Abstract
Non-small cell lung cancer (NSCLC) patients with sensitizing oncogenic driver mutations benefit from targeted therapies. Tyrosine kinase inhibitors are highly effective against classic sensitizing epidermal growth factor receptor (EGFR) mutations, such as exon 19 deletions and exon 21 L858R point mutations. Conversely, EGFR exon 20 insertions (exon20ins) are resistant to the traditional EGFR tyrosine kinase inhibitors (TKIs). In May 2021, the US Federal Drug Administration (FDA) provided accelerated approval to amivantamab (Rybrevant) in adults with locally advanced or metastatic NSCLC with EGFR exon20ins after treatment with platinum-based chemotherapy. Amivantamab was the first EGFR/MET bispecific antibody to be approved specifically for EGFR exon20ins where there was an unmet need. Furthermore, amivantamab is being evaluated in additional settings such as post osimertinib in sensitizing EGFR mutations as well as in MET altered NSCLC. Here we discuss amivantamab in regard to its mechanism of action, preclinical and clinical data, and clinical impact for patients with EGFR exon20ins NSCLC and beyond.
Similar content being viewed by others
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related mortality worldwide [1, 2]. Increased access to DNA sequencing for targetable mutations and rapid advancements in targeted therapeutic options have improved outcomes in many subtypes of NSCLC [3,4,5]. Drugs targeting molecular oncogenic drivers have improved efficacy and tolerability of treatment for NSCLC patients. EGFR is a transmembrane cell surface receptor with downstream effects that regulate cell proliferation and apoptosis [6]. In normal cells, EGFR signaling is responsible for DNA synthesis and cellular proliferation, however, surplus activity results in uncontrolled cellular growth and tumorigenesis [7]. EGFR mutations generally favor the active state leading to pro-survival and antiapoptotic signals, even without the presence of a ligand [8, 9]. EGFR is an attractive target for therapeutic development as EGFR-mutated tumors become dependent on the EGFR pathway and its downstream effects for survival [6]. EGFR-mutated NSCLC is found on sequencing of 20% of Caucasians, up to 50% of Asian patients [4, 10]. and globally, EGFR mutations account for 23–30% of NSCLC activating mutations [11, 12].
Classic EGFR mutations (exon 19 deletions or exon 21 L858R substitutions) represent 85% of EGFR mutations [13]. EGFR exon20ins (exon20ins) consist of either point mutations or insertions of 3–21 base pairs [14]. and are present in 4-to-12% of EGFR-mutated NSCLC [15]. NSCLC driven by EGFR exon20ins portends a worse prognosis and shorter overall survival than classic sensitizing EGFR mutations such as exon 19 deletions and exon 21 L858R point mutations [16, 17]. Because of its structure, the active conformation with the C-helix in an inward position, forming a rigid and inflexible structure that locks the EGFR molecules in active conformation without ligand binding [12 Yasuda], EGFR exon20ins are classically resistant to first-, second-, and third-generation EGFR tyrosine kinase inhibitors (TKIs) and prior to approval of amivantamab in 2021, there were no Federal Drug Administration (FDA) approved targeted therapeutic options. In May 2021, the FDA granted accelerated approval to amivantamab (Rybrevant) in adult NSCLC patients with locally advanced or metastatic EGFR exon20ins-positive disease following platinum-based chemotherapy.
Mesenchymal-epithelial transition (MET) is a tyrosine kinase receptor for the ligand hepatocyte growth factor (HGR) and is frequently expressed by epithelial cells of solid organs. Dysregulation of the MET pathway results in proliferation, survival, invasion, and metastasis of tumor cells. MET activation is both a primary oncogenic driver mutation and could be a secondary mechanism of drug resistance, making the MET pathway an attractive therapeutic target [18, 19]. MET aberrations can occur as overexpression, amplification or mutations. MET is overexpressed in 20%, [20] amplified in 1–5%, [21] and exon 14 skipping mutations (METex14) occur in 3–4% of NSCLC tumors [22,23,24]. MET rearrangements have been detected in several cancer types including NSCLC and glioblastomas [25, 26]. In chromosomal translocations, the fusion typically includes a dimerization domain resulting in constitutive activation. Although the TPR-MET fusion was first identified, [27] a ST7-MET fusion was reported as an acquired resistance mechansim to the third-generation TKI lorlatinib in a NSCLC patient with dual ALK-MET aberrations [28]. Within NSCLC tumors, METex14 skipping mutations are most frequent in sarcomatoid carcinoma (4.9–31%), adenosquamous carcinoma (5%), adenocarcinoma (3%), and squamous cell carcinoma (2%) [29,30,31,32,33] and are more common in patients over 70 years old, women, and never-smokers. MET aberrations are associated with poor prognosis [34,35,36]. MET exon 14 skipping mutations occur at high allele frequency and can co-occur with TP53, MDM2, CDK4, and HMGA2 co-amplifications while MET-amplified patients have co-occurring NRAS and KRAS mutations [37]. Conversely, one study of 30 patients with METex14 aberrations found no overlap with mutations in KRAS, EGFR, ERBB2, ALK, ROS1, or RET [29]. METex14 skipping mutations are associated with worse overall survival [38].
MET amplification has been shown to bypass EGFR signaling pathways and confer resistance to osimertinib [39, 40]. MET amplification was found in 15% of samples at disease progression on osimertinib [41]. MET amplification has been described in cases of rapid and prolonged response to crizotinib [42]. MET-mutated tumors are also associated with a worse prognosis [43]. Additional MET aberrations included impaired MET receptor degradation, MET fusion, and MET overexpression.
