Abstract
Background
Lung cancers arising in never smokers have been suggested to be substantially different from lung cancers in smokers at an epidemiological, genetic and molecular level. Focusing on non-small cell lung cancer (NSCLC), we characterized lung cancer patients in China looking for demographic and clinical differences between the smoking and never-smoking subgroups.
Methods
In total, 891 patients with NSCLC, including 841 with adenocarcinoma and 50 with squamous cell carcinoma, were recruited in this study. Association of smoking status with demographic and clinical features of NSCLC was determined, and risk factors for lymph node metastasis and TNM stage were evaluated using Multivariate logistic regression analysis.
Results
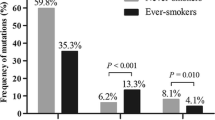
In patients with adenocarcinoma, never smokers showed a younger age at diagnosis (54.2 ± 12.7vs. 59.3 ± 9.4, padjusted<0.001), a lower risk for lymph node metastasis than smokers (7,6% vs. 19.5%, padjusted<0.001) and less severe disease as indicated by lower percentages of patients with TNM stage of III or IV (5.5% vs. 14.7%, padjusted<0.001 ). By contrast, these associations were not observed in 50 patients with squamous cell carcinoma. Multivariate logistic regression analysis showed that smoking status was a risk factor for lymph node metastasis (OR = 2.70, 95% CI: 1.39–5.31, p = 0.004) but not for TNM stage (OR = 1.18, 95% CI: 0.09–14.43, p = 0.896) in adenocarcinoma.
Conclusion
This study demonstrates that lung adenocarcinoma in never smokers significantly differ from those in smokers regarding both age at diagnosis and risk of lymph node metastasis, supporting the notion that they are distinct entries with different etiology and pathogenesis.
Similar content being viewed by others
Introduction
By far, lung cancer is the leading cause of cancer deaths worldwide making up 18% of all cancer deaths [1]. Despite cigarette smoking being the major risk factor for lung cancer, the degree of its association with different histological types varies considerably. The association is much stronger in squamous cell carcinoma (SQC) and small cell lung cancer (SCLC) than in adenocarcinoma (ADC) [2]. Consistently, smoking cession results a higher risk-reduction in SQC and SCLC than in ADC [3]. Epidemiological studies have shown that rates of lung cancers arising in never smokers have increased during recent decades [4,5,6]. As compared with lung cancers arising in smokers, those arising in never smokers show substantial differences at epidemiological, genetic and molecular levels, implying that they might be biologically distinct entities [7,8,9,10]. There is also a growing body of evidence showing differences in metastatic patterns in tumors harboring driver mutations [11]. In comparison, little is known about patterns of locoregional spread in early stage ADC in smokers vs. never smokers. As screening for lung cancer in some non-smoking persons has started to show promise [12], and targeted adjuvant therapies are becoming available, the clinical relevance of these differences will increase rapidly over the coming years.
Interestingly, the proportion of never smokers in patients with non-small cell lung cancer (NSCLC) varies highly across populations. For example, proportions of never smokers in patients with ADC in France and UK are less than 5% [13–14] , while these values in Japan and China are reportedly higher than 40% [15,16,17]. The high proportion of never smokers in patients in East Asia populations provides an opportunity to compare never smokers and smokers in NSCLC. In this study, we compared smoking and never-smoking patients with lung NSCLC and investigated differences in their demographical and clinical characteristics.
Methods
Patients
All patients with NSCLC in this study were recruited from the Department of Thoracic Surgery of the First Affiliated Hospital of Xiamen University, Xiamen, China from 2015 to 2021. Diagnosis of lung cancer was based on histology according to the 2015 World Health Organization Classification of Lung Tumors [18]. Patients were selected for lung cancer surgery according to the Chinese guidelines for diagnosis and treatment of primary lung cancer [19]. Briefly, the absolute indication of lung cancer surgery is the T1 − 3_N0 − 1_M0 disease, the relative indication of the surgery is part of T4_N0 − 1_M0 disease, the controversial indication is the T1 − 3_N2_M0 disease, and exploratory surgical indications for lung cancer include part of stage T1 − 3_N0 − 1_M1 with solitary metastases. All patients underwent segmentectomy or lobectomy with lymph node resection within two weeks after the diagnosis based on imaging and/or histopathological examination, and only patients with well-characterized demographic, clinical and pathological information as well as smoking status were recruited. Demographical, clinical and pathological information, including age at diagnosis, gender, smoking status, histological type, size of primary tumor, lymph node metastases and metastasis to distant organs were retrospectively collected from electronic medical records. To avoid the effect of treatment on the clinical features, patients receiving chemotherapy, radiotherapy, or biological therapies before surgery were excluded for the analysis. This study was performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards, and the approval was obtained from the Ethic of Committees of the First Affiliated Hospital of Xiamen University. Since this retrospective study contains anonymous patient information, informed consent was waived by the institutional review board (IRB).
