Abstract
Background
Airway fibrosis is one of the pathological characteristics of severe asthma. Transforming growth factor (TGF)-β has been known to promote epithelial-mesenchymal transition formation and to play a role in the progression of tissue fibrosis. Cellular communication network factor 2 (CCN2) and fibronectin (FN) are well-known markers of EMT and fibrosis. However, whether AREG is involved in TGF-β-induced CCN2 and FN expression in human lung epithelial cells is unknown.
Methods
AREG and FN were analyzed by immunofluorescence staining on ovalbumin-challenged mice. CCN2 and FN expression were evaluated in human lung epithelial (A459) cells following TGF or AREG treatment for the indicated times. Secreted AREG from A549 cells was detected by ELISA. Cell migration was observed by a wound healing assay. Chromatin immunoprecipitation was used to detect the c-Jun binding to the CCN2 promoter.
Results
AREG and FN expression colocalized in lung tissues from mice with ovalbumin-induced asthma by immunofluorescence staining. Moreover, TGF-β caused the release of AREG from A549 cells into the medium. Smad3 siRNA down-regulated AREG expression. AREG also stimulated CCN2 and FN expression, JNK and c-Jun phosphorylation, and cell migration in A549 cells. AREG small interfering (si) RNA inhibited TGF-β-induced expression of CCN2, FN, and cell migration. Furthermore, AREG-induced CCN2 and FN expression were inhibited by EGFR siRNA, a JNK inhibitor (SP600125), and an activator protein-1 (AP-1) inhibitor (curcumin). EGFR siRNA attenuated AREG-induced JNK and c-Jun phosphorylation. Moreover, SP600125 downregulated AREG-induced c-Jun phosphorylation.
Conclusion
These results suggested that AREG mediates the TGF-β-induced EMT in human lung epithelial cells through EGFR/JNK/AP-1 activation. Understanding the role of AREG in the EMT could foster the development of therapeutic strategies for airway remodeling in severe asthma.
Similar content being viewed by others
Introduction
Asthma affected about 262 million people and caused 461,000 deaths around the world in 2019 [1]. Airway remodeling, inflammation, and lung fibrosis are common pathological features of severe asthma [2, 3]. Lung fibrosis results in fibroblast proliferation, extracellular matrix (ECM) deposition (e.g., fibronectin (FN) and collagen), and alveolar epithelial-mesenchymal transition (EMT) [4]. Moreover, transforming growth factor (TGF)-β is a well-known cytokine in the process of airway inflammation and remodeling, as well as EMT [4, 5]. However, there are few therapeutic strategies for the alveolar EMT in severe asthma.
Amphiregulin (AREG) is a membrane protein that is a member of the epidermal growth factor (EGF) family. In addition, AREG can be proteolyzed by a disintegrin and metalloprotease 17 (ADAM17), which results in a soluble form of the ligand [6,7,8]. Soluble AREG acts as a ligand for the EGF receptor (EGFR) and is responsible for cell survival, growth, and migration [7, 9]. Previous studies showed that AREG participates in inflammation and injury in different tissues such as the lungs, liver, and intestines [10,11,12]. AREG was found to lead to the proliferation of airway epithelial cells and airway smooth muscle cells through EGFR activation, thereby affecting airway remodeling in severe asthma [13, 14].
EMT is a process of epithelial cell transition into mesenchymal cells in tissue injury that leads to organ fibrosis [15]. Fibroblast-like mesenchymal cells can express FN, collagens, and α-smooth muscle actin (α-SMA) as EMT markers [15, 16]. Previous studies have shown that TGF-β can induce EMT by activating either the classical SMAD 2/3 or a non-classical signaling pathway [17, 18]. Increasing evidence has revealed that EMT of alveolar epithelial cells can be observed in lung fibrosis [4, 15]. However, the role of AREG in the EMT of alveolar tissues is still unknown.
Cellular communication network factor 2 (CCN2), a fibrotic marker, is a well-known mediator of tissue remodeling and fibrosis [19]. Numerous studies have reported that increased CCN2 can induce ECM deposition, myofibroblast differentiation, and EMT [20,21,22]. CCN2 expression has been demonstrated to be increased by several stimulators such as TGF-β, hypoxia, and vascular endothelial growth factor in lung fibroblasts [19, 21, 23]. Thus, CCN2 plays a crucial role during tissue fibrosis or EMT. Nonetheless, the mechanism of AREG-induced CCN2 expression has yet to be identified.
Activator protein (AP)-1, a c-Jun/c-Fos complex, is a c-Jun N-terminal kinase (JNK) downstream transcription factor. The JNK/AP-1 pathway is involved in regulating cell proliferation, differentiation, survival, and apoptosis [24, 25]. Our previous study showed that JNK and AP-1 participate in lung fibrosis [21]. Also, AREG can activate the phosphorylation of mitogen-activated protein kinase (MAPK) in the abnormal proliferation of vascular smooth cells [26]. However, whether JNK/AP-1 mediates the AREG-induced EMT needs to be identified.
In this study, we found that AREG and FN were co-expressed in the lung tissues of asthmatic mice. Moreover, AERG induced CCN2 expression in A549 cells via the EGFR/JNK/AP-1 pathway.
