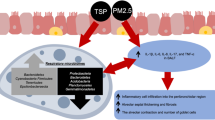
Abstract
As the public health burden of air pollution continues to increase, new strategies to mitigate harmful health effects are needed. Dietary antioxidants have previously been explored to protect against air pollution-induced lung injury producing inconclusive results. Inhaled (pulmonary or nasal) administration of antioxidants presents a more promising approach as it could directly increase antioxidant levels in the airway surface liquid (ASL), providing protection against oxidative damage from air pollution. Several antioxidants have been shown to exhibit antioxidant, anti-inflammatory, and anti-microbial properties in in vitro and in vivo models of air pollution exposure; however, little work has been done to translate these basic research findings into practice. This narrative review summarizes these findings and data from human studies using inhaled antioxidants in response to air pollution, which have produced positive results, indicating further investigation is warranted. In addition to human studies, cell and murine studies should be conducted using more relevant models of exposure such as air–liquid interface (ALI) cultures of primary cells and non-aqueous apical delivery of antioxidants and pollutants. Inhalation of antioxidants shows promise as a protective intervention to prevent air pollution-induced lung injury and exacerbation of existing lung disease.
Graphical Abstract

Similar content being viewed by others
Introduction
Long-term exposure to air pollution is associated with increased risk of cardiopulmonary and neurological diseases, cancer, and overall mortality [1]. While long-term exposure increases the risk of lung diseases such as chronic obstructive pulmonary disease (COPD) and asthma, especially in adolescents, short-term exposure can cause airway inflammation, hyperreactivity, decreased pulmonary function, susceptibility to microbial infection, and exacerbation of existing lung diseases. In 2019 the World Health Organization (WHO) found that 99% of the world population live in places where WHO air quality guidelines are not met, and caused an estimated 4.2 million premature deaths worldwide resulted from ambient air pollution, with low-and middle-income countries accounting for 89% of the estimated deaths [2]. Given the ever-increasing burden of air pollution, new strategies to mitigate its adverse health effects are needed. Further investigation into dietary and pharmacologic interventions to mitigate air pollution-induced adverse health effects may provide strategies for public health officials to overcome these challenges. One promising strategy is to increase the concentration of antioxidants (e.g., vitamin E, glutathione, etc.) in the lung, particularly at the surface of the respiratory epithelium, to counteract air pollution-induced oxidative stress. This narrative review will discuss the biochemical basis of this strategy, current research, and mixed results of studies using oral and dietary delivery of antioxidants. As future direction, we present evidence showing that treatment using several antioxidants through respiratory delivery, both intranasal and intrapulmonary, may offer improved success over oral and dietary delivery.
Composition of air pollutants and mechanisms of lung injury
Air pollution consists of gaseous components (ozone, volatile organic compounds, carbon monoxide, nitrogen oxides) and particulate matter (PM). PM is classified by particle size, ranging from ultrafine (PM0.1), fine (PM2.5), to coarse (PM10-2.5). PM0.1, particles with an aerodynamic equivalent diameter (AED) ≤ 0.1 µm, have high surface area, travel deep into the small airways, and can reach systemic circulation, making them especially harmful to inhale. In general, smaller particles (< 10 µm) can reach the lower airways and larger particles (> 10um) mostly deposit in the upper airways [3]. PM can be composed of metals, carbon, sulfates, nitrates, polycyclic aromatic hydrocarbons (PAHs), biological compounds, and can also form secondary PM through the nucleation and coagulation of gaseous pollutants onto primary PM [4]. Sources of primary PM include natural processes such as volcanic eruptions, wildfires, erosion, and through anthropogenic processes such as cigarette smoke, traffic, mining, construction, farming, power plants, and any process involving combustion of fuel [4].
Air pollution composition is heterogenous and varies across regions and with atmospheric aging; however, one of the unifying characteristics of inhaled PM and gases is their potential to cause oxidative stress in the airway epithelium [5]. Oxidative stress occurs when oxidation–reduction homeostasis is perturbed by an accumulation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) [6]. ROS and RNS are endogenously produced by inflammatory cells, during cellular respiration, and by enzymes, but are carefully controlled by antioxidant systems. Introduction of oxidants, free radicals, or redox catalysts through inhalation of air pollutants can overwhelm these systems, resulting in damage to DNA, membranes, and proteins via oxidation and eventually cytotoxicity [6]. Transition metals found in particulate matter are also capable of generating further oxidants through Fenton-like reactions [7].
Interactions of air pollutants with the respiratory mucosa
The respiratory system is a primary route of exposure to airborne environmental insults; as such, the respiratory mucosa has several mechanisms to protect against injury from inhaled toxicants. The initial line of protection is the airway surface liquid (ASL) layer, which acts as a physical barrier, helps expel pathogens through mucociliary clearance, and contains biochemical defenses [8]. ASL has two distinct physical layers, the superficial mucus layer, and the lower periciliary layer. The mucus layer traps inhaled pathogens with secreted mucins (MUC5AC, MUC5B) while the periciliary layer facilitates ciliary movement with the help of tethered mucins (MUC1, MUC4, MUC16) [9]. ASL also contains cytokines, antimicrobial peptides, antiviral interferons, leukocytes, and several types of antioxidants [10, 11].
High concentrations of antioxidants including antioxidant enzymes (dismutase, catalase, peroxidase, oxygenase) and small-molecule compounds (vitamin C, vitamin E, glutathione, uric acid, β-carotene) which act as free radical scavengers are present in the ASL [12]. Inhaled pollutants have been found to deplete ASL antioxidants and inhibit antioxidant enzymes, allowing for production of secondary oxidants through reaction with proteins, lipids, and carbohydrates in the ASL [13]. Because of this, it has been hypothesized that supplementation of ASL antioxidants through diet or inhalation could bolster antioxidant defenses and mitigate air-pollution induced oxidative stress.
Search strategy
The literature search was performed using Pubmed/MEDLINE and Google Scholar with no time frame restriction using the following antioxidant search terms: antioxidant, vitamin C, ascorbic acid, vitamin E, tocopherol, vitamin D, calciferol, glutathione, GSH, N-acetyl cysteine, NAC; respiratory system search terms: lung, airway, nasal, inhalation, bronchial; and pollutant search terms: pollutant, diesel exhaust particle, DEP, house dust mite, HDM, wood smoke, smoke, ozone, gas, particulate matter, PM. These terms were used in the following combinations: [antioxidant term] AND [respiratory system term] AND [pollutant term], and if few results were found including air pollutant search terms, [antioxidant term] AND [respiratory system term].
