Abstract
Background
There is an argument whether the delayed intubation aggravate the respiratory failure in Acute respiratory distress syndrome (ARDS) patients with coronavirus disease 2019 (COVID-19). We aimed to investigate the effect of high-flow nasal cannula (HFNC) failure before mechanical ventilation on clinical outcomes in mechanically ventilated patients with COVID-19.
Methods
This retrospective cohort study included mechanically ventilated patients who were diagnosed with COVID-19 and admitted to the intensive care unit (ICU) between February 2020 and December 2021 at Asan Medical Center. The patients were divided into HFNC failure (HFNC-F) and mechanical ventilation (MV) groups according to the use of HFNC before MV. The primary outcome of this study was to compare the worst values of ventilator parameters from day 1 to day 3 after mechanical ventilation between the two groups.
Results
Overall, 158 mechanically ventilated patients with COVID-19 were included in this study: 107 patients (67.7%) in the HFNC-F group and 51 (32.3%) in the MV group. The two groups had similar profiles of ventilator parameter from day 1 to day 3 after mechanical ventilation, except of dynamic compliance on day 3 (28.38 mL/cmH2O in MV vs. 30.67 mL/H2O in HFNC-F, p = 0.032). In addition, the HFNC-F group (5.6%) had a lower rate of ECMO at 28 days than the MV group (17.6%), even after adjustment (adjusted hazard ratio, 0.30; 95% confidence interval, 0.11–0.83; p = 0.045).
Conclusions
Among mechanically ventilated COVID-19 patients, HFNC failure before mechanical ventilation was not associated with deterioration of respiratory failure.
Similar content being viewed by others
Background
A high-flow nasal cannula (HFNC) is a noninvasive respiratory support with the clinical benefits of improving oxygenation and preventing intubation in patients with hypoxemic respiratory failure [1, 2]. In patient with coronavirus disease 2019 (COVID-19) pneumonia leading to acute respiratory distress syndrome (ARDS), oxygen therapy by HFNC has been commonly considered to avoid mechanical ventilation [3,4,5]. As the number of COVID-19 cases is increasing rapidly, it is known that COVID-19-related ARDS is different from other forms of ARDS [6]. Patients with COVID-19-related ARDS have two different subtypes of ARDS: type L, low elastance similar to isolated viral pneumonia, and type H, high elastance similar to classic ARDS [7]. There is an argument that patients with COVID-19 should be intubated early, because type-H patients with HFNC might undergo self-inflicted lung injury (SILI) without the clinical benefits of HFNC and type-L patients could tolerate strain without the risk of ventilator-related lung injury (VILI) [8]. In a recent clinical trial, a significant reduction in intubation rates with high-flow oxygen among patients with respiratory failure due to COVID-19 compared with standard oxygen therapy were observed, however HFNC oxygen did not reduce the mortality rate at day 28 [9]. Although it is known that delayed intubation due to failure of HFNC generally caused worse outcomes in patients with respiratory failure, the effects on COVID-19 patients remained unclear [10]. Therefore, we aimed to compare lung physiology and clinical outcomes between mechanically ventilated COVID-19 patients immediately and those after HFNC failure to investigate the effects of HFNC failure.
Methods
Study participants
This retrospective cohort study examined patients diagnosed with COVID-19 and admitted to the intensive care unit (ICU) between February 2020 and December 2021 at Asan Medical Center. Patients who met the following criteria were eligible for inclusion: (1) adults (age ≥ 18 years); (2) diagnosis of COVID-19 by detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid; (3) admission to ICU for the treatment of COVID-19; and (4) patients who underwent mechanical ventilation during their ICU stay. We excluded patients who were not admitted to the ICU and those who did not receive mechanical ventilation.
Data collection and outcomes
We collected data from electronic medical records, including demographic characteristics, medical history, laboratory results, and medications. The patients were divided into HFNC failure (HFNC-F) and mechanical ventilation (MV) groups according to the use of HFNC before mechanical ventilation. The HFNC-F group consisted of patients who initially received HFNC oxygen but finally underwent mechanical ventilation due to HFNC failure. In contrast, patients in the MV group received immediate mechanical ventilation without HFNC oxygen therapy.