Materials and methods
Materials
Dulbecco's modified Eagle medium (DMEM)/F-12 (12,500,062), Opti medium (31,985,070), and antibiotic–antimycotic (100 ×) (15,240,062) were purchased from GIBCO (Waltham, MA, USA). Control small interfering (si)RNA (scrambled), AREG siRNA, EGFR siRNA, Smad3 siRNA, fetal bovine serum (FBS) (F2442), a human AREG enzyme-linked immunosorbent assay (ELISA) kit (RAB0019), and an α-tubulin antibody were obtained from Sigma-Aldrich (St. Louis, MO, USA). Lipofectamine 3000 reagent, Lipofectamine Plus reagent (L3000015), and an AREG antibody (PA5-109404) were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). Recombinant human TGF-β (100-21) and recombinant human AREG (100-55B) were obtained from PeproTech (Cranbury, NJ, USA). An FN (ab2413) antibody and mounting medium with DAPI (ab104139) were purchased from Abcam (Cambridge, UK), while c-Jun phosphorylated (phospho) at Ser63 (#9261), JNK (#9252), phospho-JNK (Thr183/Tyr185) (#9251), and CCN2 (#86641) were purchased from Cell Signaling Technology (Danvers, MA, USA). A phospho-EGFR (Tyr1086) antibody (36-9700) was purchased from ThermoFisher Scientific (Waltham, MA, USA). EGFR (sc-373746) and c-Jun (sc-74543) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Goat anti-rabbit immunoglobulin G (IgG) (H + L)-horseradish peroxidase (HRP) (C04003), and goat anti-mouse IgG (H + L)-HRP (C04001) were acquired from Croyez Bioscience (Taipei, Taiwan).
Cell culture
Human lung epithelial cells (A549) were acquired from American Type Culture Collection (Manassas, VA, USA). Cells were grown in a DMEM/F-12 nutrient mixture, which contained 10% FBS, 100 U/ml penicillin G, and 100 μg/ml streptomycin, in a humidified incubator with 5% CO2 and 95% N2 at 37 °C. After A549 cells reached 80% confluence, cells were seeded into 6-cm dishes for immunoblotting.
OVA-induced asthma in mice
The 6–8-week-old C57B/6 female mice were intraperitoneally injected with 200 μl of 50 μg OVA combined with the 4 mg of aluminum hydroxide in phosphate-buffered saline (PBS) on days 0, 7, and 14. After sensitization with OVA, mice were challenged with OVA aerosol (5% in PBS) or PBS aerosol for 30 min, 2 days/week for 9 weeks.
siRNA transfection
Cells were transfected for 24 h with control siRNA, AREG siRNA, ADAM17, or EGFR siRNA (50 nM) using Lipofectamine® 3000 reagent with Opti medium. Cells were treated with TGF-β or AREG (10 ng/ml) at the indicated time intervals after 24 h of siRNA transfection. Results were analyzed by Western blotting.
ChIP assay
A549 cells were exposed to AREG (10 ng/ml) for 30 min and fixed with formaldehyde for 10 min. AP-1 binding to the CCN2 promoter region was detected through a ChIP assay, as previously described [21]. A polymerase chain reaction (PCR) of the AP-1 response elements on the CCN2 promoter region was performed using the following primers: 5′- TTC TGG AAA CAT TGA TGG -3′ (sense) and 5′- TAT AGG CTC TTG AAA CTC -3′ (antisense).
ELISA
The concentrations of AREG in the culture medium were determined by ELISA Sigma-Aldrich (St. Louis, MO, USA) according to the manufacturer's instructions. Following TGF-β stimulation for the indicated time, 100 μl of culture medium was added to a 96-well plate coated with AREG capture antibody. After 4 washes with wash buffer, 100 μl of AREG detection antibody was added to a 96-well plate at room temperature with shaking at 500 rpm for 1 h. Following 4 additional washes, 100 μl of conjugate antibody (HRP) was added for 45 min. After the final 4 washes, 100 μl of substrate buffer was added for 20 min in the dark. The reaction was terminated with stop solution. The relative absorbance was then measured using an ELISA reader to represent the concentration.
Western blotting
The Western blot analysis was as previously described [27]. Cells were cultured in 6-cm dishes. After cell lysis and protein extraction from A549 cells, proteins were transferred to a polyvinylidene difluoride (PVDF) membrane from sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE). The PVDF membrane was blocked by 5% bovine serum albumin (BSA) and then incubated with a specific primary antibody and an HRP-conjugated secondary antibody. Enhanced chemiluminescence was used to detect the immunoreactivity. The intensity of the signal was quantified using Image-Pro software (Eastman Kodak, Rochester, NY, USA).
Immunofluorescence (IF) staining
Serial 4-μm paraffin sections from OVA- or PBS-treated mice were used to detect AREG and FN by IF staining, as described previously [27]. In brief, slides were incubated with PBS containing 0.25% Triton X-100 for permeabilization. Slides were blocked with 5% BSA and incubated with specific antibodies such as AREG (PA5-109,404; Invitrogen, 1:1000) or FN (ab2413; Abcam, 1:1000) in 1% BSA overnight, and were in turn incubated with a fluorescein isothiocyanate (FITC)-conjugated secondary antibody for 1 h in the dark at room temperature. Slides were incubated with 4′,6-diamidino-2-phenylindole (DAPI) staining (ab104139; Abcam) for counter staining. Fluorescent images were captured under a confocal fluorescence microscope (Leica TCS SP5, Wetzlar, Germany).
