suspected. Intra-procedural CBCT would not be performed during the TBB and TBC. After confirming the location of the PPLs, at least six forceps biopsies were performed.
Subsequently, TBC was performed using a flexible cryoprobe (Erbe Elektromedizin GmbH, Tübingen, Germany). Due to the licensing available at our institution, we routinely used a 1.7-mm cryoprobe before August 2021. In September 2021, we started to use a 1.1-mm cryoprobe. The freeze activation time was 3–4 s for the 1.7-mm cryoprobe and 8–10 s for the 1.1-mm cryoprobe. The cryoprobes, which had the frozen tissue specimen at their tip, were removed from the airway along with the bronchoscope as a single unit. The frozen specimen was submerged in saline to rapidly thaw and release it. The bronchoscope was re-inserted into the airway to stop the wound from oozing. The occlusion balloon was not utilized for bleeding prevention. At least two specimens were retrieved via TBC. The severity of bleeding was classified according to the “Delphi Consensus Statement” as follows [24]: Grade 1, which requires suctioning or wedging for ≤ 1 min; Grade 2, suctioning for > 1 min, re-wedging, instillation of cold saline, or vasoactive substances; Grade 3, selective intubation or occlusion balloon for ≤ 20 min; Grade 4, selective intubation for > 20 min, transfer to the intensive care unit (ICU), blood transfusion, or resuscitation.
Rapid on-site cytologic evaluation (ROSE) using a rapid method (Hemacolor; Merck KGaA, Darmstadt, Germany) was always available to confirm lesion access during TBB and TBC. We defined a positive ROSE result as malignant cells on the slide. The lack of malignant cells on the slide revealed a negative ROSE result. We compared ROSE results to the formal pathologic reports on the biopsy specimen. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of ROSE were calculated via standard definitions. All histological specimens obtained through TBB and TBC were finally placed individually in 10% formalin for histological evaluation. The area of the histologic specimen was measured using a digital camera attached to a microscope. Tissue culture and irrigated washing fluid were re-taken for microbiological analysis. The CBCT scan was repeated after completing the bronchoscopy exam to detect any possible pneumothorax. Bronchial brushing, transbronchial needle aspiration, and guide sheath were not used during the procedure.
Outcomes
The histopathologic diagnostic accuracy was the primary outcome, and we defined the diagnostic accuracy as “correctly biopsy-proved class/total testing class”. The diagnostic criteria for PPLs were established based on cytopathological evidence, microbiological analyses, or clinical follow-up. Histological diagnoses that were inconclusive, such as nonspecific fibrosis, chronic inflammation, or atypia, were deemed non-diagnostic. “Suspicious” findings were also considered as negative in our analysis. Benign inflammations, which could not be determined through cytopathological or microbiological means, were confirmed through radiological and clinical follow-up at least one year after bronchoscopy.
We also evaluated the TBC failure population. The failure of TBC occurred when either we attempted TBC but couldn’t extract a histological specimen, or we didn’t perform TBC because the cryoprobe couldn’t reach the target lesions. To access the possible reasons for the failure of TBC, we also recorded the following data: the location of the lesions (upper lobe position and non-upper lobe position), and the lesions with thick texture after cancer treatment.
Statistical analysis
We calculated the propensity score using logistic regression. The regression model included indication, location, lesion size, pleural distance, visibility on the radiograph, lesion appearance, probe position, and malignant diagnosis, to reduce the impact of confounding bias. We paired each patient from the 1.7-mm cryoprobe group (1.7 group) with a patient from the 1.1-mm cryoprobe group (1.1 group). To achieve matching, we utilized a caliper width 0.2 times the standard deviation of the propensity score without replacement [25]. After creating the propensity-matched cohort, we conducted an intention-to-treat analysis of the data and calculated the point estimates and 95% confidence intervals (95% CI) of the treatment effect.
After matching, we conducted comparisons using Student’s t test or one-way analysis of variance (ANOVA) for continuous variables, and the Chi-squared test or Fisher’s exact test for categorical variables. A significance level of 0.05 was used to determine statistical significance. We utilized SPSS version 22.0 (IBM, SPSS, Chicago, IL) to conduct statistical analysis.