Since this study’s object was to investigate whether delayed intubation after HFNC worsen lung physiology, the primary outcome of this study was to compare the worst values of mechanical ventilation parameters, including tidal volume (TV), predicted body weight (PBW), peak airway pressure, PEEP, arterial oxygen partial pressure (PaO2)/ fractional inspired oxygen (FiO2) ratio (PF ratio), and dynamic compliance, from day 1 to day 3 after the initiation of mechanical ventilation between the HFNC-F and MV groups. Dynamic compliance was calculated as TV/(peak airway pressure-PEEP). Secondary outcomes included ICU mortality, ICU discharge, and successful ventilator weaning by day 28, which were the composite outcomes of ICU discharge or ventilator weaning combined with a competing risk event and mortality within 28 days. If patients died within 28 days regardless of weaning from ventilator, these events did not contribute to the time-to-event analysis of successful weaning by setting the time to zero days [11]. We also performed the comparison of the rates of prone position, extracorporeal membrane oxygenation (ECMO), and continuous renal replacement therapy (CRRT) at 28 days between the two groups as secondary outcomes. In addition, the total duration of mechanical ventilation, length of stay in ICU and length of hospital stay was evaluated.
Statistical analysis
Data are presented as numbers and proportions for categorical variables and means ± standard deviations (SD) or medians (interquartile range [IQR]) for continuous variables with normal distribution or non-normal distribution, respectively. The chi-squared test or Fisher’s exact test was used to compare categorical variables, while the Student’s t-test or Mann–Whitney U-test was used to compare continuous variables with a normal or non-normal distribution. For time-to-event analysis, the Kaplan– Meier method was used, whereas a log-rank test was used to test the significance of the differences. The time-to-event analysis was right-censored at 28 days. Adjustments for secondary outcomes were performed using the multivariable Cox proportional hazards regression model with covariables. We selected covariables with statistical differences for comparison between groups or p-values < 0.10 in the univariable analysis, considering the problem of collinearity. The proportional hazard assumption was assessed by inspecting the Schoenfeld residuals. Two-sided p-values < 0.05 were considered to indicate significance. All analyses were performed using SPSS version 26.0 (IBM Corporation, Armonk, NY, USA).
Results
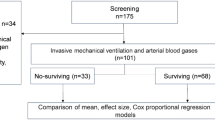
We screened 319 eligible patients for inclusion, of whom 161 were excluded for several reasons: 97 were not admitted to the ICU and 64 did not undergo mechanical ventilation (Fig. 1). Overall, 158 mechanically ventilated patients with COVID-19 pneumonia were included in the present study: 107 patients (67.7%) in the HFNC-F group and 51 (32.3%) in the MV group.
Baseline characteristics
The baseline demographics, including age, sex, body mass index, and comorbidities, of the two groups are shown in Table 1. There were no differences of age, sex, body mass index, and clinical frailty score between the two group, but significant differences were found between the HFNC-F and MV groups in terms of smoking status (never smoker: 60.8% in MV group vs. 74.8% in HFNC-F group, p = 0.008), diabetes mellitus (43.1% in MV group vs. 23.4% in HFNC-F group, p = 0.011), C-reactive protein level (12.3 mg/dL in MV group vs. 9.3 mg/dL in HFNC-F group, p = 0.020), and procalcitonin level (0.4 ng/mL in MV group vs. 0.1 ng/mL in HFNC-F group, p < 0.001). The profile of COVID-19 including the interval until hospital admission, status of vaccination, and treatment was similar between the two groups, except for the proportion of patients who received antithrombotic therapy (84.3% in the HFNC-F group vs. 97.2% in the MV group, p = 0.005). There were significant differences in pH at hospital admission (7.38 [7.31–7.44] vs. 7.46 [7.43–7.48], p < 0.001), PaCO2 (36.00 [31.10–42.70] vs. 32.00 [28.80–35.00], p = 0.001), and PaO2/FiO2 ratio (80.78 [53.00–154.20] vs. 110.17 [78.50–150.17], p = 0.047) between the HFNC-F and MV groups. The median interval of initiation of invasive ventilation from admission differed between the two groups (0.0 days in the MV group vs. 1.0 days [1.00–4.00] in the HFNC-F group, p-value < 0.001). In the HFNC-F group, the median interval from HFNC treatment to mechanical ventilation was 1.0 (1.00–3.00) days (supplemental Table 1). The median respiratory rate and respiratory rate oxygenation (ROX) index at the start of HFNC treatment was 24.0 [20.50–29.50] per minute and 4.13 [3.45–5.68], respectively. The detailed profiles according to type of respiratory support after mechanical ventilation were shown in Supplemental Table 2.