Cell migration assay
Cell migration was observed by the wound-healing assay. Wound-healing experiments were analyzed as previous described [28]. Cells were seeded into Culture-Insert 2 Well 24 (Ibidi, Gräfelfing, Germany). After 24 h, the Culture-Insert 2 Well was removed, and the cells were treated with TGF-β or AREG (10 ng/ml) for another 24 h at 37 °C with 5% CO2. The images of the wound area were observed by TS100 microscopy (Nikon, Tokyo, Japan), and captured by Lead View 2000AIO (Nikon, Tokyo, Japan). The area of square wound was quantified by image J software.
Study approval
All animal protocols were approved by the Animal Ethics Committee of Taipei Medical University (approval no. LAC-2016-0361).
Statistical analysis
Results were analyzed by one-way analysis of variance (ANOVA) with Dunnett's test as a post-test to compare at least three groups. An unpaired-sample t-test was performed to compare between two groups. Cell migration assay was analyzed using two-way ANOVA with Bonferroni’s multiple comparisons test. Data are presented as the mean ± standard error of the mean (SEM), and a p value of < 0.05 was considered statistically significant.
Results
Overexpression of AREG in OVA-induced lung fibrosis in mice
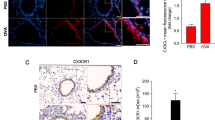
To identify the role of AREG in lung fibrosis in mice with OVA-induced asthma, we observed thickened interstitial alveoli and airway remodeling in sections of lung tissues from OVA-challenged mice. Moreover, FN and AREG expressions were higher and colocalized in OVA-challenged mice according to dual-label IF staining (Fig. 1A–C). TGF-β also induced the release of AREG from A459 cells with a peak at 8 h (Fig. 1D). Smad3 siRNA (50 nM) attenuated TGF-β-induced AREG expression (Fig. 1E). Moreover, we used AREG siRNA (50 nM) to investigate the role of AREG in TGF-β-induced fibrosis. After transfection of A549 cells with AREG siRNA, we found that AREG siRNA had a functional effect of reducing AREG expression (Fig. 1F). Furthermore, AREG siRNA inhibited TGF-β-induced CCN2 and FN expression (Fig. 1G, H).
Amphiregulin (AREG) is involved in transforming growth factor (TGF)-β-induced cellular communication network factor 2 (CCN2) and fibronectin expression in A549 human lung epithelial cells. A Lung tissue sections from ovalbumin (OVA)- or phosphate-buffered saline (PBS)-treated C57B/L6 mice were observed by immunofluorescence staining. Representative images of AREG (red) and fibronectin (green) from PBS- (n = 6) and OVA-treated (n = 5) mice. Nuclei were detected by 4′,6-diamidino-2-phenylindole (DAPI) staining (blue) (original magnification, 20 ×). Quantification of (B) fibronectin and (C) AREG from Fig. 1A. Data are presented as the mean ± SEM (PBS, n = 6 and OVA, n = 5). *p < 0.05, relative to PBS-treated mice. D A549 cells were stimulated with transforming growth factor (TGF)-β (10 ng/ml) for 0 ~ 24 h. Amounts of AREG in supernatants were investigated by an AREG-ELISA. Results are presented as the mean ± SEM, *p < 0.05, n = 3, compared to the control group. Furthermore, cells were transfected with 50 nM of control siRNA (con siRNA), Smad3 siRNA, or AREG siRNA for 24 h, and then stimulated with TGF-β (10 ng/ml) for the indicated time. Levels of (E and F) AREG, α-tubulin, (G) CCN2, α-tubulin, (H) fibronectin, and α-tubulin in cell lysates were detected by immunoblots. The results are expressed as the mean ± SEM, *p < 0.05, n = 3, relative to the TGF-β-stimulated with the control siRNA group
AREG induced fibrotic protein expression in human alveolar epithelial cells
Since AREG is highly expressed in lung sections from OVA-treated mice, we hypothesized that AREG can induce expressions of fibrotic proteins such as CCN2 and FN in human alveolar epithelial cells. As illustrated in Fig. 2A and B, AREG upregulated CCN2 expression in the time- and dose-dependent manners in A549 cells. Moreover, treatment with AREG (10 ng/ml) decreased the level of E-cadherin and induced FN protein expression in a time-dependent manner (Fig. 2C, D). We also performed a wound-healing assay to assess the role of AREG in the TGF-β-induced EMT process. Figure 2E showed that AREG (10 ng/ml) promoted cell migration compared to the control group at 24 h. AREG siRNA attenuated TGF-β-induced A549 cell migration (Fig. 2F).