Upregulation of the EGFR signaling pathway has been shown as a mechanism of resistance to MET TKIs [44, 45]. MET amplification is associated with resistance in 50–60% of first- and second-generation EGFR TKIs [46,47,48] and 15–19% of third-generation EGFR TKIs [41, 49]. EGFR and MET are co-expressed in 70% of EGFR mutations [50, 51]. In contrast, normal cells almost never concomitantly express both receptors [52, 53]. Interactions between EGFR and MET signaling pathways is well documented in the literature and are involved in both oncogenic signaling as well as tumor microenvironment remodeling [54,55,56]. Both EGFR and MET signal through the same pathways, possibly explaining frequent resistance upon inhibition of only one of these receptors [57]. The interplay between these pathways suggests that simultaneously inhibiting both oncogenes may reduce resistance to MET- or EGFR-targeted agents.
The first MET inhibitor, crizotinib, was approved in 2011 for ALK-rearranged NSCLC and since that time, MET-targeted drugs including capmatinib and tepotinib have been approved for NSCLC harboring MET exon 14 skipping mutations. Capmatinib is a type 1b MET inhibitor with a mechanism similar to that of crizotinib. The phase II GEOMETRY study, reported an objective response rate (ORR) of 41% (95% CI 29–53) in pretreated patients with MET exon 14 skipping mutations and 68% (95% CI 48–84) in treatment naïve patients [58]. The duration of response (DOR) was 9.7 months and 12.6 months, respectively. Among patients with MET amplification and a gene copy number of 10 or higher, OR was seen in 29% (95% CI, 19 to 41) of pretreated patients and in 40% (95% CI, 16 to 68) of treatment naïve patients. [51]. Tepotinib is a TKI that selectively binds MET to promote tumor cell death and in the phase II VISION trial, tepotinib had an ORR 46% (95% CI 36–57) with median DOR 11.1 months (95% CI 7.2-NR) [59].
In this review, we discuss the unique structure, pharmacodynamics, and pharmacokinetics of amivantamab as well as its indication towards EGFR exon20ins and beyond, focusing on dual inhibition of EGFR and MET, which could be employed for the treatment of MET-altered tumors as well as those with sensitizing EGFR mutations who have progressed on EGFR TKIs.
Structural characteristics and mechanism of action of amivantamab
Amivantamab (JNJ-61,186,372, Rybrevant, Janssen Biotech, Inc) is a fully human Fc-active immunoglobulin G1 (IgG1) bispecific antibody against both epidermal growth factor (EGF) and MET receptors. Amivantamab consists of two arms; one binds the extracellular domain of EGFR to block binding between the receptor and its ligand EGF while the other arm blocks HGF ligand from binding to the MET receptor. Amivantamab induces degradation of both receptors in vivo, broadening its mechanism of action to include ligand-independent driven disease [15, 60]. This results in stopping downstream signaling of pro-growth and pro-survival proteins. Amivantamab simultaneously inhibit EGFR as well as one of the more common mechanisms of resistance to EGFR targeting therapy through the MET pathway. This combined inhibition has the potential to enhance depth and duration of response for patients with these mutations.
In addition to the direct inhibitory effects as a bispecific antibody, amivantamab also appears to work with the human immune system. Indeed, amivantamab has a low fructose backbone to enhance binding to FcYRIIIa/CD16a [61]. The FcYRIIIa/CD16a receptor on NK cells, monocytes, and macrophages triggers antibody-dependent cell-mediated cytotoxicity (ADCC) of NSCLC cells. This unique structural design permits amivantamab to eliminate antigen-expressing tumor cells through ADCC, induce trogocytosis as well as antibody dependent cellular phagocytosis and antibody dependent cytokine release. This activity results in receptor-antibody complex endocytosis and removal via lysosomal trafficking [61].
Pharmacodynamic properties
Amivantamab has been shown to bind the extracellular domains of EGFR and MET receptors with binding affinity (KD) of 1.43 and 0.04 nM, respectively in preclinical studies. Amivantamab binds human EGFR and MET with EC50 values of 0.38 nM 0.27 nM, respectively. Amivantamab was selected from a panel of bispecific anti-EGFR and anti-MET molecules. Bispecifics with higher affinity to MET were favored to reduce binding to cells with normal EGFR expression. The affinity for MET was seen with equilibrium dissociation constant [Kd] of 40pmol/L. It is proposed that the high affinity for MET plus low affinity for EGFR helps overcome resistance while also decreasing wild-type EGFR-associated toxicity.
In vitro studies also found at doses ≥ 700 mg, complete and durable saturation of both EGFR and MET receptors occurred [62].
Pharmacokinetics
Data from in vivo trials showed at doses between 350 and 1750 mg, amivantamab exposure increased proportionally. Steady state was achieved by the ninth infusion. Amivantamab concentration increased rapidly during cycle 1 with steady state observed by cycle 4 [62]. The half-life of amivantamab was 11.3 (± 4.53) days with a median volume of distribution of 5.13 L.
Dosing recommendations are based on target saturation that were calculated using baseline body weight. For patients with baseline body weight < 80 kg, the recommended dose of amivantamab is 1050 mg. For patients above 80 kg at baseline, the recommended dose is 1400 mg. Amivantamab is administered intravenously weekly for the first five weeks then every two weeks until disease progression or toxicity. There was no clinically meaningful difference in amavantamab exposure across age, gender, race, creatinine clearance, or hepatic impairment.
Preclinical studies
Preclinical studies have confirmed the unique characteristics of amivantamab. The low fructose backbone of amivantamab appears to enhance ADCC through stronger binding to the F-c domain [63]. Indeed, studies in mice confirmed that tumors treated with amivantamab had lower EGFR and MET receptor expression as a result of receptor internalization and trogocytosis [60].