Scope of review
Given the topic of this review and the scarcity of literature on inhaled delivery of antioxidants, we chose to present this information as a narrative review. Introductory sections on air pollution and the respiratory mucosa are intended to provide succinct overviews of air pollution-induced pathological responses and justify the need for further investigation into inhaled supplementation of antioxidants. The discussion of human studies on oral supplementation was kept brief and largely summarized findings from previous reviews of the topic as the focus is on inhaled delivery. For antioxidants with few studies on protective effects against pollutants (vitamins C and E), studies of their effects on respiratory diseases, immune responses, and airway biology were included if links to air pollution exposure could be made.
Vitamin D
Vitamin D, or cholecalciferol, is a nutrient involved in calcium regulation and phosphate homeostasis, playing a role in immune and musculoskeletal health. Vitamin D is obtained through diet and cutaneous synthesis from ultraviolet B radiation, followed by metabolism to its active form, 1,25-dihydroxyvitamin D, or calcitriol. Both cholecalciferol and calcitriol will be referred to as vitamin D in this review as primary airway cells are able to metabolize cholecalciferol into the active form [14]. Studies in cell lines often use calcitriol, as cell lines such as A549 cells express low levels of 1α-hydroxylase (CYP27B1), the enzyme responsible for vitamin D activation, and are unable to convert cholecalciferol to its active form [14].
Several studies have explored co- or pre-treatment with vitamin D in response to various pollutants. In cigarette smoke (CS)-exposed 16HBE cells, vitamin D exhibited anti-inflammatory effects, but not in primary hBECs [15]. 16HBEs treated with lipopolysaccharide (LPS) had attenuated ROS and DNA damage when co-treated with vitamin D, a result confirmed in a murine model. Serré et al. found that in LPS-exposed mice with normal vitamin D levels, nebulized treatment with vitamin D reduced inflammatory cells in bronchoalveolar lavage fluid (BALF) and significantly protected against epithelial barrier damage [16]. This study also found that vitamin D inhalation did not change serum vitamin D levels, indicating that inhalation could provide local therapeutic effects and minimal system effects. This is one of the few studies to investigate aerosolization of vitamin D to protect against exposure-induced inflammation.
The protective effects of vitamin D are most apparent in PM exposure. Transcriptome analysis of primary hBECs exposed to urban PM saw reduced expression of IL6, a gene encoding a pro-inflammatory cytokine, and increased expression of G6PD, an antioxidant pathway gene, with vitamin D treatment [17]. The same study also observed reduced lipid oxidation, increased antioxidant response, and reduced levels of IL-6. An earlier study from the same group found that urban PM caused an increase in pro-inflammatory T helper 17 (TH17) cell response driven by IL-23 in a myeloid dendritic cell-memory CD4+ T cell co-culture system. Co-exposure with vitamin D was able to attenuate this effect through a reduction in IL-17a+ and IFN-γ+ cells [18]. Tao et al. investigated lung injury in an in vivo (intramuscular dosing of mice) and in vitro (BEAS-2B) model through exposure to silicon dioxide-containing PM [19]. Both models showed induction of autophagy via Nrf2 after vitamin D treatment. Similar results were produced in another study using undifferentiated hBECs exposed to PM, showing reduced inflammation through the p38/NF-κB/NLRP3 pathway [20]. Reduced inflammation was also demonstrated by Bolcas et al. using a murine model of co-exposure to diesel exhaust particles (DEP) and HDM [21]. In this model, treatment with vitamin D attenuated accumulation of TH17 and TH2 cells and development of airway hyperresponsiveness. Vitamin D has been found to have therapeutic effects in models of epithelial barrier dysfunction, characteristic of COPD, caused by cigarette smoke and toluene diisocyanate [22, 23]. Vitamin D also suppressed development of pulmonary emphysema, epithelial‑mesenchymal transition (EMT), and fibrogenesis, all of which are associated with the development of COPD and are exacerbated by toxicant exposure [24, 25]. Altogether, these data indicate vitamin D has possible utility in preventing air pollution-induced oxidative stress, immune responses, and microbial infection.
Of the antioxidants discussed in the review, vitamin D is the most well-studied in the airways due to its antibacterial and antiviral properties. Vitamin D has been found to significantly increase gene expression and protein levels of cathelicidin, an antimicrobial peptide, in airway cell lines, immune cells, and primary airway epithelial cells (bronchial and tracheobronchial) [26,27,28,29,30,31,32]. Vitamin D provided protection from infection to rhinovirus, respiratory syncytial virus (RSV), influenza, and mycobacterium tuberculosis through suppression of inflammation and altered expression of viral and bacterial receptors. However, there are conflicting results on the effect of vitamin D on viral replication, a more functional marker of antiviral properties, between undifferentiated vs. fully differentiated human bronchial epithelial cells (hBECs) through media dosing [33].
In addition to the study from Serré et al., two other studies have investigated inhaled vitamin D. The studies did not involve pollutant exposure and instead looked at the ability of vitamin D to enhance neonatal lung maturation and to promote anti-tumor immune activity, where it was also found that despite exhibiting therapeutic effects in the lung, treatment did not affect serum levels of vitamin D or calcium, indicating inhaled vitamin D most likely does not pass through the epithelial membrane or cause hypercalcemia [16, 34, 35]. Mathyssen et al. expanded on this by examining the transcriptional profile of vitamin D associated enzymes in lung tissue [36]. They found that CYP24A1, the inactivating enzyme of vitamin D, was highly expressed in lung endothelial cells, preventing circulating vitamin D from reaching the lungs. Conversely, this could also explain why inhaled vitamin D does not reach circulation. In the same study it was shown that the vitamin D receptor was expressed in apical epithelial cells, making it an ideal target for inhaled delivery.
It is important to note that the status of vitamin D as an antioxidant is controversial and could not be confirmed in a recent systematic review [37]. It is possible that the protective effects of vitamin D are not produced through scavenging of free radicals or induction of antioxidant enzymes and are instead due to interactions with the vitamin D receptor. However, the studies presented here indicate that vitamin D has antioxidant properties in the lung.
Glutathione
Glutathione (GSH) is a thiol tripeptide found at high concentrations in most cells and is the primary non-enzymatic antioxidant found in ASL [38]. The ratio of GSH, the reduced form of glutathione, to GSSG, the oxidized form, is a biomarker of cellular redox status, with the ratio dropping after exposure to oxidant stress [12, 39]. Notably, GSH is also the only antioxidant with a higher concentration in ASL than in plasma and is altered in several lung disease including cystic fibrosis (CF), idiopathic pulmonary fibrosis (IPF), and COPD [40]. Because of this, GSH inhalation has been evaluated as a treatment for these conditions.