Amphiregulin (AREG)-induced cellular communication network factor 2 (CCN2) and fibronectin expressions in A549 human lung epithelial cells. A A549 cells were stimulated with 0 ~ 100 ng/ml AREG for 6 h, and the expression of communication network factor 2 (CCN2) reached its highest level with 10 ng/ml AREG. Cell lysates were prepared and immunodetected with specific antibodies for CCN2 and α-tubulin. Results are expressed as the mean ± SEM, *p < 0.05, n = 3, compared to the group without AREG treatment. B Cells were incubated with AREG (10 ng/ml) for 0 ~ 8 h, and then the CCN2 and α-tubulin proteins were determined by immunoblots. Data are presented as the mean ± SEM of three experiments. *p < 0.05, compared to the control. Cells were treated with AREG (10 ng/ml) for the indicated time interval. E-cadherin and α-tubulin proteins, (C) Fibronectin and α-tubulin proteins (D) were determined by immunoblots. Data are presented as the mean ± SEM of three experiments. *p < 0.05, compared to the control. E Cells were treated with AREG (10 ng/ml) for 0 ~ 24 h, and wound area (yellow square area) was quantified by image J. Data are presented as the mean ± SEM, n = 3. *p < 0.05, compared to the control. F Cells were transfected with AREG siRNA (50 nM) for 24 h, and then incubated with TGF-β (10 ng/ml) for 0 ~ 24 h. The wound area (yellow square area) was quantified by image J. Data are presented as the mean ± SEM, n = 3. *p < 0.05, relative to the TGF-β-stimulated with the control siRNA group
Involvement of the EGFR in AREG-induced activation of JNK/c-Jun and expressions of fibrotic proteins in human alveolar epithelial cells
To confirm whether EGFR is associated with the AREG-induced EMT through the JNK/c-Jun signaling pathway in human alveolar epithelial cells, we analyzed AREG-induced CCN2 and FN expressions after transfection of EGFR siRNA (50 nM) into A549 cells. As shown in Fig. 3A and B, transfection of EGFR siRNA reduced AREG-induced CCN2 and FN expressions. Similarly, EGFR siRNA decreased AREG-induced phosphorylation of JNK/c-Jun (Fig. 3C, D).
Epidermal growth factor receptor (EGFR) mediates amphiregulin (AREG)-induced cellular communication network factor 2 (CCN2) and fibronectin expressions in A549 human epithelial cells. Cells were transfected with 50 nM of control siRNA (Con siRNA) and EGFR siRNA. After 24 h, cells were stimulated with AREG (10 ng/ml) for the indicated time. Cell lysates were detected using Western blotting and immunodetected with specific antibodies for (A) levels of CCN2, α-tubulin, (B) fibronectin, α-tubulin, (C) phospho-c-Jun N-terminal kinase (JNK) (Thr183/Tyr185), JNK, (D) phospho-c-Jun (serine 63), and c-Jun. Results are expressed as the mean ± SEM, *p < 0.05, n = 3, relative to the AREG-stimulated with the control siRNA group
Involvement of JNK in APRG-induced CCN2 expression in human alveolar epithelial cells
To identify the role of JNK in AREG-induced CCN2 expression, we treated A549 cells with 10 ng/ml AREG for various time intervals. AREG significantly provoked JNK Thr183/Tyr185 phosphorylation at 20 and 30 min (Fig. 4A). As illustrated in Fig. 4B, the JNK inhibitor, SP600125 (10 μM), reversed phosphorylation of c-Jun at Ser 63 by AREG in A549 cells. Furthermore, AREG-induced FN and CCN2 expression were inhibited by SP600125 (10 μM) (Fig. 4C, D).
c-Jun N-terminal kinase (JNK) regulates amphiregulin (AREG)-induced cellular communication network factor 2 (CCN2) and fibronectin expressions in A549 human epithelial cells. A A549 cells were exposed to AREG (10 ng/ml) for 0 ~ 30 min. Levels of phospho-JNK (Thr183/Tyr185) and JNK in cell lysates were assessed by immunoblotting. Data are shown as the mean ± SEM, n = 3. *p < 0.05, relative to unstimulated cells. A549 cells were pretreated with SP600125 (10 μM) for 30 min and then treated with AREG (10 ng/ml) for the indicated time. Levels of (B) phospho-c-Jun (serine 63), and c-Jun, n = 3, (C) fibronectin, α-tubulin, n = 4, (D) CCN2, α-tubulin, n = 3. were detected by immunoblotting. Data are shown as the mean ± SEM, *p < 0.05, relative to AREG-treated cells
Mediation of c-Jun in AREG-induced CCN2 expressions in human alveolar epithelial cells
C-Jun is a well-known substrate for JNK phosphorylation [29]. A549 cells were stimulated with AREG (10 ng/ml) for 0, 10, 20, 30, 60, and 120 min to examine the role of c-Jun Ser63 in the process of AREG-induced EMT and CCN2 expression, and the phosphorylation of c-Jun was examined using a Western blotting. AREG stimulated c-Jun phosphorylation at Ser63 at 20 min in A549 cells (Fig. 5A). Then, we treated curcumin, an AP-1 inhibitor, to understand whether c-Jun is involved in the AREG-induced CCN2 expression in A549 cells. Figure 5B showed that curcumin decreased AREG-induced CCN2 expression. Furthermore, AREG induced c-Jun binding to the promoter of CCN2 in A549 cells (Fig. 5C).