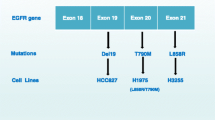
Preclinical studies of Ba/F3 cell lines containing EGFR exon20ins have found that amivantamab decreased EGF and MET receptor expression [15]. All five exon20ins studied (V769_D770insASV, D770delinsGY, H773_V774insH, Y764_V765insHH, and D770_N771ins- SVD) demonstrated a dose-dependent decrease in viability. The proposed mechanism of action is inhibition of cell proliferation through decreased pERK, pAkt, and p-S6 [15]. Additionally, amivantamab induced apoptosis via upregulating proapoptotic proteins including BCL2-interacting mediator of cell death (BIM) and cleaved caspase-3.
HCC827 is a lung adenocarcinoma cell line with an acquired E746_A750 deletion in the EGFR tyrosine kinase exon 19 domain. Data from HCC827 cell lines have found superior antitumor activity of amivantamab compared to TKI erlotinib and the MET inhibitor crizotinib. By day 34, tumor growth was inhibited 99.8% (p < 0.05) with a durable response 8 weeks after amivantamab discontinuation [64]. In experiments with xenograft models amivantamab was more efficacious than either cetuximab or poziotinib [15].
Additionally, amivantamab has preclinical data from resistant cell lines. Within cell lines of EGFR activating mutations (i.e. L858R), with EGFR resistance mutations (i.e. T790M)or MET amplification, amivantamab blocked ligand from binding its receptor [65]. This showed the antitumor activity of amivantamab even in tumors with mechanisms of resistance to EGFR targeted therapy as well as the historically difficult to target MET amplification. Further, amivantamab results in decreased cell surface EGFR and MET receptors both in vitro and in vivo [60]. Importantly, amivantamab remains effective when bound to either EGFR or MET receptors alone [60, 63].
Clinical trials
EGFR exon20ins
Phase I
Amivantamab was granted accelerated FDA approval to those with NSCLC patients with tumors harboring EGFR exon20ins post platinum based therapy on May 21, 2021. This was based on CHRYSALIS, a phase I, multicenter, open label, dose-escalation clinical trial (NCT02609776) [62]. Patients received amivantamab IV weekly for five weeks then every two weeks until disease progression or untolerable toxicity. The primary outcome was overall response rate (ORR) with secondary outcomes of interest including clinical benefit rate, duration of response (DOR), progression-free survival (PFS), and overall survival (OS). The trial overall enrolled 362 patients with median age of 62 years, 48% women, and 49% Asian. All study participants were platinum-pretreated with a median of 2 (range 1–7) previous therapies. During the dose escalation portion, no maximum tolerated dose was identified up to the highest studied dose of 1750 mg. Weight-based dosing was selected at a dose of 1050 mg for patients under 80 kg or 1400 mg for patients 80 kg or more due to the safety, pharmacokinetic, and pharmacodynamic properties. In those with NSCLC harboring EGFR exon20ins post platinum-based therapy (N = 114), the ORR was 40% (95% CI: 29–51%) with a median DOR of 11.1 months (95% CI: 6.9-NR) [Sabari WCLC 2021]. 4% reached a complete response to treatment. The clinical benefit rate was 74% (95% CI: 63–83%). Median progression-free survival was 8.3 months (95% CI: 6.5–10.9) and median overall survival was 22.8 months (95% CI: 14.6-NR). Responses were seen in patients with a variety of different EGFR exon20ins, regardless of site of insertion type [62].
As a non-randomized single-arm study, CHRYSALIS leaves some clinical questions for the application of amivantamab across heterogeneous patient populations. Real-world analyses may allow for comparison of the study agent to current standard of care practices and may be able to examine if the efficacy endpoints would be clinically meaningful. In a real-world follow-up of 81 amivantamab-treated patients compared to 125 controls all with EGFR exon20ins, the authors examined a subset of patients from the CHRYSALIS trial in addition to patients from three United States based-databases (ConcertAI, COTA and Flatiron) Demographics were similar to those of the original trial with median age of 62 years, 60% female, 59% never-smokers. The population included 40% with baseline brain metastases and a median of two lines of prior therapy in metastatic disease. These demographics were similar to established characteristics of exon20ins NSCLC patients from the CHRYSALIS study. Authors showed ORR of 40% with amivantamab versus 16% in the control group [66]. Patients in the amivantamab arm demonstrated longer PFS of 8.3 versus 2.9 months (95% CI 0.34–0.65) and OS of 22.8 months versus 12.8 months (95% CI 0.31–0.77). These results were consistent in subanalysis comparing amivantamab to commonly used treatments in the control arm including non-platinum-based chemotherapy, immunotherapy, EGFR TKIs, and platinum-based chemotherapy.
Another real-world analysis of patients from the United States as well as Europe showed a similar response with ORR of 37% vs. 17% with amivantamab compared to control [67]. This trial looked at 349 patients, 61% of whom were female. Similarly, patients had a median of two prior lines of therapy and 26% had brain metastases at the time of enrollment. The median PFS and OS were also similar at 12.5 months and 22.8 months, respectively. This study found that clinicians prescribe multiple treatments including EGFR TKIs to NSCLC patients with EGFR exon20ins despite known poor response rates, demonstrating the need for additional treatment options and further collaboration with practitioners. These real-world analyses provide prognostic information useful for patients who may be excluded from clinical trials, such as those with brain metastases. Across heterogeneous patient populations from the United States and within the European Union, amivantamab showed statistically significant benefit in ORR, OS, and PFS. These findings support the generalizability of the original study results in EGFR exon20ins NSCLC.
Currently there are no approved treatments for patients with triple EGFR mutations. Although fourth generation EGFR TKIs are being studied in the context of triple mutations in cis, it will likely take several years before these agents are available for patient use [68]. A case report has described the use of amivantamab in this scenario. Briefly, the patient was diagnosed at age 67 with stage IV EGFR L858R NSCLC and was found to have EGFR T790M mutation upon progression on erlotinib as well as a G796S mutation upon progression on osimertinib. Given amivantamab’s efficacy across a range of EGFR mutations and benefit in chemotherapy-refractory EGFR exon 20 insertions, [69] it was trialed in her refractory L858R/T790M/G796S EGFR mutations [70]. The patient demonstrated lower symptom burden, mutation allele frequency, and CEA level with ongoing response at follow-up over 100 days later. This shows that amivantamab may be active against one of the most commonly acquired triple EGFR mutations in cis.