A literature review looking specifically at inhaled GSH determined that it has potential as a treatment for cystic fibrosis (CF), chronic otitis media with effusion (OME), HIV seropositive individuals, IPF, and chronic rhinitis [40]. Randomized placebo-controlled trials found GSH improved oxygenation and clinical parameters in CF, although the effect may not be due to correction of oxidant/antioxidant balance. However, improvements in oxidant/antioxidant balance were observed in a nonrandomized IPF trial. Based on current evidence, inhaled GSH cannot be recommended for emphysema/COPD or asthma, the latter of which exhibited notable side effects (e.g., breathlessness, bronchoconstriction, cough), most likely due to co-occurrence of asthma and sulfite sensitivity. The author also recommended further research be conducted on other conditions linked to impaired antioxidant systems such as Farmer’s lung, multiple chemical sensitivity disorder, and exercise-induced oxidative stress. Although not mentioned, air pollution-induced lung injury, which occurs due to depletion of ASL antioxidants, could also potentially be inhibited by glutathione inhalation.
Several studies have explored the effects of polymorphisms in the genes encoding glutathione S-transferase (GST), an enzyme responsible for conjugation of GSH to xenobiotics, on air pollution exposure [41]. GSTM1 and GSTP1 polymorphisms have been associated with increased respiratory issues in response to ambient ozone and combined ragweed pollen/DEP exposure. The GSTM1 null-phenotype was also shown to regulate DEP-induced inflammation in vitro [42]. Jaspers et al. found that GSH-ethylester was able to reverse DEP-induced susceptibility to influenza infection in well-differentiated respiratory epithelial cells [43].
Given that GSH concentrations initially decrease and then recover in ASL after air pollution exposure, it can be concluded that GSH is vital for first line defense against inhaled oxidants [13]. Despite this, GSH treatment has been sparingly investigated for mitigating the effects of air pollution exposure. Glutamine, a glutathione precursor, supplementation has been explored as a therapeutic for several lung diseases, most notably acute respiratory distress syndrome (ARDS); and despite promising results through parenteral administration, few studies have explored inhaled supplementation [44].
N-acetylcysteine
N-acetylcysteine (NAC), another precursor to glutathione, is a prescription drug used to treat acetaminophen overdoses and as a mucolytic for muco-obstructive lung diseases [45]. NAC has direct antioxidant activity but also increases intracellular levels of cysteine which facilitates GSH synthesis [45]. NAC’s mucolytic properties stem from its the ability to break down disulfide bonds crosslinking mucus glycoproteins, reducing mucus viscosity and elasticity. This makes it an attractive option for inhaled treatment as it could potentially restore antioxidant capacity and alleviate mucus hypersecretion. Due to the number of benefits over glutathione, NAC has been investigated in several lung diseases and to protect against air pollution exposure.
Although taken orally for COPD, inhaled NAC has been approved for use in CF and has been shown to be effective as an adjunct therapy for IPF [46, 47]. In a controlled exposure of human subjects to DEP, oral NAC pre-treatment reduced airway responsiveness in hyperresponsive individuals [48]. However, as previously mentioned with vitamin C, NAC and vitamin C oral pre-treatment was found to augment DEP-induced vasoconstriction [49]. This indicates oral antioxidants may lead to unwanted systemic side effects in combination with air pollution exposure. No human studies were found using inhaled NAC to protect against air pollution; however, several in vitro cell culture and in vivo animal model studies have investigated the direct treatment of airway cells with NAC.
A recent study from Oh et al. explored using NAC-loaded microparticles to adsorb and remove particulate matter containing nitrates. The microparticles were found to effectively adsorb nitrate and were able to be cleared following intratracheal instillation in mice. The results from this study are promising and warrant further testing using more functional markers of protection (e.g., BALF inflammatory cells, oxidative stress, lung function). Therapeutic effects have also been reported in the context of ozone and nitrogen dioxide exposure. Intravenous NAC pretreatment was found to prevent ozone-induced mucociliary dysfunction in sheep, and post-exposure intraperitoneal NAC treatment reversed airway hyperresponsiveness in mice [50, 51]. NAC was also able to abrogate cytokine release caused by combined rhinovirus infection and oxidant gas exposure [52].
Despite seemingly positive results in the previously mentioned studies, a review from 2007 on the induction of antioxidant enzymes to protect against the adverse effects of DEPs briefly mentioned the idea of using inhaled NAC to protect against inhaled oxidants; however, they reported that preliminary studies from their laboratory using inhaled NAC did not see any protective effects against DEPs in an in vivo human nasal model [53]. Given no other information or data was provided, further investigation into this concept is warranted. Another study using well-differentiated primary hBECs from a COPD cohort at an air–liquid interface (ALI) found that basolateral treatment with NAC following DEP exposure did not provide any significant therapeutic effects, but did show trends in reducing IL-8 secretion and antioxidant gene expression [54]. Other studies of DEP and PM, mostly in in vivo murine models and submerged cell cultures, reported anti-inflammatory and antioxidant effects of NAC pre- and post-exposure treatment [55,56,57,58,59]. Similar effects were also seen in response to benzo[a]pyrene-induced acute lung injury [60].
Interestingly, in an in vitro experiment comparing several antioxidants, including vitamin C, vitamin E, and NAC, all three reduced protein and lipid peroxidation, but NAC was the only one that improved the ratio of reduced to oxidized glutathione [61]. Considering NAC directly contributes to production of glutathione, this result is not surprising. A more unbiased measure of protective effects could provide more insightful data such as a copper-based total antioxidant capacity or cellular ROS assay. The same study also found that NAC protected ovalbumin-sensitized mice against DEP exposure, although vitamin C and E were not examined in this experiment.
Melatonin
Melatonin is an endogenous hormone primarily synthesized in the pineal gland from tryptophan [62]. Melatonin biosynthesis is synchronized with the cycle of light and dark with serum melatonin concentrations following a circadian rhythm, playing a direct role in the body’s sleep cycle and thermoregulation [63]. Due to its antioxidant and anti-inflammatory properties, it has been investigated as a therapeutic for asthma, COPD, and allergic airway inflammation [63, 64].
Few human studies have been conducted on the effect of melatonin in the lungs. Cavalcante et al. conducted a randomized, double-blind, placebo-controlled study of oral melatonin in COPD patients which showed that melatonin administration reduced oxidative stress and relieved dyspnea (difficulty breathing) [65]. Notably, in a previous study using a similar dose and time of administration, melatonin improved sleep in moderate to severe COPD patients, with no detrimental effects on daytime alertness, lung function, and exercise capacity [66].
Although not as extensively studied as the previously mentioned antioxidants, melatonin has been shown to exhibit protective effects against PM and ozone. Intragastric melatonin alleviated PM2.5-induced lung injury, edema, ferroptosis, and lipid peroxidation through expression of Nrf2 [67]. Lee et al. found intraperitoneal melatonin protected against PM2.5 and acute ischemic reperfusion injury through reduction of oxidative stress, inflammation, and tracheal immune cell infiltration in mice [68]. Protective effects were also seen in PM2.5-exposed guinea pigs, along with a reduction of chronic cough following melatonin treatment [69]. In a murine model of ozone exposure, oral melatonin reduced oxidative stress and stabilized the Nrf2 pathway [70].