Results
Patients
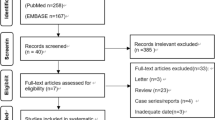
In total, 305 consecutive patients who underwent CBCT-AF and EBUS-TBB for the diagnosis of PPLs were included in our study. Initially, 21 patients were excluded from the study. There were 284 patients, with 172 in the 1.7 group and 112 in the 1.1 group, before propensity score matching. We eventually obtained 99 pairs of patients in both groups. The process for selecting study subjects is outlined in Fig. 1. There were no significant differences in their baseline characteristics or procedure-related complications after matching (Table 1). The final diagnoses for both groups are presented in Table 2.
Accuracy between different sized cryoprobes
In terms of the overall diagnostic accuracy of TBC, the 1.1-mm cryoprobe was found to be more accurate than the 1.7-mm cryoprobe (80.8% vs. 69.7%, P = 0.050). In the subgroup analysis, the 1.1-mm cryoprobe demonstrated higher accuracy when used with PPLs located in the upper lobe (85.2% vs. 66.1%, P = 0.020). If the PPLs were smaller than 20 mm, the 1.1-mm cryoprobe had a higher diagnostic accuracy (78.8% vs. 48.8%, P = 0.008), and still provided a benefit when the lesion was diagnosed as malignant (90.2% vs. 75%, P = 0.010). In addition, the 1.1 group retained a better accuracy when the PPLs were needed to confirm its location under intra-procedural CBCT (42.3% vs. 76.2%, P = 0.020) (Table 3).
In the 1.7 group, the accuracy of TBC also showed a statistically significant difference between lesion sizes (85.7% for sizes > 20 mm vs. 48.8% for sizes ≤ 20 mm, P < 0.001) and bronchus sign subgroups (76.1% for presence of a bronchus sign vs. 53.6% for absence of a bronchus sign, P = 0.028). Lower accuracy of TBC happened when intra-procedural CBCT needed to be used (42.3% vs. 79.5%, P = 0.001). Diagnosis of malignant PPLs had a higher accuracy than that of benign PPLs in both 1.1 and 1.7 subgroups.
Subgroup analysis of the TBC failure population
More patients had TBC failure in the 1.7 group than in the 1.1 group (20/99, 20.2% vs. 3/99, 3.0%, P < 0.001). There was a statistically significant difference between the two groups only in the upper lobe location of the PPLs (15.2% vs. 0%, P < 0.001). The detail reasons for TBC failure in the 1.7 group and 1.1 group were showed in Table 4.
After excluding those in the study population who experienced TBC failure, the histologic specimen in both groups was found to be significantly larger with TBC than with TBB (40.8 mm2 vs. 7.8 mm2, P < 0.001 in the 1.7 group; 22.0 mm2 vs. 5.3 mm2, P < 0.001 in the 1.1 group). The mean sample area of the specimen via TBC was not significantly different between the 1.7 and 1.1 groups (40.8 mm2 vs. 22.0 mm2, P = 0.283), nor did the diagnostic accuracy of TBC and TBB show a significant difference between the two groups (Table 5). The ROSE results in both 1.1 and 1.7 groups (175 patients) were showed in Table 6. The sensitivity, specificity, PPV, NPV and diagnostic accuracy of ROSE were 94.9, 89.7, 97.0, 83.3 and 93.7%, respectively.
Discussion
This retrospective study found that TBC guided by CBCT-AF and EBUS had comparable diagnostic accuracy to TBB, with larger tissue specimens. When comparing cryoprobes of different sizes, the use of a 1.1-mm cryoprobe resulted in better diagnostic accuracy than a 1.7-mm cryoprobe, particularly for PPLs located in the upper lobe, small lesion sizes, and intra-procedural CBCT was needed to be used. The size of the tissue specimens did not show a statistically significant difference between the 1.7 and 1.1 groups.