Statistical analyses
All statistical analyses were performed with R software (R, version 4.1.1). For quantitative variables, data were shown as the mean ± SD (standard deviation). The Kolmogorov-Smirnov normality test or Shapiro-Wilk test were performed to examine if variables are normally distributed. To determine statistical significance, quantitative data in normal distribution were compared using the student t-test, otherwise the Mann-Whitney U test was used. For the categorical variables, data were expressed as number (percentage), and p values were calculated with Chi-Square test or Fisher’s exact test. P values were adjusted by Benjamini-Hochberg (BH) method for multiple comparisons and a p value less than 0.05 was considered as statistically significant. In addition, multivariate logistic regression (method ENTER) analysis was used to examine the influence of categorical variables such as smoking status (ever smoker/never smoker) and gender (male/female) as well as numeric variables including age on lymph node metastasis (LNMets) and TNM stages. For the multivariate logistic regression analysis, stages of primary tumor size, LNMets and metastasis were regarded as numeric variables. Odds ratios (OR), 95% CIs and p values were calculated for each variable.
Results
Clinical and demographic characteristics of patients
In total, 891 patients with NSCLC were recruited, including 841 with ADC and 50 with SQC (Table1). Mean age of patients with ADC was 55.2 ± 12.3 years (mean ± SD), and mean age of patients with SQC was 63.1 ± 6.8 years). Patients with ADC patients consisted of more females (58.3%) than males, while SQC patients were predominantly males (92%). As expected, majority of patients with SQC were smokers (68%), including current smokers and former smokers. By contrast, only 17% patients with ADC smoked. Proportion of never smokers in patients with ADC (83%) was approximately 2.6 times as high as that in patients with SQC (32%). Among the 891 patients with NSCLC, 66 were diagnosed with carcinoma in situ, and they were all ADC. When patients were categorized according to the size of primary tumor, the percentage of SQC patients with T2 to T4 was higher than that in ADC patients (61.9% vs. 16.6%). Also, the percentage of patients with lymph node metastasis (LNMets) in SQC was roughly 3 times as high as in ADC (28.6% vs. 9.6%), while no significant differences were observed regarding tissue metastasis between these two histological sub-types of NSCLC. With regards to TNM stages, the proportion of patients with severe disease (with a TNM stage III and IV) in SQC was higher than that in ADC (20.4% vs. 7.0%). (Table1)
Association of smoking status with characteristics of ADC
We first examined the association of smoking status with demographical and clinical characteristics of patients with ADC. Among 841 patients with ADC, 698 were never smokers and 143 were ever smokers, including 5 former smokers and 138 current smokers. Notably, the mean age at diagnosis of never smokers was approximately 5 years younger than that of ever smokers (54.2 ± 12.6vs. 59.3 ± 9.4, padjusted<0.001) (Table2 and supplementary Fig.1A). Significant association of smoking status with gender was also observed. In smokers 91.2% were male, while in non-smokers only 31.2% were male (padjusted<0.001). Percentages of ADC in situ were comparable between smokers and never smokers, and no significant difference was observed in size of primary tumors between the two subgroups. However, significant a difference was observed regarding LNMets; the proportion of patients showing LNMets was approximately 2.5 times in smokers than in never smokers (19.6% vs. 7.6%, padjusted<0.001). In addition, smokers showed a higher percentage of patients with severe disease as defined by TNM stages of III and IV than never smokers (14.7% vs. 5.49%, padjusted<0.001). (Table2)
Association of smoking status with characteristics of SQC
Having demonstrated the association of smoking status with age at diagnosis, gender, LNMets and TNM stage, we sought to investigate whether the association is ADC specific. For this purpose, we examined the effect of smoking status on SQC. The 50 patients with SQC consisted of 34 smokers and 16 never smokers. In contrast to ADC, age at diagnosis in SQC patients who never smoked was older, although not significant after adjustment for multiple comparison, than that in patients who were smokers (66.0 ± 7.1 vs 61.7 ± 6.3, p = 0.035) (Table3 and supplementary Fig.1B). Similar to ADC, SQC was also characterized by a significantly higher male-to-female ratio in ever smokers than in never smokers (padjusted=0.003). Regarding LNMets, 29.4% of SQC patients who were smokers showed lymph node spreading, which was not significantly different to the prevalence of lymph node involvement (26.67%) in never smokers. In addition, no significant difference was observed in disease severity as indicated by TNM stages between the two groups.