Safety, tolerability, and adverse events
From the EGFR exon20ins post platinum-based therapy cohort in the CHRYSALIS study, the most common adverse events include rash (86%), infusion-related reaction (65%), and paronychia (42%) [62]. Rare adverse events include stomatitis, pruritis, hypoalbuminemia, increased ALT, fatigue, and cellulitis. The side effects are associated with the unique properties of this drug as rash, paronychia, stomatitis, pruritis, and diarrhea resulting from EGFR inhibition while MET inhibition is associated with hypoalbuminemia and peripheral edema (Table 1). 16% of patients experienced grade 3 or higher treatment-related adverse events. The most common of which include rash (4%), infusion-related reaction (3%), and neutropenia (3%). Serious treatment-related adverse events included infusion-related reactions (2%), and diarrhea (2%). Interstitial lung disease occurred in 4% of patients. Significant side effects resulted in amivantamab dose-reduction in 13% and drug discontinuation in 4% of participants. Almost all (94%) of infusion-related reactions occurred during the first infusion.
Signs of infusion-related reaction include chills, dyspnea, flushing, nausea, chest discomfort, and emesis. These reactions were mitigated in the CHRYSALIS trial by holding of infusion (56%), reinitiating at a slowed rate (53%), or aborting infusion (14%) [71]. As a result, amivantamab is typically administered as a slow infusion over two days for the first cycle. On day 1 amivantamab is administered at 25 mL/h for the first two hours then increased to 50 mL/h for the remainder of the 350 mg dose. With this regimen, the median time to infusion-related reaction onset is 45 min and the majority of reactions that occur are grade 1–2. No predisposing risk factors for which patients will develop infusion-related reactions were identified.
In further safety analysis of 302 patients with any EGFR mutation who received at least 1 dose of amivantamab, side effects occurred in approximately 20% of patients [65]. These side effects were consistent with those reported earlier and include infusion-related reactions, rash, paronychia, stomatitis, and edema. Of those with infusion-related reactions, 97% were grade 1 or 2.
A subcutaneous form of amivantamab is being studied in the PALOMA trial (NCT04606381). Preliminary results show subcutaneous route is well-tolerated and reduced infusion-related reactions to 18.2% [72]. This route allows for decreased administration time to less than 5 min and maintained approximately 65% of the bioavailability seen with intravenous dosing [73]. Saturation of free EGFR and MET receptors was seen after the first dose.
Both anti-EGFR and anti-MET therapies are associated with dermatologic toxicities. An analysis of cutaneous side effects from patients enrolled in the phase I CHRYSALIS trial noted acneiform rash and paronychia in 100% of patients [74]. Other adverse events include hypertrichosis in 50% of men, hirsutism in 80% of women, skin abrasians of scalp (71%), and skin fissure (57%). Amivantamab administered at the higher dose of 1400 mg was associated with both higher grade and more rapid skin toxicity. Secondary prevention of cutaneous manifestations should utilize tetracycline, moisturizers, and hygienic measures at least 14 days prior to treatment initiation. It is essential to ask patient about skin reactions to therapy as these can have a psychological impact.
In a small review focusing on dermatologic side effects, lesions associated with amivantamab use may appear more severe with unique features and distribution due to the dual EGFR and MET inhibition [75]. Patients were noted to have severe crusted plaques of the scalp which may be the result of MET activity which is known to impact follicle growth [76, 77]. The dermatologic effects of amivantamab are reduced 50% when patients use proactive therapy with moisturizers, sunscreen, topical corticosteroids, and oral tetracycline [78].
EGFR exon 19 deletion and L858R mutation post osimertinib
This cohort was designed to combine lazertinib, a potent, CNS-penetrant, third-generation EGFR TKI with amivantamab which has the potential to target the two most common resistance mechanisms to TKIs – secondary EGFR mutations and MET amplification. Preliminary results presented at ASCO 2022 were from the 45 patients who were chemotherapy naïve but progressed on osimertinib. Analysis showed ORR 33% (95% CI 26–41) with PFS 5.1 months (95% CI 4.2–6.9) [79]. Median DOR was 9.6 months (95% CI 7.0-NR) and median OS 14.8 months (95% CI 12.1-NR). Of note, intracranial ORR was 26% (7/27). The most common treatment-related adverse events include infusion-related reactions (67%), paronchia (52%), and rash (44%). Grade 3 or higher adverse events seen include dyspnea (8%), infusion-related reaction (8%), and hypoalbuminemia (7%).
NSCLC with MET exon 14 skipping mutation
Given amivantamab’s higher affinity for MET over EGFR, the phase I CHRYASLIS trial is also looking at amivantamab in MET amplification (Cohort MET-1) and MET exon 14 skipping mutations (Cohort MET-2). Preliminary data within the MET exon 14 skipping mutations cohort (n = 55) was presented at ASCO 2022. Results revealed ORR 33% (15/45) with a median PFS 6.7 months (95% CI 2.9–15.3) [72]. The response was most pronounced in treatment-naïve patients (ORR 57%) compared to those who were pretreated without MET inhibitors (47%) and with prior MET inhibitors (17%). Within responders, 10/15 demonstrated response greater than 6 months and median DOR was not reached. Most common toxicities include infusion-related reactions (69%), dermatitis (40%), and paronchia (38%). Grade 3 and higher AEs include dyspnea (7%), infusion-related reactions (5%), and hypoalbuminemia (4%). Treatment-related adverse events resulted in dose reduction or drug discontinuation in three patients.