To achieve a consensus on PPLs diagnosis, at least three major steps: navigation, confirmation, and acquisition, are required to implement the transbronchial procedure [4, 5]. In this study, we utilized CBCT-AF for precise and nearly real-time navigation, radial-EBUS to confirm target lesions, and ROSE to immediately affirm the adequacy of the diagnostic samples acquired. Diagnostic accuracy with TBB was 78.5% in the 1.7 group and 80.2% in the 1.1 group. This confirms our previous results that using CBCT-AF, ROSE, and EBUS simultaneously has a relatively high accuracy for TBB diagnosis [9]. There was no statistically significant difference in accuracy between TBC and TBB in both groups after excluding TBC failure population. In addition, the tissue specimen was significantly larger with TBC than with TBB in both of our study groups. Previous literature has reported that tissue specimens obtained via cryobiopsy are significantly larger than those obtained via forceps biopsy, resulting in a higher detection rate for gene analysis [11]. Therefore, the combination of TBC with EBUS and CBCT-AF is an effective method for diagnosing PPLs.
The acquisition of larger specimens can lead to greater destruction of lung parenchyma, thereby increasing the risk of bleeding and pneumothorax [14, 26]. The cryoprobe has to be removed together with the endoscope during TBC procedure. This necessitates re-insertion of the bronchoscope might also raise the risk of severe bleeding. In the present study, the rate of severe bleeding (grade 3 and grade 4) was approximately 2% in both the 1.7 and 1.1 groups. One patient in each group experienced grade 4 bleeding, requiring transfer to the ICU for monitoring. Two patients in the 1.7 group suffered from mild pneumothorax and did not require further drainage. Severe bleeding and pneumothorax were uncommon in our study population, and this may be attributed to the routine combination of radial-EBUS and CBCT-AF in all procedures. Radial-EBUS can detect vessels within PPLs, thereby decreasing the risk of severe bleeding by avoiding injury to large vessels [27, 28]. CBCT-AF can provide real-time imaging and help prevent pleural injury [5, 9]. Previous literature has also reported that severe bleeding and pneumothorax rarely occurred after TBC [20, 29]. Though serious complication causing by TBC still require vigilance, the incidence could be minimized and more controllable when CBCT-AF and radial-EBUS are used together.
However, performing TBC in strongly bent bronchi, such as the upper lobe bronchus or distal small airway, is challenging due to the rigidity of usual-sized cryoprobes [30]. This can lead to difficulties in accurately targeting lesions that are located in the upper lobe, close to the pleura, non-solid pattern, small size, negative bronchus sign, or invisible in radiographs. Intra-procedural CBCT might also be required to confirm the location of the target PPLs in these difficultly diagnosed conditions. As a result, cryoprobe may have difficulty approaching the lesion, leading to lower accuracy rates [31,32,33,34]. In the 1.7 group, smaller lesion sizes, the absence of bronchus signs, and intra-procedural CBCT used were associated with lower TBC accuracy rates. Nineteen patients (19.2%) could not undergo TBC due to cryoprobe failure upon arrival at the PPLs. The majority of these patients (15/19, 78.9%) had upper lobe locations. There was also a trend of decreasing accuracy in TBC diagnosis when PPLs were located in the upper lobe, had pleural distension shorter than 10 mm, had non-solid appearance, or were invisible in radiographs. Furthermore, four cases experienced scope injury with working channel leakage before propensity score matching, and two cases after propensity score matching following the procedures. All of the injuries occurred due to a reluctance to advance to the upper lobe PPLs, even though TBC was successfully performed. These situations indicated that a cryoprobe of usual size is too bulky to access PPLs in difficult areas.
The thin cryoprobe has a small outer diameter and is easily bendable, making it easier to expand to a farther range than usual-sized cryoprobes [29]. As a result, the failure rate of the cryoprobe reaching the target PPLs was significantly lower in the 1.1 group than in the 1.7 group (1.0% vs. 19.2%, P < 0.001). The thin cryoprobe demonstrated higher diagnostic accuracy not only in the overall population but also in PPLs with small lesion size, upper lobe location, and intra-procedural CBCT used. Although there was no statistical significance, there was still a trend towards increased accuracy in TBC when using a 1.1-mm cryoprobe with shorter pleural distension, non-solid patterns and absence of bronchus sign. In addition, the diagnostic accuracy of the factors, which we mentioned above were no significant differences in the 1.1 group. This result is similar to Kim’s report [35]. No episode of scope injury was also observed in the 1.1 group. Using a thin cryoprobe appears to make TBC easier to perform.