Melatonin has also shown promise in models of lung disease exacerbated by pollutant exposure. Two studies from Shin et al. have found that melatonin is able to attenuate MUC5AC secretion and gene expression in H292 cells and a murine model of asthma via intraperitoneal treatment [71, 72]. MUC5AC is one of the predominant mucins overexpressed in muco-obstructive lung diseases and in response to inhaled pollutants [73, 74]. The same group saw similar effects in vitro and in vivo in a cigarette smoke extract (CSE) and LPS model of COPD, along with suppression of pulmonary fibrosis [75]. This murine model was also used in a transcriptomic study of oral melatonin which found that melatonin alleviated lung damage and inflammation and reduced necroptosis [76]. Melatonin has also exhibited antiviral properties in various tissue. Huang et al. showed that oral melatonin inhibited lung oxidative stress, proinflammatory cytokine production, and inflammatory injury in RSV-infected mice [77].
Vitamin C
Vitamin C, or ascorbic acid, is a water-soluble nutrient vital for proper immune function that cannot be synthesized endogenously. As one of the antioxidants found in ASL, many early studies investigating the effect of diet on susceptibility to air pollution included vitamin C. Several epidemiologic and exposure chamber studies that analyzed ozone pollution in healthy and asthmatic subjects found that oral supplementation of vitamin cocktails containing vitamin C, E, and/or β-carotene above the daily minimum requirement may provide protection against ozone-induced decreases in lung function and bronchoconstriction [78]. However, an in vivo exposure chamber study of diesel exhaust found that vitamin C and N-acetylcysteine (NAC) supplementation increased vasoconstriction caused by exposure, and another study of acute exposure to ambient PM and/or ozone found no effects on cardiovascular outcomes. Most importantly, it has been shown that dietary vitamin C supplementation does not significantly affect ASL concentrations of ascorbic acid [79, 80]. Together, these data do not provide strong evidence for the use of dietary vitamin C supplementation to protect against cardiovascular and pulmonary injury induced by air pollution.
Preliminary investigations of vitamin C treatment prior to air pollution exposure in vitro have shown more promising results. Studies from Lee et al. in house dust mite (HDM) stimulated H292 cells, a pulmonary mucoepidermoid cell line, and Jin et al. in PM2.5 stimulated 16HBE cells found that co-exposure of HDM/PM2.5 with ascorbic acid reduced ROS levels and inhibited inflammatory responses [81, 82]. Despite little evidence of effectiveness through oral supplementation, the few in vitro studies of direct vitamin C treatment onto airway cells show possible antioxidant and antiviral properties.
Although there are few studies looking at vitamin C to protect against air pollution in vitro, several other studies exhibit relevant effects without pollutant exposure. A study in human nasal epithelial cells (hNECs) exhibited an initial increase in cilia beat frequency following vitamin C treatment [83]. This suggests that intranasal administration of vitamin C may be capable of improving mucociliary clearance of inhaled pollutants. Transcriptional analysis of vitamin C-treated BEAS-2B cells, a bronchial epithelial cell line, found that pathways associated with antiviral activity were upregulated while pathways associated with lung injury, inflammation, oxidative stress were downregulated [84]. In the same model it was found that treatment increased responses to polyinosinic:polycytidylic acid (poly I:C), an antiviral ligand, and type I interferons, further supporting the antiviral properties of vitamin C in airway cells and potentially providing a way to combat air pollution-induced dysregulation of antiviral immune responses.
Vitamin E
Vitamin E is a fat-soluble antioxidant used by humans primarily in the form of α-tocopherol as well as other tocopherols such as γ-tocopherol, and is exclusively obtained through diet [85]. In the context of the lungs, alpha-tocopherol has been most commonly studied as an inhibitor of allergic inflammation in allergies and asthma [86]. Dietary intervention studies using vitamin E almost all included vitamin C as mentioned previously, but in a controlled ozone exposure study of oral vitamin E alone, no significant changes in lung function were observed; however, vitamin E is one of the few antioxidants to have been investigated through in vivo aerosol delivery.
Gao et al. conducted a study pre-treating human subjects with an intranasally delivered cocktail of antioxidant oils (soy oil, coconut oil, orange oil, aloe vera oil, peppermint oil, and vitamin E oil) followed by acute ozone exposure [87]. Oil treatment was able to attenuate ozone-induced nasal inflammatory response and increase baseline levels of antioxidant gene HO-1 in the nasal mucosa. In the same study, the antioxidant oil was applied to a lung epithelial cell line, inducing expression of several antioxidant genes, activating NRF2, and mitigating pro-inflammatory endotoxin signaling.
Another study in mice saw similar upregulation of Nrf2 and Ho-1, as well as alleviation of ozone-induced lung injury and oxidative stress [88]. Vitamin E also exhibits protective effects against other pollutants such as Benzo[a]pyrene (BaP), acrolein, and aluminum nanopowder by preventing oxidative stress [89,90,91]. In addition to airway epithelial cells, vitamin E supplementation has been shown to prevent allergen-induced NRF2 suppression in asthmatic alveolar macrophages and reduced ozone-induced cell death in fibroblasts [92, 93]. Several murine studies have also found that dietary α-tocopherol can improve immune function, preventing lung injury and susceptibility to bacterial infection [94,95,96].
Limitations of current research
The majority of clinical trials and in vivo murine experiments investigating antioxidant treatment to protect against air pollution-induced lung injury use either dietary supplementation or systemic delivery of antioxidants. Aerosol delivery presents several benefits for this purpose. Inhalation avoids hepatic first-pass metabolism and the lungs have lower enzymatic activity than other organs [97]. This prevents premature drug degradation and allows for lower doses to be used for direct delivery to the lungs. Direct delivery and absorption also aid in preventing unwanted system effects. Inhaled delivery presents a more convenient and less invasive option than intravenous delivery while also allowing for more direct treatment than oral delivery.
Current literature is also limited to mostly submerged cell lines and murine experiments using non-inhalational routes o exposure. Submerged cell cultures lack many of the physiological features of the airways such as the over 40 different cell types and variations in epithelial thickness, among others [98]. Exposure also presents a challenge as normally air-borne particles and gases are added directly into the cell culture media allowing for agglomeration of particles, reaction with the media, and difficulty assessing dose. A more physiologically relevant model is the culturing of primary airway cells at an air–liquid interface using permeable membrane supports. Although immortalized cell lines can be cultured at an ALI, primary cells are able to differentiate, forming a pseudostratified epithelium. Several exposure systems now exist that allow for apical deposition of air pollution onto ALI cultures and should be used to validate past results from submerged systems. ALI cultures, combined with in vitro nebulizer systems would allow for the most relevant model of inhaled delivery and air pollutant exposure through apical delivery of both pollutants and antioxidant aerosols [99].