Discussion/future directions
Traditional EGFR targeted therapies such as gefitinib, erlotinib and osimertinib which are effective against those with EGFR sensitizing mutations, were not as effective against EGFR exon20ins-mutated NSCLC, leaving an unmet need for a significant percentage of lung adenocarcinoma patients with EGFR mutations. Approximately 90% of exon20ins mutations occur after the C-helix of the tyrosine kinase domain, wedging the C-helix in front of the drug binding pocket resulting in active kinase formation making it difficult for drug binding [9, 80]. This may explain resistance to first generation TKIs [81]. Second generation TKIs in exon20ins are limited by significant toxicity at plasma concentrations below the efficacy threshold required to inhibit signaling pathways [14]. The third generation EGFR TKI, osimertinib, was ineffective in EGFR exon20ins with a low overall response rate of 5% [81, 82]. Patients with newly diagnosed EGFR exon20ins-driven NSCLC have a median OS of 16.2 months (95% CI: 11.0-19.4) [83] compared to a median OS of 38.6 months in those with exon 19 deletions and 21 mutations based on the FLAURA study of front-line osimertinib [84].
Promising results from the phase I CHRYSALIS trial led to the FDA accelerated approval of amivantamab in those with EGFR exon20ins post platinum-based therapy. Amivantamab is the first FDA-approved bispecific molecule for treatment of solid malignancies. The investigators found an ORR of 40% with a median PFS of 8.3 months. These findings are clinically meaningful considering that relapsed metastatic or unresectable NSCLC has a 5-year survival rate of less than 10% [62]. While mobocertinib also received FDA accelerated approval for the same indication with potentially similar efficacy profile, [85] as the confirmatory phase 3 EXCLAIM-2 study did not meet its primary endpoint, Takeda has announced its voluntary withdrawal.
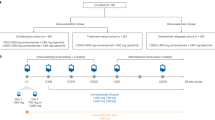
Further enhancing the efficacy with combination therapy with chemotherapy may be attractive to patients especially those with an initially high tumor burden. The ongoing phase III PAPILLON trial (NCT04538664) is studying the efficacy and safety of carboplatin-pemetrexed chemotherapy with or without amivantamab in the first-line treatment setting of metastatic NSCLC with EGFR exon20ins. This design is particularly favorable, as the standard of care first line treatment is platinum doublet, ensuring patients a “no-risk” approach, while the addition of amivantamab from first line may have a chance to induce even better efficacy results. The primary outcome of interest is PFS at 18 months with secondary outcomes including ORR, DOR, and tolerability (Table 2). Additional evidence is needed for patients with baseline brain metastases using combinations of amivantamab with chemotherapy, targeted agents, and radiation along with careful evaluation of the associated toxicity profiles.
Amivantamb, given its broad-spectrum coverage against EGFR and the fact that it is a bispecific against MET, which alterations are known to be part of the mechanism of resistance against osimertinib, is also being evaluated in the first line setting for those with EGFR exon 19 deletions and L858R mutations as well as post progression on osimertinib.
Of 20 treatment-naïve patients with classical EGFR mutations treated with amivantamab plus lazertinib in the CHRYSALIS (NCT02609776) study, the ORR was 100% [86]. In the post osimertinib setting, preliminary data of CHRYSALIS-2 showed promising results with ORR 36%, clinical benefit rate of 58% including one complete response. Although the median DOR was not reached, 39% of participants had a durable response at a median follow-up of 8.3 months [79]. Sub-analysis of the heavily-pretreated population found ORR 29% with clinical benefit rate 55% and median DOR 8.6 months. Also, among eight patients with baseline brain lesions, antitumor activity was reported.
Currently, the CHRYSALIS-2 trial (NCT04077463) is studying amivantamab and lazertinib in EGFR-NSCLC (Table 2). While the phase III MARIPOSA study (NCT04487080) is investigating the safety and efficacy of amivantamab in combination with lazertinib versus lazertinib alone or lazertinib alone in NSCLC patients with classic EGFR mutations in the front line setting, MARIPOSA-2 study (NCT04988295) is evaluating three arms (lazertinib + amivantamab + carboplatin + pemetrexed, carboplatin + pemetrexed, amivantamab + carboplatin + pemetrexed) post progression on lazertinib in those with the classic EGFR mutations. The primary outcome of interest is PFS with secondary endpoints of ORR, OS, DOR, intracranial PFS among others.
The safety profile of amivantamab combined with lazertinib is similar to that of amivantamab monotherapy [86]. Most common adverse events include rash (78%), infusion-related reactions (61%), paronychia (42%), stomatitis (31%), and pruritis (24%). Grade 3 or higher adverse events were reported in 7% of participants. Similar rates of infusion-related reaction (65%), paronychia (49%), rash (41%), and stomatitis (39%) were seen in CHRYSALIS-2 [79]. This combination has the benefit of amivantamab’s activity against extracellular EGFR with lazertinib’s intracellular EGFR TKI efficacy. Lazertinib also crosses the blood-brain barrier, making this combination favorable for NSCLC patients with brain metastases who have limited effective treatment options. Within the CHRYSALIS cohort, only 7% of patients on combination therapy had documented central nervous system progression compared to 17% with amivantamab monotherapy [87]. Additional ongoing trials with amivantamab are listed in Table 2.
Currently there are no FDA approved targeted agents for MET amplified cancers. Although new therapeutics targeting MET include capmatinib and tepotinib for MET exon 14 skipping mutations have become available, additional treatment options are needed. Amivantamab has shown early promising data in MET exon 14 skipping mutations with ORR 33% in all patients and 57% in treatment-naïve patients [72]. The median PFS was 6.7 months and was generally well tolerated.