Risk factors for LNMets and severe disease
Given the above results showed that smoking status was associated with LNMmet and TNM stage in ADC, we next investigated whether cigarette smoking is an independent risk factor for the two clinical characteristics using multivariate logistic regression analysis. As shown in Table4, size of primary tumor was the strongest factor associated with the risk of LNMets, where a 1-stage increase in tumor grade was associated with a roughly 3 times higher risk for LNMets (OR = 3.81, 95% CI: 2.74-5,34, p < 0.001). Besides size of primary tumor, smoking status was another risk factor for LNMets. Smokers showed an increased risk for LNMets, with an OR value of 2.70 (95% CI: 1.39–5.31, p = 0.004). By contrast, LNMets was not significantly associated with age and gender of patients. As expected, primary tumor size, LNMets and metastasis were the main independent risk factors for severe disease (TNM stage III or IV). In addition, age at diagnosis was identified to be a risk factor for severe disease (OR = 0.92, 95% CI: 0.84–0.99, p = 0.04). Although smoking status was significantly associated with severe disease, the multivariate logistic regression analysis showed it was not an independent risk factor (OR = 1.18, 95% CI: 0.09–14.43, p = 0.896). (Table5)
Discussion
In the present study, we investigated the role of smoking status, age, and gender in a clinical cohort of patients with NSCLC who underwent thoracic surgery. Since it has been shown that CT Screening promotion is associated with lung cancer overdiagnosis among Asian women [20], precise staging is a strength of the surgical cohorts in the current study. We demonstrate that ever smokers show a higher age at diagnosis, a higher male-to-female ratio, an increased risk of LNMets and more severe disease than never smokers in patients with ADC. Moreover, multiple multivariate logistic regression analysis shows that smoking is an independent risk factor for LNMets.
Notably, 83% of patients with lung ADC in the present study are never smokers. This finding is line with the observation that proportion of never smokers in ADC patients is much higher than in East Asian than in Europe [21–22] . The difference between East Asia and Europe suggests that population-specific genetic and environmental factors contribute to the development of ADC in never smokers [23]. In line with this notion, genetic studies have shown that lung ADC arising in never smokers is associated with different genetic variants in Asian populations compared to Caucasian populations [24]. Besides genetics factor, multiple environmental risk factors contributing to the development of COPD vary considerably cross the world[25] .
As expected, most smokers with ADC are males, while roughly two-thirds of never smokers with ADC are females. This result is in consistent with a previous population-based study in Japan, where Seki and colleagues reported that percentages of male in ever smokers and never smokers in patients with lung ADC are 87% and 16%, respectively [26]. The male predominance in ever smokers can only be explained in part by an extremely low female smoking prevalence in China. According to a previous study, only 3.2% are ever regular smokers among 302 669 women enrolled in 2004–08, while the male smoking prevalence in 210 222 men enrolled in the same time period is approximately 70% in China [27]. The female predominance in never smokers in ADC might be due to at least two factors. The dramatic difference in smoking prevalence between men and women leads to a high female-to-male ratio in never smokers in general population, which partially accounts for the predominance of female in lung ADC patients arising in never smokers. In addition, the female predominance might also be due, in part, to the higher risk of lung cancer in women than in men, a hypothesis supported by epidemiological evidence [28]. A comprehensive molecular characterization for instance of by determining EGFR, K-RAS and other mutations by sequencing, droplet-PCR or mutation-specific antibodies w has been completed in similar contexts in other cohorts and suggests differences in the underlying molecular events [29,30,31].