Primary outcomes
The two groups had similar profiles of ventilator parameters from day 1 to day 3 after mechanical ventilation (Table 2). The PF ratio in the first day had a mean of 92.7 ± 8.2 in the MV group and 151.3 ± 64.7 in the HFNC-F group (p = 0.875). There were no differences in dynamic compliance on the first day between the two groups (34.1 ± 13.4 in the MV group vs. 31.5 ± 9.4 in the HFNC-F group, p = 0.359). However, patients in the HFNC-F group tended to have a higher dynamic compliance on day 3 (31.9 ± 9.2) than those in the MV group (28.1 ± 10.0, p = 0.032).
Secondary outcomes
The ICU mortality, ICU discharge, and ventilator weaning on day 28 did not differ significantly between the two groups (Fig. 2). Also, the two groups had similar rates of CRRT (p = 0.114) and prone positioning (p = 0.106) at 28 days. However, the HFNC-F group had a lower rate of ECMO at 28 days than the MV group (p = 0.021). Even after adjustment for covariables, the only association between the HFNC therapy and the lower likelihood of ECMO at 28 days (adjusted hazard ratio, 0.30; 95% confidence interval, 0.11–0.83; p = 0.045) were observed (Table 3). A detailed description of the multivariate Cox regression models for secondary outcomes was provided in supplemental Tables 3–8. There were no significant differences between the two groups concerning the length of the mechanical ventilator (13.00 days [7.00–32.00] in the MV group and 12.0 days [6.00–25.00] in the HFNC-F group, p = 0.849), length of stay in ICU (14.00 days [8.00–35.00] in the MV group and 16.0 days [8.00–34.00] in the HFNC-F group, p = 0.762), or length of hospital stay (27.00 days [16.00–95.00] in the MV group and 31.0 days [17.00–46.00] in the HFNC-F group, p = 0.320). The results of outcomes according to the prespecified subgroups and propensity-score matched cohort were shown in supplemental Tables 9–15 and Figs. 1 and 2.
Secondary outcomes at day 28 according to the use of high-flow nasal cannula before mechanical ventilation. These Kaplan-Meier curves depict (A) intensive care unit (ICU) survival until day 28 as well as cumulative incidence of (B) ICU discharge, (C) successful ventilator weaning, (D) CRRT, (E) Prone position, and (F) Extracorporeal membrane oxygenation at day 28. MV; mechanical ventilation, HFNC-F; high-flow nasal cannula failure, ICU; intensive care unit, CRRT; continuous renal replacement therapy, ECMO; extracorporeal membrane oxygenation
Discussion
In this retrospective, single-center study, we observed that the use of HFNC before mechanical ventilation in COVID-19 patients resulted in similar changes of ventilator parameters and clinical course compared with those who immediately underwent mechanical ventilation without the use of HFNC. Furthermore, the use of HFNC was associated with a reduction in the risk of ECMO at 28 days even though there were no significant associations between the use of HFNC and clinical outcomes in mechanically ventilated COVID-19 patients. In all cases of respiratory failure, the failure of HFNC may not necessarily worsen physiological parameters and clinical outcomes.
Although several studies have analyzed the benefits of HFNC in COVID-19 patients with ARDS, most have focused on the influence of HFNC on reducing the risk of intubation. A previous study reported a decreased risk of intubation with HFNC compared with standard oxygen [10] A randomized study showed a decreased risk of intubation with HFNC compared to standard oxygen, while another study reported no difference in intubation rates between HFNC and standard oxygen [12, 13]. Recently, a randomized clinical trial conducted in France reported that the intubation rate was significantly lower with HFNC than with standard oxygen, without superiority to 28-day mortality [9]. However, the effect of HFNC failure before mechanical ventilation in patients with COVID-19 remains unknown. Although there was a difference in the PaO2/FiO2 ratio at the time of hospital admission between the two groups in our study, no difference in mechanical ventilation parameters was observed at day 1 of mechanical ventilation. In addition, patients who underwent mechanical ventilation after the failure of HFNC had a similar prognosis, including ICU mortality on day 28, to those who received invasive ventilation immediately. Thus, failure of HFNC therapy may not have a significant impact on the prognosis of COVID-19 patients with ARDS.