Similarly, with murine models, nose-only and whole-body inhalation chambers can be used to test antioxidant aerosol pre-treatment followed by air pollution exposure in a whole body gas or PM exposure chambers [100, 101]. Mouse models would also provide useful data on immune effects as well as other systemic effects of antioxidant inhalation, as evidenced by previous studies using vitamin D. However, mice have several key lung morphological and physiological differences compared to humans such as breathing pattern (mice are primarily nose-breathers), cell composition (mice largely lack submucosal glands except in the trachea), and eosinophil function and distribution [102]. The gold standard to evaluate the ability of antioxidant inhalation to protect against air pollution would be to conduct controlled studies in environmental exposure chambers with human subjects. Epidemiological cohort studies utilizing ambient air quality data and various antioxidant treatment groups could also provide useful data regarding chronic, low-dose exposures. The same methodology previously used for dietary antioxidant studies can be applied to inhaled antioxidants, although inter-subject differences in inhalation technique, breathing patterns, and variability in nebulizer output present unique challenges to aerosol treatment.
Considerations for inhaled antioxidant therapies
Regarding inhaled therapies, it is important to acknowledge that agents may have different effects when they are inhaled than when administered through oral or dermal routes of exposure. For example, flavoring compounds and humectants used in vaping products, although generally recognized as safe for oral consumption, can cause adverse effects when inhaled, especially following heated aerosolization and subsequent degradation/oxidation. Most notably is the example of inhaled vitamin E acetate, a compound safe for oral and dermal routes of exposures, but linked to e-cigarette or vaping use-associated lung injury (EVALI) as the potential causative component [103]. There are also examples of oral medications that when being used experimentally via inhalation cause significant toxicity, such as the death of a research participant following inhalation of hexamethonium [104]. Additionally, given the important role of redox homeostasis and the various beneficial roles of ROS/RNS in cellular function (signaling, oxidative burst, phagocytosis, mitogenic responses), antioxidant therapies are not guaranteed to produce positive results in human studies and may even result in detrimental effects [105,106,107,108]. For example, the Carotene and Retinol Efficacy Trial (CARET), a study of beta-carotene and retinyl palmitate supplementation, was stopped prematurely due to an increase in lung cancer risk and death from lung cancer in participants who received the intervention [109]. As such, critically evaluating local and system effects of inhaled antioxidant therapies is imperative.
Conclusions and future directions
Based on the current body of evidence, antioxidants have the potential to provide protection against air pollution. In addition to directly scavenging ROS, several of these antioxidants also exhibit anti-inflammatory and antimicrobial properties, indicating they could alleviate oxidative stress as well as susceptibility to viral and bacterial respiratory infections caused by exposure to air pollution. Despite mixed results from dietary antioxidant studies, a limited number of studies using intranasal and intrapulmonary delivery show that these routes may prove more effective. Additionally, inhaled delivery avoids first pass metabolism, provides direct treatment, and potentially limits systemic off-target effects making it ideal for pulmonary protection [97]. Future clinical work should be done to examine the effects of inhaled antioxidants on air pollution exposure in controlled environmental exposure chamber studies and cohort studies using ambient air conditions. Additional in vitro work should be done to elucidate mechanisms of these antioxidants, especially in well-differentiated primary airway cells at an ALI as many previous studies were in cell lines and undifferentiated primary cells. Primary airway cells at ALI can also use cells from specific donors, such as asthmatics, which would provide information on protective effects for susceptible populations. In summary, antioxidant inhalation has promise as a possible preventative intervention for people with lung diseases like COPD, asthma, and cystic fibrosis, in which ASL antioxidant composition is altered and therefore warrants further investigation.
Availability of data and materials
Not applicable.
Abbreviations
- ASL:
-
Airway surface liquid
- ALI:
-
Air–liquid interface
- COPD:
-
Chronic obstructive pulmonary disease
- WHO:
-
World health Organization
- PM:
-
Particulate Matter
- AED:
-
Aerodynamic equivalent diameter
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- NAC:
-
N-acetylcysteine
- hNEC:
-
Human nasal epithelial cell
- poly I:C:
-
Polyinosinic:polycytidylic acid
- HDM:
-
House dust mite
- BaP:
-
Benzo[a]pyrene
- CYP27B1:
-
1α-Hydroxylase
- RSV:
-
Respiratory syncytial virus
- hBEC:
-
Human bronchial epithelial cell
- LPS:
-
Lipopolysaccharide
- BALF:
-
Bronchoalveolar lavage fluid
- TH17:
-
T helper 17
- DEP:
-
Diesel exhaust particle
- EMT:
-
Epithelial-mesenchymal transition
- CSE:
-
Cigarette smoke extract
- GSH/GSSG:
-
Glutathione (reduced/oxidized)
- CF:
-
Cystic fibrosis
- IPF:
-
Idiopathic pulmonary fibrosis
References
Yang W. Air pollutants, oxidative stress and human health. Mutation Res Genetic Toxicol Environ Mutagenesis (MRGTEM). 2009;674:45.
Ambient (outdoor) air pollution who.int: World Health Organization (WHO); Available from: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health.
Glencrossab DA, Ho T-R, Camiñab N, Hawrylowicza CM, Pfeffer PE. Air pollution and its effects on the immune system. Free Radic Biol Med. 2020;151:56–68.
Falcon-Rodriguez CI, Osornio-Vargas AR, Sada-Ovalle I, Segura-Medina P. Aeroparticles, composition, and lung diseases. Front Immunol. 2016.
Mudway IS, Kelly FJ, Holgate ST. Oxidative stress in air pollution research. Free Radic Biol Med. 2020;151:2–6.
Huff RD, Carlsten C, Hirota JA. An update on immunologic mechanisms in the respiratory mucosa in response to air pollutants. J Allergy Clin Immunol. 2019;143(6):1989–2001.
Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011: 487074.
Aghapour M, Ubags ND, Bruder D, Hiemstra PS, Sidhaye V, Rezaee F, et al. Role of air pollutants in airway epithelial barrier dysfunction in asthma and COPD. Eur Respir Rev. 2022;31(163): 210112.
Hill DB, Button B, Rubinstein M, Boucher RC. Physiology and pathophysiology of human airway mucus. Physiol Rev. 2022;102(4):1757–836.
Hiemstra PS, McCray PB, Bals R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur Respir J. 2015;45(4):1150–62.
Lewis BW, Patial S, Saini Y. Immunopathology of airway surface liquid dehydration disease. J Immunol Res. 2019;2019:1–16.
Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533(1–3):222–39.
Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: a review. Eur Respir J. 2008;31(1):179–97.
Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense1. J Immunol. 2008;181(10):7090–9.
Mathyssen C, Serré J, Sacreas A, Everaerts S, Maes K, Verleden S, et al. Vitamin D modulates the response of bronchial epithelial cells exposed to cigarette smoke extract. Nutrients. 2019;11(9):2138.