Interestingly, although the smoking status is associated with both with LNMets and TNM stages in ADC, multivariate logistic regression analysis demonstrates that cigarette smoking is only an independent risk factor for LNMets, with an OR of 2.70. This finding suggests that the association of smoking status with TNM stages is due to the contribution of cigarette smoking to LNMets. In a previous multicenter retrospective study, Chen et al. examined risk factors for LNMets in 10 885 patients with clinical T1 NSCLC [32]. Their analyses demonstrated that smokers showed a significant higher prevalence of LNMets than never smokers (22.8% vs. 13.6%, p < 0.001), while the multivariate analyses failed to show smoking status as a significant risk factors for LNMets [33]. A possible explanation for the discrepancy between our results and the findings from Chen‘s study could be the difference in types of NSCLC investigated. In the present study, we examined the impact of smoking status on LNMets in ADC and SQC separately, while Chen and colleagues combined these two entities for the analysis. As shown in the current study, significant association of cigarette smoking with an increased risk of LNMets was only observed in ADC, but not in SQC. Therefore, combination of ADC and SQC likely reduces the statistical power of the analysis. Besides LNMets, age at diagnosis is also associated with cigarette smoking in both the discovery and replication cohorts in the present study, where never smokers are roughly 5 years younger than smokers. This difference was also observed by Lam and colleagues who reported that median ages of never smokers and smokers in 115 patients with lung ADC are 54 and 60 years, respectively, although the difference is not statistically significant due to the small sample size [34].
Given that LNMets develop during cancer progression [35], it is conceivable to speculate that smokers might have symptomatic comorbid illnesses which might lead to a delayed lung cancer diagnosis and consequent high prevalence of LNMets in lung ADC. However, this speculation is contradicted by two findings from the present study. First, the primary tumor size was comparable between smokers and never smokers in ADC, indicating no evidence of a delay of diagnosis in ever smokers compared to never smokers. Second, never smokers and smokers in patients with SQC showed comparable prevalence of LNMets, suggesting that the association of cigarette smoking with LNMets might be ADC-specific. However, due to the sample size of the SQC cohort in the current study, the association of smoking status with LNMets in SQC needs to be further elucidated. A possible explanation for the association of smoking status with age at diagnosis and the risk of LNMets is that lung ADC arising in never smokers differs from that arising in smokers in disease etiology and pathogenesis. This notion is supported by the evidence that prevalence of oncogenic driver mutations in lung ADC in never smokers is significantly higher than that in smokers [36–37]. Until very recently molecular testing was not clinically indicated in early stage, surgically treated NSCLC, and so this data is not available for our cohorts. However, other data sets have reliably described clinically relevant differences in this context and further studies are planned to characterise the available tissue samples, with a focus on both molecular driver alterations and differences in immune response.
LNMets are initiated by the migration of cancer cells into lymphatic capillaries, and the development of LNMets likely depends on a combination of intrinsic properties of cancer cells and microenvironment of LN [38–39]. Cigarette smoke is a complex mixture of numerous chemicals including multiple lung carcinogens [40]. Nicotine, a component responsible for tobacco addiction, also plays a role in the invasion and metastasis of various cancers, including lung cancer [41]. Using a mouse model of lymphatic metastasis, Shimizu et al. demonstrated that nicotine promotes LNMets in head and neck squamous cell carcinoma by inducing nuclear accumulation of phosphorylated epidermal growth factor (pEGFR) and activation of Akt signaling in neck squamous cell carcinoma cells [42]. Therefore, it is intriguing to examine whether the nicotine induced pEGFR-Akt signaling pathway also contributes to the risk effect of cigarette smoking mediated LNMets in lung ADC.