Two phenotypes of COVID-19-related ARDS have been proposed based on the severity of the infection, ventilator responsiveness of the patients to hypoxemia, and time interval from the onset of the disease [14]. One of the phenotypes is type-L, which may be considered as the early stage of COVID-19-related ARDS, presenting with only sub-pleural ground-glass densities on computed tomography scan, high compliance, low ventilation-to-perfusion ratio, and low lung recruitability [15]. In contrast, type H may have the key features of low compliance, high right-to-left shunt, and high lung recruitability. The transition from type L to type H may be induced by interstitial lung edema due to negative inspiratory intrathoracic pressure [16]. In patients with type-L, dyspnea leads to this phenomenon according to SILI [17]. In our study, no deterioration in lung physiology after the failure of HFNC was observed, and the use of HFNC before mechanical ventilation was even associated with a reduction in the probability of ECMO. We observed that median values of PaCO2 between initial and worst values in the HFNC-F group were similar, even though median values of FiO2 increased from the start of HFNC to the period before mechanical ventilation. Considering these findings, thus, we considered that the use of HFNC in type L patients with dyspnea may attenuate the transition to type H by decreasing SILI-induced interstitial pulmonary edema. Higher dynamic compliance on day3 in the HFNC group than that in the MV group may support our hypothesis. In contrast, several complications due to early endotracheal intubation, including aspiration of gastric contents, disruption of the natural airway defense, sedatives induced hemodynamic instability, and increased risk of VILI might have decreased dynamic compliance on day3 and increased the rate of ECMO in the MV group [18].
Mechanical ventilation often increases the risk of kidney injury [19]. Positive pressure ventilation leads to damage to the alveolar-capillary membrane, resulting in the release of proinflammatory cytokines [20]. This propagation of inflammatory cascades induces ventilator-induced kidney injury (VIKI) [21]. In our study, there was no difference in the CRRT rate according to the use of HFNC. The similarity of parameters at the initiation of mechanical ventilation as well as the same duration of mechanical ventilation between the two groups may provoke a similar impact of VIKI.
This study has several limitations that should be considered when interpreting the results. First, this was a retrospective study conducted at a single center; a study bias may have influenced the results of this study. The imbalances in baseline characteristics between the two groups may bias the study interpretation, although we adjusted for outcomes. Second, the actual effects of HFNC on SILI are unknown because there is no objective indicator of SILI. Third, the intubation in HFNC-F group was done by intensivist’s decision rather than the objective criteria such as ROX index though COVID-19 patients were managed by the dedicated ICU team. Finally, chest tomography was not conducted at admission to evaluate the type of COVID-19-related ARDS in all patients; however, chest X-rays in most patients presented similar findings, such as diffuse ground glass opacity and focal consolidation in both lungs.
Conclusions
In conclusion, the use of HFNC before mechanical ventilation in COVID-19 patients had similar clinical outcomes compared with those with immediately mechanical ventilation in a retrospective analysis of HFNC in 158 patients with COVID-19. The failure of HFNC before mechanical ventilation may not worsen lung physiology and clinical outcomes in the case of respiratory failure with low elastance, as seen in COVID-19.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Papazian L, Corley A, Hess D, Fraser JF, Frat JP, Guitton C, et al. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. 2016;42(9):1336–49.
Ferreyro BL, Angriman F, Munshi L, Del Sorbo L, Ferguson ND, Rochwerg B, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA. 2020;324(1):57–67.
Demoule A, Vieillard Baron A, Darmon M, Beurton A, Géri G, Voiriot G, et al. High-Flow nasal cannula in critically III patients with severe COVID-19. Am J Respir Crit Care Med. 2020;202(7):1039–42.