A thin cryoprobe would obtain a smaller sample than a standard-sized cryoprobe during an endobronchial biopsy. To compensate the situation, we used longer freeze activation time in the 1.1 group than in the 1.7 group. There is still a trend of larger sample size obtained in 1.7 group, but there is no statistically different between our two study groups (40.8 mm2 vs. 22.0 mm2, P = 0.283). In addition, the tissue specimen was significantly larger with TBC than with TBB in both of our study groups. We believed the use of a thin cryoprobe with appropriate freeze time appears to be effective in obtaining sufficient tissue during TBC procedures.
Apart from the failure of the cryoprobe to arrive during the lesion approach, another reason for failure in our study was the presence of PPLs with a thick texture. Tumor fibrosis may occur after cancer treatment [36, 37], resulting in a thicker texture that makes it more difficult to extract tissue specimens during re-biopsy. It is more difficult to extract large specimens via TBC than small specimens via TBB because more strength is needed to break through the surrounding fibrotic tissue. In our study, one patient in the 1.7 group and two patients in the 1.1 group were unable to undergo the procedure due to lesions with thick textures. However, all of these patients were successfully diagnosed through forceps biopsy. We also found that the accuracy of TBB remained acceptable in the TBC failure population, with a success rate of 75% in the 1.7 group and 100% in the 1.1 group. TBC cannot completely replace TBB in these situations.
Some study revealed that the diagnostic accuracy of re-biopsy would be decreased because of tumor fibrosis and necrosis occurred after cancer treatment [36, 37]. This might increase the heterogenous in the study population; therefore, many studies excluded the re-biopsy population. To minimize the impact, we used indication (initial diagnosis vs. re-biopsy) as one factor for matching and the proportion of re-biopsy population was no different in the two study groups. In addition, the accuracy was also similar between the population for initial diagnosis or for re-biopsy. The reason is that ROSE with high accuracy helps immediate feedback on the quality of the biopsy sample, thereby confirms the adequate biopsy site [3]. We believe our study population is more similar in the real world, and our results can be applied to the clinical practice.
Our study has several limitations. First, this was a retrospective study. Although we used propensity score matching to minimize the bias, we could not account for the potential confounding factors. Positive findings that are actually meaningful could also have been missed. It is also difficult for us to standardize the protocol of TBC procedure in all our study population. Some factors, such as the use of different types of bronchoscopes, and the use of intra-procedural CBCT might also influence the diagnostic accuracy of TBC. However, we found that there was no statistic difference after the propensity score matching. We believe the effect might be minimized in this situation and we can still apply our results in the clinical practice. Second, this was a single-center study, and our study population was relatively homogeneous. More than 80% of the study population received a final diagnosis of malignancy. We know that the accuracy of diagnosing malignant PPLs is actually higher than that of benign processes [38]. However, there is a risk that this may not be generalizable to patients in other institutions. Therefore, a prospective randomized study with a comprehensive study group is warranted.
Third, the introduction of the 1.1-mm cryoprobe in our institution came later than the introduction of the 1.7-mm cryoprobe. It is inappropriate for us to ignore the possibility that the technical familiarity with the use of the 1.1-mm cryoprobe might boost the diagnostic performance of the 1.7-mm cryoprobe. In addition, the diagnostic accuracy of TBC in Tanaka’s study was relatively high, and all 1.7-mm cryoprobe could be advanced to the PPLs located upper lobe [39]. All operators in the study were experts with at least 100 cases of experience performing cryobiopsy using conventional cryoprobes (1.9-mm cryoprobe). We believe the experience of the operator might also influence the success rate of TBC. Forth, all patients underwent TBB followed by TBC in a single procedure; therefore, it was difficult to distinguish between the complications of TBB and those of TBC. In addition, one study supposed that initial TBB might alter the surrounding environments of PLLs, such as the formation of organized blood clots or an edematous change of the target bronchus, thus reducing the accuracy of TBC [40]. Though the situation was not reported in other studies, the possible impact remained to be cautious. Unlike other studies, we did not exclude individuals who attempted TBC but ultimately failed. Although this may decrease the diagnostic accuracy, we can still observe the challenges of TBC in the real world.