Serré J, Mathyssen C, Ajime TT, Heigl T, Verlinden L, Maes K, et al. Local nebulization of 1α,25(OH)2D3 attenuates LPS-induced acute lung inflammation. Respir Res. 2022;23(1).
Pfeffer PE, Lu H, Mann EH, Chen Y-H, Ho T-R, Cousins DJ, et al. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS ONE. 2018;13(8): e0200040.
Mann EH, Ho TR, Pfeffer PE, Matthews NC, Chevretton E, Mudway I, et al. Vitamin D counteracts an IL-23-dependent IL-17A(+)IFN-gamma(+) response driven by urban particulate matter. Am J Respir Cell Mol Biol. 2017;57(3):355–66.
Tao S, Zhang H, Xue L, Jiang X, Wang H, Li B, et al. Vitamin D protects against particles-caused lung injury through induction of autophagy in an Nrf2-dependent manner. Environ Toxicol. 2019;34(5):594–609.
Xin L, Che B, Zhai B, Luo Q, Zhang C, Wang J, et al. 1,25-Dihydroxy vitamin D3 attenuates the oxidative stress-mediated inflammation induced by PM2.5via the p38/NF-kappaB/NLRP3 pathway. Inflammation. 2019;42(2):702–13.
Bolcas PE, Brandt EB, Zhang Z, Biagini Myers JM, Ruff BP, Khurana Hershey GK. Vitamin D supplementation attenuates asthma development following traffic-related particulate matter exposure. J Allergy Clin Immunol. 2019;143(1):386-94.e3.
Li W, Dong H, Zhao H, Song J, Tang H, Yao L, et al. 1,25-Dihydroxyvitamin D3 prevents toluene diisocyanate-induced airway epithelial barrier disruption. Int J Mol Med. 2015;36(1):263–70.
Zhang R, Zhao H, Dong H, Zou F, Cai S. 1α,25-dihydroxyvitamin D3 counteracts the effects of cigarette smoke in airway epithelial cells. Cell Immunol. 2015;295(2):137–43.
Ricca C, Aillon A, Viano M, Bergandi L, Aldieri E, Silvagno F. Vitamin D inhibits the epithelial-mesenchymal transition by a negative feedback regulation of TGF-β activity. J Steroid Biochem Mol Biol. 2019;187:97–105.
Hu G, Dong T, Wang S, Jing H, Chen J. Vitamin D3-vitamin D receptor axis suppresses pulmonary emphysema by maintaining alveolar macrophage homeostasis and function. EBioMedicine. 2019;45:563–77.
Crane-Godreau MA, Clem KJ, Payne P, Fiering S. Vitamin D deficiency and air pollution exacerbate COVID-19 through suppression of antiviral peptide LL37. Front Public Health. 2020;8:232.
Dhawan P, Wei R, Sun C, Gombart AF, Koeffler HP, Diamond G, et al. C/EBPα and the vitamin D receptor cooperate in the regulation of cathelicidin in lung epithelial cells. J Cell Physiol. 2015;230(2):464–72.
Greiller CL, Suri R, Jolliffe DA, Kebadze T, Hirsman AG, Griffiths CJ, et al. Vitamin D attenuates rhinovirus-induced expression of intercellular adhesion molecule-1 (ICAM-1) and platelet-activating factor receptor (PAFR) in respiratory epithelial cells. J Steroid Biochem Mol Biol. 2019;187:152–9.
Telcian AG, Zdrenghea MT, Edwards MR, Laza-Stanca V, Mallia P, Johnston SL, et al. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral Res. 2017;137:93–101.
Vargas Buonfiglio LG, Cano M, Pezzulo AA, Vanegas Calderon OG, Zabner J, Gerke AK, et al. Effect of vitamin D(3) on the antimicrobial activity of human airway surface liquid: preliminary results of a randomised placebo-controlled double-blind trial. BMJ Open Respir Res. 2017;4(1): e000211.
Wang T-T, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression1. J Immunol. 2004;173(5):2909–12.
Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D3. J Cyst Fibros. 2007;6(6):403–10.
Gayan‐Ramirez G, Janssens W. Vitamin D actions: the lung is a major target for vitamin D, <scp>FGF23</scp> , and Klotho. JBMR Plus. 2021;5(12).
Taylor SK, Sakurai R, Sakurai T, Rehan VK. Inhaled vitamin D: a novel strategy to enhance neonatal lung maturation. Lung. 2016;194(6):931–43.
Bianchi F, Sommariva M, Le Noci V, Camelliti S, Gagliano N, Giussani M, et al. Aerosol 1,25-dihydroxyvitamin D3 supplementation: a strategy to boost anti-tumor innate immune activity. PLoS ONE. 2021;16(3): e0248789.
Mathyssen C, Aelbrecht C, Serré J, Everaerts S, Maes K, Gayan-Ramirez G, et al. Local expression profiles of vitamin D-related genes in airways of COPD patients. Respir Res. 2020;21(1):137.
Tagliaferri S, Porri D, De Giuseppe R, Manuelli M, Alessio F, Cena H. The controversial role of vitamin D as an antioxidant: results from randomised controlled trials. Nutr Res Rev. 2019;32(1):99–105.
Pizzorno J. Glutathione! Integr Med (Encinitas). 2014;13(1):8–12.
Kelly FJ. Gluthathione: in defence of the lung. Food Chem Toxicol. 1999;37(9):963–6.
Prousky J. The treatment of pulmonary diseases and respiratory-related conditions with inhaled (nebulized or aerosolized) glutathione. Evid-Based Complementary Altern Med. 2008;5: 975283.
Kelly FJ, Fussell JC. Air pollution and airway disease. Clin Exp Allergy. 2011;41(8):1059–71.
Wu W, Peden DB, McConnell R, Fruin S, Diaz-Sanchez D. Glutathione-S-transferase M1 regulation of diesel exhaust particle-induced pro-inflammatory mediator expression in normal human bronchial epithelial cells. Part Fibre Toxicol. 2012;9:31.
Jaspers I, Ciencewicki JM, Zhang W, Brighton LE, Carson JL, Beck MA, et al. Diesel exhaust enhances influenza virus infections in respiratory epithelial cells. Toxicol Sci. 2005;85(2):990–1002.
Oliveira GP, De Abreu MG, Pelosi P, Rocco PRM. Exogenous glutamine in respiratory diseases: myth or reality? Nutrients [Internet]. 2016;8(2):76.
Tenório M, Graciliano NG, Moura FA, Oliveira ACM, Goulart MOF. N-Acetylcysteine (NAC): impacts on human health. Antioxidants (Basel). 2021;10(6):967.
Feng F, Zhang J, Wang Z, Wu Q, Zhou X. Efficacy and safety of N-acetylcysteine therapy for idiopathic pulmonary fibrosis: an updated systematic review and meta-analysis. Exp Ther Med. 2019;18(1):802–16.