While amivantamab is an excellent agent towards EGFR exon20ins and beyond, we must be cognizant of the adverse event profile and the inconvenience to patients. To circumvent the infusion time (and potentially infusion related reactions), multiple studies are looking at utilizing the subcutaneous version of amivantamab in NSCLC and solid tumors (PALOMA: NCT04606381, PALOMA2 NCT05498428 and PALOMA3 NCT05388669). Preliminary results look promising with a remarkably lower rate of infusion-related reactions.
Conclusion
The unique structural design of amivantamab with simultaneous binding to both EGFR and MET with addition of a low fructose backbone for enhanced ADCC provide increased selectivity and efficacy with decreased toxicity compared to other targeted therapies for EGFR exon20ins NSCLC [53]. Amivantamab, especially in combination with lazertinib and appears to have promising activity beyond EGFR exon20ins. These indications may include classic EGFR mutations in the front line setting and in the post osimertinib failure scenario as well as those with MET alterations. Additional studies are warranted to not only document and improve on the clinical efficacy of amivantamab in different settings but also to reduce the toxicities and inconvenience of therapy.
Data Availability
Not applicable to this study.
Abbreviations
- ADCC:
-
antibody dependent cell-mediated cytotoxicity
- ALK:
-
anaplastic lymphoma kinase
- AKT:
-
protein kinase B
- BIM:
-
BCL2-intearcting mediator of cell death
- CI:
-
confidence interval
- CNS:
-
central nervous system
- DOR:
-
duration of response
- ERK:
-
extracellular signal-related kinase
- EGF:
-
epidermal growth factor
- EGFR:
-
epidermal growth factor receptor
- Exon20ins:
-
exon 20 insertion mutations
- FDA:
-
federal drug administration
- HGF:
-
hepatocyte growth factor
- MAPK:
-
mitogen-activated protein kinase
- MET:
-
mesenchymal-epithelial transition factor
- NSCLC:
-
non-small cell lung cancer
- ORR:
-
overall response rate
- OS:
-
overall survival
- PFS:
-
progression free survival
- PI3K:
-
phosphatidylinositol 3 kinase
- TKI:
-
tyrosine kinase inhibitor
- STAT:
-
signal transducer and activator of transcription
References
Sung H, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Thai AA, et al. Lung cancer. The Lancet. 2021;398(10299):535–54.
Reck M, Rabe KF. Precision diagnosis and treatment for Advanced Non-small-cell Lung Cancer. N Engl J Med. 2017;377(9):849–61.
Kris MG, et al. Using multiplexed assays of oncogenic drivers in Lung Cancers to select targeted Drugs. JAMA. 2014;311(19):1998–2006.
Swanton C, Govindan R. Clinical implications of genomic discoveries in Lung Cancer. N Engl J Med. 2016;374(19):1864–73.
Sharma SV, et al. Epidermal growth factor receptor mutations in Lung cancer. Nat Rev Cancer. 2007;7(3):169–81.
Wee P, Wang Z. Epidermal growth factor receptor cell Proliferation Signaling pathways. Cancers. 2017;9(5):52.
Kumar A, et al. Structure and clinical relevance of the epidermal growth factor receptor in human cancer. J Clin Oncol. 2008;26(10):1742–51.
Eck MJ, Yun CH. Structural and mechanistic underpinnings of the differential drug sensitivity of EGFR mutations in non-small cell Lung cancer. Biochim Biophys Acta. 2010;1804(3):559–66.
Travis WD, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–60.
Zhang YL, et al. The prevalence of EGFR mutation in patients with non-small cell Lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48):78985–93.
Sholl LM, et al. Multi-institutional Oncogenic Driver Mutation Analysis in Lung Adenocarcinoma: the Lung Cancer Mutation Consortium Experience. J Thorac Oncol. 2015;10(5):768–77.
Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell Lung cancer. Sem Cancer Biol. 2020;61:167–79.
Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell Lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13(1):e23–31.
Yun J, et al. Antitumor Activity of Amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in Diverse models of. Cancer Discov. 2020;10(8):1194–209.
Vyse S, Huang PH. Targeting EGFR exon 20 insertion mutations in non-small cell Lung cancer. Signal Transduct Target Therapy. 2019;4(1):5.
Oxnard GR, et al. Natural history and molecular characteristics of Lung Cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8(2):179–84.
Gentile A, Trusolino L, Comoglio PM. The Met tyrosine kinase receptor in development and cancer. Cancer Metastasis Rev. 2008;27(1):85–94.
Zhang YW, et al. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc Natl Acad Sci U S A. 2003;100(22):12718–23.
Molnarfi N, et al. Hepatocyte growth factor: a regulator of inflammation and autoimmunity. Autoimmun Rev. 2015;14(4):293–303.
Glodde N, et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET Limit Cancer Immunotherapy. Immunity. 2017;47(4):789–802e9.
Liu X, et al. A novel kinase inhibitor, INCB28060, blocks c-MET-dependent signaling, neoplastic activities, and cross-talk with EGFR and HER-3. Clin Cancer Res. 2011;17(22):7127–38.
Baltschukat S, et al. Capmatinib (INC280) is active against models of Non-small Cell Lung Cancer and other Cancer types with defined mechanisms of MET activation. Clin Cancer Res. 2019;25(10):3164–75.
Frampton GM, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple Tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. 2015;5(8):850–9.
Plenker D, et al. Structural alterations of MET trigger response to MET kinase inhibition in Lung Adenocarcinoma patients. Clin Cancer Res. 2018;24(6):1337–43.
Ferguson SD, et al. Targetable Gene fusions Associate with the IDH Wild-Type Astrocytic Lineage in Adult Gliomas. J Neuropathol Exp Neurol. 2018;77(6):437–42.
Park M, et al. Mechanism of met oncogene activation. Cell. 1986;45(6):895–904.