c-Jun is involved in amphiregulin (AREG)-induced cellular communication network factor 2 (CCN2) and fibronectin expressions in A549 human epithelial cells. A A549 cells were incubated with AREG (10 ng/ml) for 0 ~ 120 min. Levels of phospho-c-Jun Ser63, and c-Jun were immunodetected with specific antibodies and assessed using Western blotting. The results are expressed as the mean ± SEM, *p < 0.05, n = 3, compared to the control group. B Cells were pretreated with curcumin for 30 min and then stimulated with AREG (10 ng/ml) for another 6 h. Western blotting was performed to assess the levels of cellular communication network factor 2 (CCN2) and α-tubulin in cell lysates, Data are shown as the mean ± SEM, n = 3. *p < 0.05, relative to AREG-stimulated cells. C Schematic diagram of activator protein (AP)-1 binding to the CCN2 promoter. A549 cells were exposed to AREG (10 ng/ml) for 30 min. The AP-1-binding site of the CCN2 promoter region was identified through a ChIP assay. Typical traces are presented in all three experiments
Discussion
Severe asthma results in goblet cell hyperplasia, airway remodeling, and lung inflammation, which in turn induces lung fibrosis [2, 30]. In particular, the alveolar EMT plays an important role in lung fibrosis [4, 15, 31, 32]. Furthermore, AREG can induce fibroblast proliferation and the expression of fibrotic genes (such as collagen 1-α1, α-SMA, and FN) [6]. In this study, we demonstrated that expressions of AREG and FN increased in lung sections from OVA-sensitized mice, which were used as an asthma model. Moreover, colocalization of AREG and FN was observed in alveolar tissues. This indicated that AREG was associated with lung fibrosis and the alveolar EMT in asthmatic mice. AREG siRNA inhibited TGF-β-induced CCN2 and FN expression in A549 cells. Taken together, these results revealed that AREG mediated TGF-β-induced alveolar EMT and contributed to asthma aggravation.
The EMT is a crucial process during fibrogenesis. Numerous studies have revealed that TGF-β1 induces EMT via multiple pathways (e.g. Smad 2/3, PI3K, and MAPK pathways) [33,34,35]. Moreover, β-catenin induces vimentin, α-SMA, and collagen-I in A549 alveolar epithelial cells during pulmonary fibrosis [36]. In this study, AREG stimulated the induction of CCN2 and FN in A549 alveolar epithelial cells. In addition, AREG promoted A549 cell migration, and AREG siRNA inhibited TGF-β-stimulated cell migration. Therefore, these results suggest that AREG has a vital role in the EMT process.
A previous study has reported that AREG acts as an important mediator in inflammation and the tissue repair process in lung diseases [37]. Furthermore, higher concentrations of AREG in sputum were observed in various lung diseases, such as cystic fibrosis, chronic obstructive pulmonary disease, and asthma [38,39,40]. In children with persistent asthma, Areg expression was up-regulated in nasal epithelial cells compared bronchial epithelium in RNA-seq analysis [41]. The severity of asthma was correlated with the concentrations of AREG in the sputum and blood [39, 42]. Novali et al. reported that AREG expression was localized in bronchial epithelial cells in airway sections of asthma patients, and was correlated with the severity of asthma [39, 43]. Airway epithelial cells produce IL-33, IL-25, and thymic stromal lymphopoietin (TSLP) under allergen stimulation in the type 2 immune reaction of asthma. These cytokines then induce the release of AREG from group 2 innate lymphoid cells for tissue repair [44]. In addition, the level of Areg was increased in OVA-specific ST2hi memory Th2 cells after IL-33 stimulation in RNA-seq data [45]. These results indicated that AREG plays a role in type 2 asthma. In our study, we found that TGF-β could induce the expression and release of AREG in human lung epithelial cells. AREG siRNA downregulated CCN2 expression, and AREG could induce EGFR phosphorylation in A459 cells. Thus, these results suggested that AREG participated in TGF-β-induced CCN2 expression in A459 cells.
CCN2 is a central mediator of tissue fibrosis and EMT [19, 46]. A previous study showed that inhibition of CCN2 could attenuate lung fibrosis in bleomycin-treated mice [47]. Moreover, CCN2 increased collagen and α-SMA expression in human lung fibroblasts through the Rac1/MLK3/JNK/AP-1 pathway [21]. Interestingly, severe acute respiratory syndrome coronavirus-2 infection increased mRNA levels of CCN2, TGF-β, ADAM17, and FN in alveolar epithelial cells [48]. A previous study indicated that CCN2 interacts with FN to induce lung fibrosis via the integrin signaling pathway [49]. In addition, we previously found that CCN2 siRNA could decrease TGF-β-induced FN expression in lung epithelial cells [50]. In the present study, TGF-β induced AREG expression, which in turn stimulated EMT markers expression through the EGFR-dependent pathway in human epithelial cells. However, the mechanism by which CCN2 regulates AREG-induced FN up-regulation still requires further investigation. Taken together, CCN2 plays a role in mediating lung fibrosis. It can be used to develop a strategy for the alveolar EMT in severe asthma.