In conclusion, combining TBC with CBCT-AF and EBUS is an effective method for diagnosing PPLs, though severe complication is cautionary but controllable. This approach provides valid accuracy, and allows for obtaining larger tissue specimens. The thin cryoprobe is more flexible, which improves the accuracy of diagnosing PPLs with small lesion sizes and difficult locations. The histologic sample size obtained through a thin cryoprobe is not inferior to that obtained through a usual-sized cryoprobe. We believe that the combination of CBCT-AF and EBUS, along with TBC using a thin cryoprobe, will be valuable tools in the era of gene-guided therapy. However, conventional biopsy devices cannot be abandoned when TBC fails to accomplish its purpose.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- 2D:
-
2-dimensional
- 3D:
-
3-dimensional
- CBCT-AF:
-
cone-beam computed tomography-derived augmented fluoroscopy
- CT:
-
computed tomography
- EBUS:
-
endobronchial ultrasound
- EBUS-TBB:
-
endobronchial ultrasound-guided transbronchial biopsy
- GGO:
-
ground glass opacity
- ICU:
-
intensive care unit
- NPV:
-
negative predictive value
- PPLs:
-
peripheral pulmonary lesions
- PPV:
-
positive predictive value
- ROSE:
-
rapid on-site cytologic evaluation
- TBB:
-
transbronchial biopsy
- TBC:
-
transbronchial cryobiopsy
References
Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J. 2002;20:972–4.
Yamada N, Yamazaki K, Kurimoto N, Asahina H, Kikuchi E, Shinagawa N, Oizumi S, Nishimura M. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest. 2007;132:603–8.
Lin CK, Jan IS, Yu KL, Chang LY, Fan HJ, Wen YF, Ho CC. Rapid on-site cytologic evaluation by pulmonologist improved diagnostic accuracy of endobronchial ultrasound-guided transbronchial biopsy. J Formos Med Assoc. 2020;119:1684–92.
Casal RF. Cone beam CT-guided bronchoscopy: Here to stay? J Bronchol Interv Pulmonol. 2018;25:255–6.
Pritchett MA, Schampaert S. Tipping point: cone beam CT with augmented fluoroscopy for the biopsy and treatment of peripheral nodules. J Bronchol Interv Pulmonol. 2019;26:e13–5.
Verhoeven RLJ, Fütterer JJ, Hoefsloot W, van der Heijden EHFM. Cone-beam CT image guidance with and without electromagnetic navigation bronchoscopy for biopsy of peripheral pulmonary lesions. J Bronchol Interv Pulmonol. 2021;28:60–9.
Casal RF, Sarkiss M, Jones AK, Stewart J, Tam A, Grosu HB, Ost DE, Jimenez CA, Eapen GA. Cone beam computed tomography-guided thin/ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study. J Thorac Dis. 2018;10:6950–9.
Yu KL, Yang SM, Ko HJ, Tsai HY, Ko JC, Lin CK, Ho CC, Shih JY. Efficacy and safety of bone-beam computed tomography-derived augmented fluoroscopy combined with endobronchial ultrasound in peripheral pulmonary lesions. Respiration. 2021;100:538–46.
Lin CK, Fan HJ, Yao ZH, Lin YT, Wen YF, Wu SG, Ho CC. Cone-beam computed tomography-derived augmented fluoroscopy improves the diagnostic yield of endobronchial ultrasound-guided transbronchial biopsy for peripheral pulmonary lesions. Diagnostics (Basel). 2021;12:41.
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, Ishikawa Y, Wistuba I, Flieder DB, Franklin W, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med. 2013;137:668–84.
Arimura K, Kondo M, Nagashima Y, Kanzaki M, Kobayashi F, Takeyama K, Tamaoki J, Tagaya E. Comparison of tumor cell numbers and 22C3 PD-L1 expression between cryobiopsy and transbronchial biopsy with endobronchial ultrasonography-guide sheath for lung cancer. Respir Res. 2019;20:185.
Udagawa H, Kirita K, Naito T, Nomura S, Ishibashi M, Matsuzawa R, Hisakane K, Usui Y, Matsumoto S, Yoh K, et al. Feasibility and utility of transbronchial cryobiopsy in precision medicine for lung cancer: prospective single-arm study. Cancer Sci. 2020;111:2488–98.