Dagogo-Jack I, et al. MET alterations are a recurring and actionable resistance mechanism in ALK-Positive Lung Cancer. Clin Cancer Res. 2020;26(11):2535–45.
Awad MM, et al. Exon 14 mutations in Non-small-cell Lung Cancer are Associated with Advanced Age and Stage-Dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016;34(7):721–30.
Schrock AB, et al. Characterization of 298 patients with Lung Cancer Harboring MET exon 14 skipping alterations. J Thorac Oncol. 2016;11(9):1493–502.
Liu X, et al. Next-generation sequencing of Pulmonary Sarcomatoid Carcinoma reveals high frequency of actionable MET gene mutations. J Clin Oncol. 2016;34(8):794–802.
Saffroy R, et al. MET exon 14 mutations as targets in routine molecular analysis of primary sarcomatoid carcinoma of the lung. Oncotarget. 2017;8(26):42428–37.
Vuong HG, et al. Clinicopathological implications of MET exon 14 mutations in non-small cell Lung cancer - A systematic review and meta-analysis. Lung Cancer. 2018;123:76–82.
Drilon A, et al. Targeting MET in Lung Cancer: will expectations finally be MET? J Thorac Oncol. 2017;12(1):15–26.
Tsakonas G, et al. c-MET as a biomarker in patients with surgically resected non-small cell Lung cancer. Lung Cancer. 2019;133:69–74.
Bubendorf L, et al. Prevalence and clinical association of MET gene overexpression and amplification in patients with NSCLC: results from the European thoracic oncology platform (ETOP) lungscape project. Lung Cancer. 2017;111:143–9.
Castiglione R, et al. Comparison of the genomic background of MET-altered carcinomas of the lung: biological differences and analogies. Mod Pathol. 2019;32(5):627–38.
Tong JH, et al. Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non-small Cell Lung Carcinoma with Poor Prognosis. Clin Cancer Res. 2016;22(12):3048–56.
Yu HA, et al. Analysis of Tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant Lung Cancers. Clin Cancer Res. 2013;19(8):2240–7.
Chabon JJ, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in Lung cancer patients. Nat Commun. 2016;7(1):11815.
Ramalingam SS, et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol. 2018;29:viii740.
Caparica R, et al. Responses to Crizotinib can occur in high-level MET-Amplified Non-small Cell Lung Cancer Independent of MET exon 14 alterations. J Thorac Oncol. 2017;12(1):141–4.
Chu QS. Targeting non-small cell Lung cancer: driver mutation beyond epidermal growth factor mutation and anaplastic Lymphoma kinase fusion. Therapeutic Adv Med Oncol. 2020;12:1758835919895756.
Migliore C, et al. miR-205 mediates adaptive resistance to MET inhibition via ERRFI1 targeting and raised EGFR signaling. EMBO Mol Med. 2018;10(9):e8746.
Fujino T, et al. J Thorac Oncol. 2019;14(10):1753–65.
Turke AB, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17(1):77–88.
Engelman JA, et al. MET amplification leads to gefitinib resistance in Lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43.
Bean J, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104(52):20932–7.
Papadimitrakopoulou VA, et al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann Oncol. 2018;29:viii741.
Charakidis M, Boyer M, Targeting MET. EGFR in NSCLC—what can we learn from the recently reported phase III trial of onartuzumab in combination with erlotinib in advanced non-small cell Lung cancer? Translational Lung Cancer Research. 2014;3(6):395–6.
Spigel DR, et al. Randomized Phase II Trial of Onartuzumab in Combination with Erlotinib in patients with Advanced non–small-cell Lung Cancer. J Clin Oncol. 2013;31(32):4105–14.
Jarantow SW, et al. Impact of cell-surface Antigen expression on Target Engagement and function of an epidermal growth factor receptor נc-MET bispecific antibody *. J Biol Chem. 2015;290(41):24689–704.
Zheng S, et al. Cross-arm binding efficiency of an EGFR x c-Met bispecific antibody. MAbs. 2016;8(3):551–61.
Jo M, et al. Cross-talk between Epidermal Growth Factor Receptor and c-Met Signal pathways in transformed cells *. J Biol Chem. 2000;275(12):8806–11.
Ortiz-Zapater E, et al. MET-EGFR dimerization in lung adenocarcinoma is dependent on EGFR mtations and altered by MET kinase inhibition. PLoS ONE. 2017;12(1):e0170798.
Tang Z, et al. Dual MET–EGFR combinatorial inhibition against T790M-EGFR-mediated erlotinib-resistant Lung cancer. Br J Cancer. 2008;99(6):911–22.
Puri N, Salgia R. Synergism of EGFR and c-Met pathways, cross-talk and inhibition, in non-small cell Lung cancer. J Carcinog. 2008;7:9.
Wolf J, et al. Capmatinib in MET exon 14–Mutated or MET-Amplified non–small-cell Lung Cancer. N Engl J Med. 2020;383(10):944–57.
Paik PK, et al. Tepotinib in non–small-cell Lung Cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931–43.
Vijayaraghavan S, et al. Amivantamab (JNJ-61186372), an fc enhanced EGFR/cMet Bispecific Antibody, induces receptor downmodulation and antitumor activity by Monocyte/Macrophage trogocytosis. Mol Cancer Ther. 2020;19(10):2044–56.
Grugan KD, et al. Fc-mediated activity of EGFR x c-Met bispecific antibody JNJ-61186372 enhanced killing of Lung cancer cells. MAbs. 2017;9(1):114–26.
Park K, et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell Lung Cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391–402.
Moores SL, et al. A novel bispecific antibody targeting EGFR and cMet is effective against EGFR inhibitor-resistant lung tumors. Cancer Res. 2016;76(13):3942–53.
Neijssen J et al. Discovery of Amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET. J Biol Chem, 2021. 296.