Ciofu O, Smith S, Lykkesfeldt J. Antioxidant supplementation for lung disease in cystic fibrosis. Cochrane Database Syst Rev. 2019;10(10):CD007020.
Carlsten C, MacNutt MJ, Zhang Z, Sava F, Pui MM. Anti-oxidant N-acetylcysteine diminishes diesel exhaust-induced increased airway responsiveness in person with airway hyper-reactivity. Toxicol Sci. 2014;139(2):479–87.
Sack CS, Jansen KL, Cosselman KE, Trenga CA, Stapleton PL, Allen J, et al. Pretreatment with antioxidants augments the acute arterial vasoconstriction caused by diesel exhaust inhalation. Am J Respir Crit Care Med. 2016;193(9):1000–7.
Li F, Wiegman C, Seiffert JM, Zhu J, Clarke C, Chang Y, et al. Effects of N-acetylcysteine in ozone-induced chronic obstructive pulmonary disease model. PLoS ONE. 2013;8(11): e80782.
Allegra L, Moavero NE, Rampoldi C. Ozone-induced impairment of mucociliary transport and its prevention with N-acetylcysteine. Am J Med. 1991;91(3):S67–71.
Spannhake EW, Reddy SPM, Jacoby DB, Yu X-Y, Saatian B, Tian J. Synergism between rhinovirus infection and oxidant pollutant exposure enhances airway epithelial cell cytokine production. Environ Health Perspect. 2002;110(7):665–70.
Wan J, Diaz-Sanchez D. Antioxidant enzyme induction: a new protective approach against the adverse effects of diesel exhaust particles. Inhalation Toxicol. 2007;19(sup1):177–82.
Vaughan A, Stevanovic S, Jafari M, Rahman M, Bowman RV, Fong KM, et al. The effect of diesel emission exposure on primary human bronchial epithelial cells from a COPD cohort: N-acetylcysteine as a potential protective intervention. Environ Res. 2019;170:194–202.
Rhoden CR. N-acetylcysteine prevents lung inflammation after short-term inhalation exposure to concentrated ambient particles. Toxicol Sci. 2004;79(2):296.
Wang J, Guo Z, Zhang R, Han Z, Huang Y, Deng C, et al. Effects of N-acetylcysteine on oxidative stress and inflammation reactions in a rat model of allergic rhinitis after PM2.5 exposure. Biochem Biophys Res Commun. 2020;533(3):275–81.
Yan Z, Wang J, Li J, Jiang N, Zhang R, Yang W, et al. Oxidative stress and endocytosis are involved in upregulation of interleukin-8 expression in airway cells exposed to PM2.5. Environ Toxicol. 2016;31(12):1869–78.
Hashimoto S, Gon Y, Takeshita I, Matsumoto K, Jibiki I, Takizawa H, et al. Diesel exhaust particles activate p38 MAP kinase to produce interleukin 8 and RANTES by human bronchial epithelial cells and N-acetylcysteine attenuates p38 MAP kinase activation. Am J Respir Crit Care Med. 2000;161(1):280–5.
Wang J, Huang J, Wang L, Chen C, Yang D, Jin M, et al. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-κB signaling pathway. J Thorac Dis. 2017;9(11):4398–412.
Zhao H, Fu L, Xiang H-X, Xiang Y, Li M-D, Lv B-B, et al. N-acetylcysteine alleviates pulmonary inflammatory response during benzo[a]pyrene-evoked acute lung injury. Environ Sci Pollut Res. 2022;29(3):3474–86.
Whitekus MJ, Li N, Zhang M, Wang M, Horwitz MA, Nelson SK, et al. Thiol antioxidants inhibit the adjuvant effects of aerosolized diesel exhaust particles in a murine model for ovalbumin sensitization. J Immunol. 2002;168(5):2560–7.
Melatonin Pharmacology, Functions and Therapeutics.
Melatonin, Circadian Rhythms, and Sleep.
Wang W, Gao J. Effects of melatonin on protecting against lung injury (Review). Exp Ther Med. 2021;21(3):228.
de Matos Cavalcante AG, de Bruin PF, de Bruin VM, Nunes DM, Pereira ED, Cavalcante MM, et al. Melatonin reduces lung oxidative stress in patients with chronic obstructive pulmonary disease: a randomized, double-blind, placebo-controlled study. J Pineal Res. 2012;53(3):238–44.
Nunes DM, Mota RMS, Machado MO, Pereira EDB, de Bruin VMS, de Bruin PFC. Effect of melatonin administration on subjective sleep quality in chronic obstructive pulmonary disease. Braz J Med Biol Res. 2008;41:926.
Guohua F, Tieyuan Z, Xinping M, Juan X. Melatonin protects against PM2.5-induced lung injury by inhibiting ferroptosis of lung epithelial cells in a Nrf2-dependent manner. Ecotoxicol Environ Saf. 2021;223:112588.
Lee FY, Lee MS, Wallace CG, Huang CR, Chu CH, Wen ZH, et al. Short-interval exposure to ambient fine particulate matter (PM2.5) exacerbates the susceptibility of pulmonary damage in setting of lung ischemia-reperfusion injury in rodent: pharmacomodulation of melatonin. Biomed Pharmacother. 2019;113:108737.
Ji Z, Wang Z, Chen Z, Jin H, Chen C, Chai S, et al. Melatonin attenuates chronic cough mediated by oxidative stress via transient receptor potential melastatin-2 in guinea pigs exposed to particulate matter 2.5. Physiol Res. 2018;67(2):293–305.
Chen Y, Wu X, Yang X, Liu X, Zeng Y, Li J. Melatonin antagonizes ozone-exacerbated asthma by inhibiting the TRPV1 channel and stabilizing the Nrf2 pathway. Environ Sci Pollut Res. 2021;28(42):59858–67.
Shin I-S, Park J-W, Shin N-R, Jeon C-M, Kwon O-K, Lee M-Y, et al. Melatonin inhibits MUC5AC production via suppression of MAPK signaling in human airway epithelial cells. J Pineal Res. 2014;56(4):398–407.
Shin IS, Shin NR, Park JW, Jeon CM, Hong JM, Kwon OK, et al. Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling. J Pineal Res. 2015;58(1):50–60.
Abnormalities in MUC5AC and MUC5B Protein in Airway Mucus in Asthma.
Bonser LR, Erle DJ. Airway mucus and asthma: the role of MUC5AC and MUC5B. J Clin Med. 2017;6(12):112.
Melatonin suppresses fibrotic responses induced by cigarette smoke via downregulation of TGF-β1.
Mao K, Luo P, Geng W, Xu J, Liao Y, Zhong H, et al. An integrative transcriptomic and metabolomic study revealed that melatonin plays a protective role in chronic lung inflammation by reducing necroptosis. Front Immunol. 2021;12: 668002.