Schumann C, Hetzel J, Babiak AJ, Merk T, Wibmer T, Möller P, Lepper PM, Hetzel M. Cryoprobe biopsy increases the diagnostic yield in endobronchial tumor lesions. J Thorac Cardiovasc Surg. 2010;140:417–21.
Ganganah O, Guo SL, Chiniah M, Li YS. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta-analysis. Respirology. 2016;21:834–41.
Maldonado F, Danoff SK, Wells AU, Colby TV, Ryu JH, Liberman M, Wahidi MM, Frazer L, Hetzel J, Rickman OB, et al. Transbronchial cryobiopsy for the diagnosis of interstitial lung diseases: CHEST guideline and expert panel report. Chest. 2020;157:1030–42.
Babiak A, Hetzel J, Krishna G, Fritz P, Moeller P, Balli T, Hetzel M. Transbronchial cryobiopsy: a new tool for lung biopsies. Respiration. 2009;78:203–8.
Griff S, Ammenwerth W, Schönfeld N, Bauer TT, Mairinger T, Blum TG, Kollmeier J, Grüning W. Morphometrical analysis of transbronchial cryobiopsies. Diagn Pathol. 2011;6:53.
Schuhmann M, Bostanci K, Bugalho A, Warth A, Schnabel PA, Herth FJ, Eberhardt R. Endobronchial ultrasound-guided cryobiopsies in peripheral pulmonary lesions: a feasibility study. Eur Respir J. 2014;43:233–9.
Sryma PB, Mittal S, Madan NK, Tiwari P, Hadda V, Mohan A, Guleria R, Madan K. Efficacy of radial endobronchial ultrasound (R-EBUS) guided transbronchial cryobiopsy for peripheral pulmonary lesions (PPL’s): a systematic review and meta-analysis. Pulmonology. 2023;29:50–64.
Matsumoto Y, Nakai T, Tanaka M, Imabayashi T, Tsuchida T, Ohe Y. Diagnostic outcomes and safety of cryobiopsy added to conventional sampling methods: an observational study. Chest. 2021;160:1890–901.
Suzuki M, Matsumoto Y, Imabayashi T, Teishikata T, Tsuchida T, Asamura H, Yatabe Y. Cryobiopsy as a reliable technique for the preoperative identification of micropapillary/solid components in early-stage lung adenocarcinoma. Lung Cancer. 2021;162:147–53.
Yarmus LB, Semaan RW, Arias SA, Feller-Kopman D, Ortiz R, Bösmüller H, Illei PB, Frimpong BO, Oakjones-Burgess K, Lee HJ. A randomized controlled trial of a novel sheath cryoprobe for bronchoscopic lung biopsy in a porcine model. Chest. 2016;150:329–36.
Verhoeven RLJ, Vos S, van der Heijden EHFM. Multi-modal tissue sampling in cone beam CT guided navigation bronchoscopy: comparative accuracy of different sampling tools and rapid on-site evaluation of cytopathology. J Thorac Dis. 2021;13:4396–406.
Folch EE, Mahajan AK, Oberg CL, Maldonado F, Toloza E, Krimsky WS, Oh S, Bowling MR, Benzaquen S, Kinsey CM, et al. Standardized definitions of bleeding after transbronchial lung biopsy: a Delphi consensus statement from the Nashville working group. Chest. 2020l;158:393–400.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–61.
Hetzel J, Eberhardt R, Herth FJ, Petermann C, Reichle G, Freitag L, Dobbertin I, Franke KJ, Stanzel F, Beyer T, et al. Cryobiopsy increases the diagnostic yield of endobronchial biopsy: a multicentre trial. Eur Respir J. 2012;39:685–90.
Kurimoto N, Murayama M, Yoshioka S, Nishisaka T. Analysis of the internal structure of peripheral pulmonary lesions using endobronchial ultrasonography. Chest. 2002;122:1887–94.
Berim IG, Saeed AI, Awab A, Highley A, Colanta A, Chaudry F. Radial probe ultrasound-guided cryobiopsy. J Bronchol Interv Pulmonol. 2017;24:170–3.