AREG is shed from epithelial cells by ADAM17, which in turn activates EGFR in fibroblasts [6]. In a previous study, profibrotic protein expression (e.g., TGF-β, CCN2) and airway fibrosis were suppressed in OVA-treated ADAM17f/f/Cre+ mice [51]. Moreover, TGF-β activates ADAM17, which in turn leads to the EMT via angiotensin-converting enzyme-2 ectodomain shedding or the RSK1/C/EBPβ pathway [50, 52]. A Previous study found that AREG has a synergistic effect on TGF-β-induced EMT via Smad 2/3 pathway in fibroblasts [53]. In this study, Smad3 siRNA could down-regulated TGF-β-induced AREG expression in A549 cells. It is reasonable to speculate that TGF-β-induced AREG expression through Smad pathway, then shedding by ADAM17 to induce EMT process in human epithelial cells. However, the more clear mechanism still needs further investigation.
EGFR expression was positively correlated with the severity of asthma [54]. Zhou et al. revealed that inhibiting EGFR reduced TGF-β-induced pulmonary fibrosis [6]. Furthermore, TGF-β induced AREG expression, which in turn stimulated fibroblast proliferation or myofibroblast transformation through EGFR/PI3K/Akt or MAPK/ERK signaling [53]. In our previous study, the EGFR was found to be involved in fibroblast differentiation and thrombin- or TGF-β-induced ECM production [51]. In this study, we found that EGFR siRNA downregulated AREG-induced CCN2 and FN expressions in human epithelial cells. These results indicated that the AREG-induced alveolar EMT was required for EGFR.
In conclusion, AREG and FN were found to be colocalized in lung sections of asthmatic mice. Moreover, AREG mediates fibrotic protein CCN2 expression through EGFR/JNK/c-Jun signaling in human epithelial cells (Fig. 6). We show for the first time how AREG regulates the expression of CCN2 and alveolar EMT in human lung epithelial cells. This will help develop strategies for the prevention and treatment of lung fibrosis in severe asthma.
Simplified diagram of the results of amphiregulin (AREG)-induced cellular communication network factor 2 (CCN2) and fibronectin via the epidermal growth factor receptor EGFR/c-Jun N-terminal kinase (JNK)/c-Jun pathway in A549 human lung epithelial cells. Transforming growth factor (TGF)-β induces the release of AREG, which in turn activates EGFR, resulting in the activation of JNK and activator protein (AP)-1. Moreover, AP-1 mediates CCN2 and FN expression through AREG stimulation in A549 human lung epithelial cells, which leads to EMT. Schematic figure created with BioRender.com (https://biorender.com/)
Availability of data and materials
Not applicable.
Abbreviations
- ADAM17:
-
A disintegrin and metalloproteinase 17
- AP-1:
-
Activator protein-1
- AREG:
-
Amphiregulin
- α-SMA:
-
α-Smooth muscle actin
- BSA:
-
Bovine serum albumin
- CCN2:
-
Cellular communication network factor 2
- DMEM:
-
Dulbecco’s modified minimal essential/Eagle medium
- ECM:
-
Extracellular matrix
- EGFR:
-
Epidermal growth factor receptor
- ELISA:
-
Enzyme-linked immunosorbent assay
- EMT:
-
Epithelial-mesenchymal transition
- FN:
-
Fibronectin
- HRP:
-
Horseradish peroxidase
- IF:
-
Immunofluorescence
- IgG:
-
Immunoglobulin G
- IL:
-
Interleukin
- JNK:
-
C-Jun N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinase
- OVA:
-
Ovalbumin
- NEAAs:
-
Nonessential amino acids
- NHLF:
-
Normal human lung fibroblast
- PBS:
-
Phosphate-buffered saline
- PCR:
-
Polymerase chain reaction
- SDS-PAGE:
-
Sodium dodecylsulfate polyacrylamide gel electrophoresis
- siRNA:
-
Small interfering RNA
- TGF:
-
Transforming growth factor
- TSLP:
-
Thymic stromal lymphopoietin
References
Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. 2020;396:1204–22.
Hough KP, Curtiss ML, Blain TJ, et al. Airway remodeling in asthma. Front Med. 2020;7:191.
Peng S, Gao J, Liu W, et al. Andrographolide ameliorates OVA-induced lung injury in mice by suppressing ROS-mediated NF-κB signaling and NLRP3 inflammasome activation. Oncotarget. 2016;7:80262.
Kim KK, Kugler MC, Wolters PJ, et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci. 2006;103:13180–5.
Chen Q, Yang W, Wang X, et al. TGF-β1 induces EMT in bovine mammary epithelial cells through the TGFβ1/Smad signaling pathway. Cell Physiol Biochem. 2017;43:82–93.
Zhou Y, Lee J-Y, Lee C-M, et al. Amphiregulin, an epidermal growth factor receptor ligand, plays an essential role in the pathogenesis of transforming growth factor-β-induced pulmonary fibrosis. J Biol Chem. 2012;287:41991–2000.
Stahler A, Stintzing S, Modest DP, et al. Amphiregulin expression is a predictive biomarker for EGFR inhibition in metastatic colorectal cancer: combined analysis of three randomized trials. Clin Cancer Res. 2020;26:6559–67.