Cho BC, et al. Amivantamab, an epidermal growth factor receptor (EGFR) and mesenchymal-epithelial transition factor (MET) Bispecific Antibody, designed to Enable multiple mechanisms of action and broad clinical applications. Clinical Lung Cancer; 2022.
Minchom A, et al. Amivantamab compared with real-world therapies in patients with advanced non-small cell Lung cancer harboring EGFR exon 20 insertion mutations who progressed after platinum-based chemotherapy. Lung Cancer. 2022;168:74–82.
Chouaid C, et al. An adjusted treatment comparison comparing Amivantamab Versus Real-World Clinical Practice in Europe and the United States for patients with Advanced Non-small Cell Lung Cancer with activating Epidermal Growth Factor Receptor Exon 20 insertion mutations. Adv Ther; 2023.
Du X et al. Acquired resistance to third-generation EGFR-TKIs and emerging next-generation EGFR inhibitors. The Innovation, 2021. 2(2).
Haura EB, et al. JNJ-61186372 (JNJ-372), an EGFR-cMet bispecific antibody, in EGFR-driven advanced non-small cell Lung cancer (NSCLC). American Society of Clinical Oncology; 2019.
Nagasaka M, et al. Amivantamab (JNJ-61186372) induces clinical, biochemical, molecular, and radiographic response in a treatment-refractory NSCLC patient harboring amplified triple EGFR mutations (L858R/ T790M/G796S) in cis. Lung Cancer. 2022;164:52–5.
Park K, et al. Management of infusion-related reactions (IRRs) in patients receiving amivantamab in the CHRYSALIS study. Lung Cancer. 2023;178:166–71.
Krebs M, et al. Amivantamab in patients with NSCLC with MET exon 14 skipping mutation: updated results from the CHRYSALIS study. J Clin Oncol. 2022;40(16suppl):9008–8.
Krebs MG, et al. Abstract CT198: subcutaneous delivery of amivantamab in patients with advanced solid malignancies: initial safety and pharmacokinetic results from the PALOMA study. Cancer Res. 2022;82(12Supplement):CT198–8.
Basse C, et al. Management of cutaneous toxicities under amivantamab (anti MET and anti EGFR bispecific antibody) in patients with metastatic non-small cell Lung cancer harboring EGFR Exon20ins: towards a proactive, multidisciplinary approach. Lung Cancer. 2022;173:116–23.
Belzer A et al. Spectrum of dermatologic adverse events Associated with Amivantamab Use. JAMA Dermatology, 2022.
Yu D, et al. Expression profiles of Tyrosine kinases in cultured follicular papilla cells < em > Versus dermal fibroblasts. J Invest Dermatology. 2004;123(2):283–90.
Lindner G, et al. Involvement of hepatocyte growth factor/scatter factor and met receptor signaling in hair follicle morphogenesis and cycling. FASEB J. 2000;14(2):319–32.
Lacouture ME, et al. Skin toxicity evaluation protocol with Panitumumab (STEPP), a phase II, Open-Label, Randomized Trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic Colorectal Cancer. J Clin Oncol. 2010;28(8):1351–7.
Shu CA, et al. Amivantamab and lazertinib in patients with EGFR-mutant non–small cell lung (NSCLC) after progression on osimertinib and platinum-based chemotherapy: updated results from CHRYSALIS-2. J Clin Oncol. 2022;40(16suppl):9006–6.
Robichaux JP, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell Lung cancer. Nat Med. 2018;24(5):638–46.
Kim TM, et al. Phase II study of osimertinib in NSCLC patients with EGFR exon 20 insertion mutation: a multicenter trial of the Korean Cancer Study Group (LU17-19). Ann Oncol. 2019;30:v628.
van Veggel B, et al. Osimertinib treatment for patients with EGFR exon 20 mutation positive non-small cell Lung cancer. Lung Cancer. 2020;141:9–13.
Girard N, et al. MA04.07 comparative clinical outcomes for patients with NSCLC Harboring EGFR exon 20 insertion mutations and common EGFR mutations. J Thorac Oncol. 2021;16(3):S145–6.
Ramalingam SS, et al. Overall survival with Osimertinib in untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020;382(1):41–50.
Zhou C, et al. OA04.03 mobocertinib in NSCLC with < em > EGFR exon 20 insertions: results from EXCLAIM and pooled platinum-pretreated patient populations. J Thorac Oncol. 2021;16(3):S108.
Cho BC, et al. 1258O Amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in combination with lazertinib, a 3rd-generation tyrosine kinase inhibitor (TKI), in advanced EGFR NSCLC. Ann Oncol. 2020;31:S813.
Leighl NB, et al. 1192MO Amivantamab monotherapy and in combination with lazertinib in post-osimertinib EGFR-mutant NSCLC: analysis from the CHRYSALIS study. Ann Oncol. 2021;32:S951–2.
Funding
No funding was secured for this report.
Author information
Authors and Affiliations
Contributions
DB provided clinical interpretation, drafted and reviewed all versions of the manuscript. MN provided clinical interpretation, drafted and reviewed all versions of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
COI disclosure: This manuscript did not receive any funding. There are no direct conflicts of interest to disclose. Dr Brazel has no potential conflicts to disclose. Dr Nagasaka has received consulting fees from Caris Life Sciences, honoraria from AstraZeneca, Daiichi Sankyo, Novartis, Lilly, Pfizer, EMD Serono, Genentech, Mirati, Takeda, Janssen, Blueprint Medicine, Regeneron and travel support from AnHeart Therapeutics.
Ethics approval and consent to participate
this report did not meet criteria for IRB approval.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Brazel, D., Nagasaka, M. The development of amivantamab for the treatment of non-small cell lung cancer. Respir Res 24, 256 (2023). https://doi.org/10.1186/s12931-023-02558-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-023-02558-4