Huang SH, Cao XJ, Liu W, Shi XY, Wei W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J Pineal Res. 2010;48(2):109–16.
Tong H. Dietary and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim Biophys Acta. 2016;1860(12):2891–8.
Behndig AF, Blomberg A, Helleday R, Kelly FJ, Mudway IS. Augmentation of respiratory tract lining fluid ascorbate concentrations through supplementation with vitamin C. Inhalation Toxicol. 2009;21(3):250–8.
Schock BC, Koostra J, Kwack S, Hackman RM, van der Vliet A, Cross CE. Ascorbic acid in nasal and tracheobronchial airway lining fluids. Free Radical Biol Med. 2004;37(9):1393–401.
Lee AJ, Lim JW, Kim H. Ascorbic acid suppresses house dust mite-induced expression of interleukin-8 in human respiratory epithelial cells. Journal of Cancer Prevention. 2021;26(1):64–70.
Jin X, Su R, Li R, Song L, Chen M, Cheng L, et al. Amelioration of particulate matter-induced oxidative damage by vitamin c and quercetin in human bronchial epithelial cells. Chemosphere. 2016;144:459–66.
Jiao J, Meng N, Wang H, Zhang L. The effects of vitamins C and B12 on human nasal ciliary beat frequency. BMC Complement Altern Med. 2013;13(1):110.
Teafatiller T, Agrawal S, De Robles G, Rahmatpanah F, Subramanian VS, Agrawal A. Vitamin C enhances antiviral functions of lung epithelial cells. Biomolecules. 2021;11(8):1148.
Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radical Biol Med. 2007;43(1):4–15.
Cook-Mills JM, Averill SH, Lajiness JD. Asthma, allergy and vitamin E: Current and future perspectives. Free Radic Biol Med. 2022;179:388–402.
Gao M, Singh A, Macri K, Reynolds C, Singhal V, Biswal S, et al. Antioxidant components of naturally-occurring oils exhibit marked anti-inflammatory activity in epithelial cells of the human upper respiratory system. Respir Res. 2011;12(1):92.
Zhu Y, Li J, Wu Z, Lu Y, You H, Li R, et al. Acute exposure of ozone induced pulmonary injury and the protective role of vitamin E through the Nrf2 pathway in Balb/c mice. Toxicol Res. 2016;5(1):268–77.
Nardinia M, Finkelstein EI, Reddy S, Valacchi G, Traber M, Cross CE, et al. Acrolein-induced cytotoxicity in cultured human bronchial epithelial cells. Modulation by alpha-tocopherol and ascorbic acid. Toxicology.
Cui H, Huang J, Lu M, Zhang Q, Qin W, Zhao Y, et al. Antagonistic effect of vitamin E on nAl2O3-induced exacerbation of Th2 and Th17-mediated allergic asthma via oxidative stress. Environ Pollut. 2019;252(Pt B):1519–31.
Zhu W, Cromie MM, Cai Q, Lv T, Singh K, Gao W. Curcumin and vitamin E protect against adverse effects of benzo[a]pyrene in lung epithelial cells. PLoS ONE. 2014;9(3): e92992.
Konings AWT. Mechanisms of ozone toxicity in cultured cells. I. Reduced clonogenic ability of polyunsaturated fatty acid-supplemented fibroblasts. Effect of Vitamin E. J Toxicol Environ Health. 1986;18(3):491–7.
Dworski R, Han W, Blackwell TS, Hoskins A, Freeman ML. Vitamin E prevents NRF2 suppression by allergens in asthmatic alveolar macrophages in vivo. Free Radical Biol Med. 2011;51(2):516–21.
Bou Ghanem EN, Clark S, Du X, Wu D, Camilli A, Leong JM, et al. The α-tocopherol form of vitamin E reverses age-associated susceptibility to streptococcus pneumoniae lung infection by modulating pulmonary neutrophil recruitment. J Immunol. 2015;194(3):1090–9.
Zheng K-C, Shinjo M, Todoriki H, Ariizumi M, Shinjo S, Adjei AA. Effect of dietary vitamin E supplementation on murine nasal allergy. Am J Med Sci. 1999;318(1):49–54.
Cook-Mills J, Gebretsadik T, Abdala-Valencia H, Green J, Larkin EK, Dupont WD, et al. Interaction of vitamin E isoforms on asthma and allergic airway disease. Thorax. 2016;71(10):954.
Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. The Lancet. 2011;377(9770):1032–45.
Upadhyay S, Palmberg L. Air-liquid interface: relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol Sci. 2018;164(1):21–30.
Horstmann JC, Thorn CR, Carius P, Graef F, Murgia X, de Souza C-W, et al. A custom-made device for reproducibly depositing pre-metered doses of nebulized drugs on pulmonary cells in vitro. Front Bioeng Biotechnol. 2021;9: 643491.
Oldham MJ, Phalen RF, Robinson RJ, Kleinman MT. Performance of a portable whole-body mouse exposure system. Inhal Toxicol. 2004;16(9):657–62.
Smith GJ, Walsh L, Higuchi M, Kelada SNP. Development of a large-scale computer-controlled ozone inhalation exposure system for rodents. Inhalation Toxicol. 2019;31(2):61–72.
Finkelman FD, Wills-Karp M. Usefulness and optimization of mouse models of allergic airway disease. J Allergy Clin Immunol. 2008;121(3):603–6.
Gordon T, Karey E, Rebuli ME, Escobar Y-NH, Jaspers I, Chen LC. E-Cigarette toxicology. Annu Rev Pharmacol Toxicol. 2022;62(1):301–22.
Savulescu J, Spriggs M. The hexamethonium asthma study and the death of a normal volunteer in research. J Med Ethics. 2002;28(1):3.
Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discovery. 2021;20(9):689–709.
Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89–96.
Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30(1):11–26.
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84.
Didier AJ, Stiene J, Fang L, Watkins D, Dworkin LD, Creeden JF. Antioxidant and anti-tumor effects of dietary vitamins A, C, and E. Antioxidants [Internet]. 2023;12(3):632.
Acknowledgements
We are grateful for the helpful editorial suggestion and input from Drs. Adam Speen and Philip Bromberg.
Funding
The authors received funding from the following Grants: T32 ES007126 (KDS), R01 ES031173 (IJ), R01 ES028269 (IJ).
Author information
Authors and Affiliations
Contributions
KDS wrote the main manuscript text and GJS provided significant input. IJ reviewed and edited the manuscript and approved the final version. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None of the authors have any competing financial or non-financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schichlein, K.D., Smith, G.J. & Jaspers, I. Protective effects of inhaled antioxidants against air pollution-induced pathological responses. Respir Res 24, 187 (2023). https://doi.org/10.1186/s12931-023-02490-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-023-02490-7