Berasain C, Avila MA. Amphiregulin. Elsevier 2014. 31–41.
Effenberger T, von der Heyde J, Bartsch K, et al. Senescence-associated release of transmembrane proteins involves proteolytic processing by ADAM17 and microvesicle shedding. FASEB J. 2014;28:4847–56.
Monticelli LA, Osborne LC, Noti M, et al. IL-33 promotes an innate immune pathway of intestinal tissue protection dependent on amphiregulin–EGFR interactions. Proc Natl Acad Sci. 2015;112:10762–7.
Perugorria MJ, Latasa MU, Nicou A, et al. The epidermal growth factor receptor ligand amphiregulin participates in the development of mouse liver fibrosis. Hepatology. 2008;48:1251–61.
Du B, Altorki NK, Kopelovich L, et al. Tobacco smoke stimulates the transcription of amphiregulin in human oral epithelial cells: evidence of a cyclic AMP-responsive element binding protein-dependent mechanism. Can Res. 2005;65:5982–8.
Inoue H, Akimoto K, Homma T, et al. Airway epithelial dysfunction in asthma: relevant to epidermal growth factor receptors and airway epithelial cells. J Clin Med. 2020;9:3698.
Enomoto Y, Orihara K, Takamasu T, et al. Tissue remodeling induced by hypersecreted epidermal growth factor and amphiregulin in the airway after an acute asthma attack. J Allergy Clin Immunol. 2009;124:913–20.
Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Investig. 2003;112:1776–84.
Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Investig. 2009;119:1429–37.
Valcourt U, Kowanetz M, Niimi H, et al. TGF-β and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell. 2005;16:1987–2002.
Xu J, Lamouille S, Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–72.
Lipson KE, Wong C, Teng Y, et al. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. BioMed Central 2012; 1–8.
Wang Y, Chang T, Wu T, et al. Connective tissue growth factor promotes retinal pigment epithelium mesenchymal transition via the PI3K/AKT signaling pathway. Mol Med Rep. 2021;23:1–13.
Lin C-H, Yu M-C, Tung W-H, et al. Connective tissue growth factor induces collagen I expression in human lung fibroblasts through the Rac1/MLK3/JNK/AP-1 pathway. Biochimica et Biophysica Acta (BBA) Mol Cell Res. 2013;1833:2823–33.
Sonnylal S, Xu S, Jones H, et al. Connective tissue growth factor causes EMT-like cell fate changes in vivo and in vitro. J Cell Sci. 2013;126:2164–75.
Cheng Y, Lin C, Chen J-Y, et al. Correction: induction of connective tissue growth factor expression by hypoxia in human lung fibroblasts via the MEKK1/MEK1/ERK1/GLI-1/GLI-2 and AP-1 pathways. PLoS ONE. 2017;12: e0188608.
Bode AM, Dong Z. The functional contrariety of JNK. Mol Carcinog. 2007;46:591–8.
Yan D, An G, Kuo MT. C-Jun N-terminal kinase signalling pathway in response to cisplatin. J Cell Mol Med. 2016;20:2013–9.
Wu Y, Zhou J, Wang H, et al. The activation of p38 MAPK limits the abnormal proliferation of vascular smooth muscle cells induced by high sodium concentrations. Int J Mol Med. 2016;37:74–82.
Cheng W-H, Chen C-L, Chen J-Y, et al. Hypoxia-induced preadipocyte factor 1 expression in human lung fibroblasts through ERK/PEA3/c-Jun pathway. Mol Med. 2021;27:1–14.
Venter C, Niesler C. Rapid quantification of cellular proliferation and migration using ImageJ. Biotechniques. 2019;66:99–102.
Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochimica et Biophysica Acta (BBA) Mol Cell Res. 2007;1773:1341–8.
Lee M-Y, Shin I-S, Jeon W-Y, et al. Bangpungtongseong-san, a traditional herbal medicine, attenuates chronic asthmatic effects induced by repeated ovalbumin challenge. Int J Mol Med. 2014;33:978–86.
Yamauchi K, Inoue H. Airway remodeling in asthma and irreversible airflow limitation—ECM deposition in airway and possible therapy for remodeling. Allergol Int. 2007;56:321–9.
Bergeron C, Al-Ramli W, Hamid Q. Remodeling in asthma. Proc Am Thorac Soc. 2009;6:301–5.
Zhou X-L, Xu P, Chen H-H, et al. Thalidomide inhibits TGF-β1-induced epithelial to mesenchymal transition in alveolar epithelial cells via Smad-dependent and Smad-independent signaling pathways. Sci Rep. 2017;7:1–10.
Yu L, Hébert MC, Zhang YE. TGF-β receptor-activated p38 MAP kinase mediates Smad-independent TGF-β responses. EMBO J. 2002;21:3749–59.
Massagué J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000;19:1745–54.
Song P, Zheng J, Xu J, et al. β-catenin induces A549 alveolar epithelial cell mesenchymal transition during pulmonary fibrosis. Mol Med Rep. 2015;11:2703–10.
Zaiss DM, Gause WC, Osborne LC, et al. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42:216–26.