Jiang S, Liu X, Chen J, Ma H, Xie F, Sun J. A pilot study of the ultrathin cryoprobe in the diagnosis of peripheral pulmonary ground-glass opacity lesions. Transl Lung Cancer Res. 2020;9:1963–73.
Imabayashi T, Uchino J, Yoshimura A, Chihara Y, Tamiya N, Kaneko Y, Yamada T, Takayama K. Safety and usefulness of cryobiopsy and stamp cytology for the diagnosis of peripheral pulmonary lesions. Cancers (Basel). 2019;11:410.
Ishida T, Asano F, Yamazaki K, Shinagawa N, Oizumi S, Moriya H, Munakata M, Nishimura M. Virtual Navigation in Japan Trial Group. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax. 2011;66:1072–7.
Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117:1049–54.
Gaeta M, Pandolfo I, Volta S, Russi EG, Bartiromo G, Girone G, La Spada F, Barone M, Casablanca G, Minutoli A. Bronchus sign on CT in peripheral carcinoma of the lung: value in predicting results of transbronchial biopsy. AJR Am J Roentgenol. 1991;157:1181–5.
Minezawa T, Okamura T, Yatsuya H, Yamamoto N, Morikawa S, Yamaguchi T, Morishita M, Niwa Y, Takeyama T, Mieno Y, et al. Bronchus sign on thin-section computed tomography is a powerful predictive factor for successful transbronchial biopsy using endobronchial ultrasound with a guide sheath for small peripheral lung lesions: a retrospective observational study. BMC Med Imaging. 2015;15:21.
Kim SH, Mok J, Kim S, Yoo WH, Jo EJ, Kim MH, Lee K, Kim KU, Park HK, Lee MK, et al. Clinical outcomes of transbronchial cryobiopsy using a 1.1-mm diameter cryoprobe for peripheral lung lesions - a prospective pilot study. Respir Med. 2023;217:107338.
Nam BD, Kim TJ, Park K, Ahn MJ, Choi YL, Chung MJ, Kim TS, Lee KS. Transthoracic rebiopsy for mutation analysis in lung adenocarcinoma: outcomes and risk factors for the acquisition of nondiagnostic specimens in 199 patients. Clin Lung Cancer. 2019;20:e309–16.
Puukila S, Thome C, Brooks AL, Woloschak G, Boreham DR. The role of radiation induced injury on lung cancer. Cancers. 2017;9:89–100.
Oki M, Saka H, Ando M, Asano F, Kurimoto N, Morita K, Kitagawa C, Kogure Y, Miyazawa T. Ultrathin bronchoscopy with Multimodal Devices for Peripheral Pulmonary lesions. A Randomized Trial. Am J Respir Crit Care Med. 2015;192:468–76.
Tanaka M, Matsumoto Y, Imabayashi T, Kawahara T, Tsuchida T. Diagnostic value of a new cryoprobe for peripheral pulmonary lesions: a prospective study. BMC Pulm Med. 2022;22:226.
Kim SH, Mok J, Jo EJ, Kim MH, Lee K, Kim KU, Park HK, Lee MK, Eom JS. The additive impact of Transbronchial Cryobiopsy using a 1.1-mm diameter cryoprobe on conventional biopsy for peripheral lung nodules. Cancer Res Treat. 2023;55:506–12.
Acknowledgements
None.
Funding
This article has no Foundation support.
Author information
Authors and Affiliations
Contributions
CKL and CCH designed the study and led the writing of the manuscript. CKL collected and analysed the data. SYR accessed and verified the data. CKL, HJF, HCC, and YTL provided additional clinical data for the individuals in the study. All authors contributed to drafting and providing critical feedback on the manuscript. CCH is the guarantor of the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Board (IRB #202305118RINA) of the National Taiwan University Cancer Center. Written informed consent was obtained from each patient prior to bronchoscopy. All aspects of the study conformed to the principals of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, CK., Ruan, SY., Fan, HJ. et al. Using cryoprobes of different sizes combined with cone-beam computed tomography-derived augmented fluoroscopy and endobronchial ultrasound to diagnose peripheral pulmonary lesions: a propensity-matched study. Respir Res 25, 65 (2024). https://doi.org/10.1186/s12931-024-02700-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-024-02700-w