Garibaldi BT, Fiksel J, Muschelli J, Robinson ML, Rouhizadeh M, Perin J, et al. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33–41.
Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–23.
Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24(1):154.
Dereli N, Babayiğit M, Koç F, Menteş O, Yildiz G, Kilinçarslan A, et al. Type-H, and Type-L COVID-19: are they different subtypes or the same? Eur Rev Med Pharmacol Sci. 2022;26(9):3367–73.
Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–30.
Frat JP, Quenot JP, Badie J, Coudroy R, Guitton C, Ehrmann S, et al. Effect of high-flow nasal cannula oxygen vs standard oxygen therapy on mortality in patients with respiratory failure due to COVID-19: the SOHO-COVID Randomized Clinical Trial. JAMA. 2022;328(12):1212–22.
Bonnet N, Martin O, Boubaya M, Levy V, Ebstein N, Karoubi P, et al. High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: a retrospective study. Ann Intensive Care. 2021;11(1):37.
Nolley EP, Sahetya SK, Hochberg CH, Hossen S, Hager DN, Brower RG, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401.
Perkins GD, Ji C, Connolly BA, Couper K, Lall R, Baillie JK, et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS Randomized Clinical Trial. JAMA. 2022;327(6):546–58.
Ospina-Tascón GA, Calderón-Tapia LE, García AF, Zarama V, Gómez-Álvarez F, Álvarez-Saa T, et al. Effect of high-flow oxygen therapy vs conventional oxygen therapy on invasive mechanical ventilation and clinical recovery in patients with severe COVID-19: a Randomized Clinical Trial. JAMA. 2021;326(21):2161–71.
Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–102.
Gando S, Wada T. Pathomechanisms underlying hypoxemia in two COVID-19-associated acute respiratory distress syndrome phenotypes: insights from thrombosis and hemostasis. Shock. 2022;57(1):1–6.
Mascheroni D, Kolobow T, Fumagalli R, Moretti MP, Chen V, Buckhold D. Acute respiratory failure following pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med. 1988;15(1):8–14.
Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of Lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195(4):438–42.
Griesdale DE, Bosma TL, Kurth T, Isac G, Chittock DR. Complications of endotracheal intubation in the critically ill. Intensive Care Med. 2008;34(10):1835–42.
van den Akker JP, Egal M, Groeneveld AB. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: a systematic review and meta-analysis. Crit Care. 2013;17(3):R98.
Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2014;370(10):980.
Hepokoski ML, Malhotra A, Singh P, Crotty Alexander LE. Ventilator-Induced kidney injury: are novel biomarkers the key to prevention? Nephron. 2018;140(2):90–3.
Acknowledgements
None.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
D.H.: Conception and design of the study; generation, collection, assembly, analysis, and/or interpretation of data; drafting or revision of the manuscript; and approval of the final version of the manuscript. J.H.: Conception and design of the study; generation, collection, assembly, analysis, and/or interpretation of data; drafting or revision of the manuscript; and approval of the final version of the manuscript. S.L.: Generation, collection, assembly, analysis, and/or interpretation of data; drafting of manuscript. approval of the final version of the manuscript. J.A.: Generation, collection, assembly, analysis, and/or interpretation of data; drafting of manuscript. approval of the final version of the manuscript. S.H.: Generation, collection, assembly, analysis, and/or interpretation of data; drafting of manuscript. approval of the final version of the manuscript. Y.K.: Generation, collection, assembly, analysis, and/or interpretation of data; drafting of manuscript. approval of the final version of the manuscript. C.L.: Generation, collection, assembly, analysis, and/ or interpretation of data; drafting of manuscript. Approval of the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Asan Medical Center Institutional Review Board (No.2022 − 1026). The requirement for informed consent was waived owing to the retrospective nature of the study. All studies were conducted under the Guidelines for Good Clinical Practice and the 1964 Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hyun, Dg., Lee, S.Y., Ahn, J.H. et al. Prognosis of mechanically ventilated patients with COVID-19 after failure of high-flow nasal cannula: a retrospective cohort study. Respir Res 25, 109 (2024). https://doi.org/10.1186/s12931-024-02671-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-024-02671-y