Stolarczyk M, Amatngalim GD, Yu X, et al. ADAM 17 and EGFR regulate IL-6 receptor and amphiregulin mRNA expression and release in cigarette smoke-exposed primary bronchial epithelial cells from patients with chronic obstructive pulmonary disease (COPD). Physiol Rep. 2016;4: e12878.
Kim KW, Jee HM, Park YH, et al. Relationship between amphiregulin and airway inflammation in children with asthma and eosinophilic bronchitis. Chest. 2009;136:805–10.
Adib-Conquy M, Pedron T, Petit-Bertron A-F, et al. Neutrophils in cystic fibrosis display a distinct gene expression pattern. Mol Med. 2008;14:36–44.
Chun Y, Do A, Grishina G, et al. Integrative study of the upper and lower airway microbiome and transcriptome in asthma. JCI insight 2020;5.
Hachim MY, Elemam NM, Ramakrishnan RK, et al. Blood and salivary amphiregulin levels as biomarkers for asthma. Front Med. 2020;7:670.
Novali M, Hirota N, Hassan M, et al. Amphiregulin expression correlates with asthma severity in human bronchial epithelial cells and is released by histamine. In: C38. AIRWAY INFLAMMATION: FROM MECHANISMS TO NON-INVASIVE BIOMARKERS. American Thoracic Society 2011. A4449–A4449.
Yasuda Y, Nagano T, Kobayashi K, et al. Group 2 innate lymphoid cells and the house dust mite-induced asthma mouse model. Cells. 2020;9:1178.
Morimoto Y, Hirahara K, Kiuchi M, et al. Amphiregulin-producing pathogenic memory T helper 2 cells instruct eosinophils to secrete osteopontin and facilitate airway fibrosis. Immunity. 2018;49:134–50.
Shafieian M, Chen S, Wu S. Integrin-linked kinase mediates CTGF-induced epithelial to mesenchymal transition in alveolar type II epithelial cells. Pediatr Res. 2015;77:520–7.
Wang X, Wu G, Gou L, et al. A novel single-chain-Fv antibody against connective tissue growth factor attenuates bleomycin-induced pulmonary fibrosis in mice. Respirology. 2011;16:500–7.
Xu J, Xu X, Jiang L, et al. SARS-CoV-2 induces transcriptional signatures in human lung epithelial cells that promote lung fibrosis. Respir Res. 2020;21:1–12.
Chen Y, Abraham DJ, Shi-Wen X, et al. CCN2 (connective tissue growth factor) promotes fibroblast adhesion to fibronectin. Mol Biol Cell. 2004;15:5635–46. https://doi.org/10.1091/mbc.e04-06-0490.
Ou S-C, Bai K-J, Cheng W-H, et al. TGF-β induced CTGF expression in human lung epithelial cells through ERK, ADAM17, RSK1, and C/EBPβ pathways. Int J Mol Sci. 2020;21:9084.
Chen J-Y, Cheng W-H, Lee K-Y, et al. Abnormal ADAM17 expression causes airway fibrosis in chronic obstructive asthma. Biomed Pharmacother. 2021;140: 111701.
Sisto M, Ribatti D, Lisi S. ADAM 17 and epithelial-to-mesenchymal transition: the evolving story and its link to fibrosis and cancer. J Clin Med. 2021;10:3373.
Lee C-M, Park JW, Cho W-K, et al. Modifiers of TGF-β1 effector function as novel therapeutic targets of pulmonary fibrosis. Korean J Intern Med. 2014;29:281.
Puddicombe SM, Polosa R, Richter A, et al. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–74.
Acknowledgements
This study was supported by a Grant (MOST111-2320-B-038-011, MOST109-2320-B-038-068 and MOST109-2320-B-038-024-MY3) from the Ministry of Science and Technology of Taiwan, R.O.C, and the Taipei Medical University (TMU) within the framework of the Higher Education Sprout Project (DP2-111-21121-01-T-02-01) by the Ministry of Education (MOE) in Taiwan, R.O.C.
Funding
MOST111-2320-B-038-011, MOST109-2320-B-038-068 and MOST109-2320-B-038-024-MY3 from the Ministry of Science and Technology of Taiwan, R.O.C. DP2-111-21121-01-T-02-01 from Ministry of Education (MOE) in Taiwan, R.O.C.
Author information
Authors and Affiliations
Contributions
Conceptualization: BCC. Data curation: WHC and SYK. Formal analysis: WHC and FSY. Funding acquisition: BCC. Investigation: WHC and SYK. Methodology: BCC,CLC, and LYL. Project administration: BCC. Resources: BCC. Software: WHC and BCC. Supervision: BCC and CHL. Validation: WHC, SYK, FSY, LYL, and BCC. Visualization: WHC, FSY, CHL, LYL, and BCC. Writing—original draft: WHC. Writing—review & editing: BCC, WHC, CLC, LYL, and CHL. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal protocols were approved by the Animal Ethics Committee of Taipei Medical University (approval nos. LAC-2016-0361).
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cheng, WH., Kao, SY., Chen, CL. et al. Amphiregulin induces CCN2 and fibronectin expression by TGF-β through EGFR-dependent pathway in lung epithelial cells. Respir Res 23, 381 (2022). https://doi.org/10.1186/s12931-022-02285-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02285-2