A major limitation of the current study is the lack of information of secondhand smoke in never smokers. For this reason, it is not possible to determine the effect of passive smoking in the development of lung cancer. According to a systematic meta-analysis performed by Du et al., approximately 16% of lung cancer cases among never smokers in China are potentially attributable to passive smoking [43]. Therefore the potential effect of passive smoking in the development of lung ADC in never smoker needs to be taken account when interpreting this study.
Conclusion
The present study demonstrates that lung ADC in never smokers differs significantly to that in smokers in age at diagnosis and the risk for LNMets, supporting the notion that they are distinct entries with different etiology and pathogenesis. Furthermore, this study also indicates that cigarette smoking could be an independent risk factor for LNMets in lung ADC. The predisposition of smoking-related ADC to spread to regional lymph nodes may indicate differences in locoregional antitumoral immune response in smokers vs. never smokers, which should be the focus of further investigation.
Data availability
All data generated or analysed during this study are included in this published article and are available from the corresponding author on reasonable request.
Abbreviations
- NSCLC:
-
non-small cell lung cancer.
- ADC:
-
adenocarcinoma.
- SQC:
-
squamous cell carcinoma.
- NS:
-
never smokers.
- FS:
-
former smokers.
- CS:
-
current smokers.
- LNMets:
-
lymph node metastasis.
Reference list
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer. 2001;31(2–3):139–48.
Khuder SA, Mutgi AB. Effect of smoking cessation on major histologic types of lung cancer. Chest. 2001;120(5):1577–83.
Cufari ME, Proli C, De SP, Raubenheimer H, Al SM, Chavan H, et al. Increasing frequency of non-smoking lung cancer: Presentation of patients with early disease to a tertiary institution in the UK. Eur J Cancer. 2017;84:55–9.
Pelosof L, Ahn C, Gao A, Horn L, Madrigales A, Cox J, et al. Proportion of Never-Smoker Non-Small Cell Lung Cancer Patients at Three Diverse Institutions. J Natl Cancer Inst 2017;109(7).
Thun MJ, Hannan LM, ms-Campbell LL, Boffetta P, Buring JE, Feskanich D, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5(9):e185.
Rudin CM, vila-Tang E, Harris CC, Herman JG, Hirsch FR, Pao W, et al. Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin Cancer Res. 2009;15(18):5646–61.
Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers–a different disease. Nat Rev Cancer. 2007;7(10):778–90.
Devarakonda S, Li Y, Martins RF, Sankararaman S, Kadara H, Goparaju C, et al. Genomic Profiling of Lung Adenocarcinoma in Never-Smokers. J Clin Oncol. 2021;39(33):3747–58.
Zhang T, Joubert P, nsari-Pour N, Zhao W, Hoang PH, Lokanga R, et al. Genomic and evolutionary classification of lung cancer in never smokers. Nat Genet. 2021;53(9):1348–59.
Hsu F, De CA, Anderson D, Nichol A, Toriumi T, Ho C. Patterns of spread and prognostic implications of lung cancer metastasis in an era of driver mutations. Curr Oncol. 2017;24(4):228–33.
Yang P. National Lung Cancer Screening Program in Taiwan: The TALENT Study. Journal of Thoracic Oncology 2021;Supplement(S58).
Capewell S, Wathen CG, Sankaran R, Sudlow MF. Lung cancer in young patients. Respir Med. 1992;86(6):499–502.
Nagy-Mignotte H, Guillem P, Vesin A, Toffart AC, Colonna M, Bonneterre V, et al. Primary lung adenocarcinoma: characteristics by smoking habit and sex. Eur Respir J. 2011;38(6):1412–9.
Chen B, Wang X, Yu X, Xia WJ, Zhao H, Li XF, et al. Lymph node metastasis in Chinese patients with clinical T1 non-small cell lung cancer: A multicenter real-world observational study. Thorac Cancer. 2019;10(3):533–42.
Cho J, Choi SM, Lee J, Lee CH, Lee SM, Kim DW, et al. Proportion and clinical features of never-smokers with non-small cell lung cancer. Chin J Cancer. 2017;36(1):20.
Seki T, Nishino Y, Tanji F, Maemondo M, Takahashi S, Sato I, et al. Cigarette smoking and lung cancer risk according to histologic type in Japanese men and women. Cancer Sci. 2013;104(11):1515–22.
Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10(9):1243–60.
Chinese guidelines for diagnosis and treatment of primary lung. cancer 2018 (English version). Chin J Cancer Res. 2019;31(1):1–28.
Gao W, Wen CP, Wu A, Welch HG. Association of Computed Tomographic Screening Promotion With Lung Cancer Overdiagnosis Among Asian Women. JAMA Intern Med. 2022;182(3):283–90.
Scagliotti GV, Longo M, Novello S. Nonsmall cell lung cancer in never smokers. Curr Opin Oncol. 2009;21(2):99–104.
Wakelee HA, Chang ET, Gomez SL, Keegan TH, Feskanich D, Clarke CA, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25(5):472–8.
Samet JM, vila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res. 2009;15(18):5626–45.
Okazaki I, Ishikawa S, Ando W, Sohara Y. Lung Adenocarcinoma in Never Smokers: Problems of Primary Prevention from Aspects of Susceptible Genes and Carcinogens. Anticancer Res. 2016;36(12):6207–24.
Global burden. of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–49.
Seki T, Nishino Y, Tanji F, Maemondo M, Takahashi S, Sato I, et al. Cigarette smoking and lung cancer risk according to histologic type in Japanese men and women. Cancer Sci. 2013;104(11):1515–22.
Chen Z, Peto R, Zhou M, Iona A, Smith M, Yang L, et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet. 2015;386(10002):1447–56.
Stapelfeld C, Dammann C, Maser E. Sex-specificity in lung cancer risk. Int J Cancer. 2020;146(9):2376–82.
Yu J, Kane S, Wu J, Benedettini E, Li D, Reeves C, et al. Mutation-specific antibodies for the detection of EGFR mutations in non-small-cell lung cancer. Clin Cancer Res. 2009;15(9):3023–8.
Zhang T, Joubert P, nsari-Pour N, Zhao W, Hoang PH, Lokanga R, et al. Genomic and evolutionary classification of lung cancer in never smokers. Nat Genet. 2021;53(9):1348–59.
Zhu G, Ye X, Dong Z, Lu YC, Sun Y, Liu Y, et al. Highly Sensitive Droplet Digital PCR Method for Detection of EGFR-Activating Mutations in Plasma Cell-Free DNA from Patients with Advanced Non-Small Cell Lung Cancer. J Mol Diagn. 2015;17(3):265–72.
Chen B, Wang X, Yu X, Xia WJ, Zhao H, Li XF, et al. Lymph node metastasis in Chinese patients with clinical T1 non-small cell lung cancer: A multicenter real-world observational study. Thorac Cancer. 2019;10(3):533–42.
Chen B, Wang X, Yu X, Xia WJ, Zhao H, Li XF, et al. Lymph node metastasis in Chinese patients with clinical T1 non-small cell lung cancer: A multicenter real-world observational study. Thorac Cancer. 2019;10(3):533–42.
Lam B, Lam WK, Lam CL, Ooi GC, Ho JC, Wong MP, et al. Adenocarcinoma of the lung in Chinese patients: a revisit and some perspectives from the literature. Postgrad Med J. 2001;77(913):708–12.
Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124(3):922–8.
Ren S, Kuang P, Zheng L, Su C, Li J, Li B, et al. Analysis of driver mutations in female non-smoker Asian patients with pulmonary adenocarcinoma. Cell Biochem Biophys. 2012;64(2):155–60.
Sun Y, Ren Y, Fang Z, Li C, Fang R, Gao B, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010;28(30):4616–20.
Jones D, Pereira ER, Padera TP. Growth and Immune Evasion of Lymph Node Metastasis. Front Oncol. 2018;8:36.
Pereira ER, Jones D, Jung K, Padera TP. The lymph node microenvironment and its role in the progression of metastatic cancer. Semin Cell Dev Biol. 2015;38:98–105.
Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health. 2009;6(2):445–62.
Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29(3):151–8.
Shimizu R, Ibaragi S, Eguchi T, Kuwajima D, Kodama S, Nishioka T, et al. Nicotine promotes lymph node metastasis and cetuximab resistance in head and neck squamous cell carcinoma. Int J Oncol. 2019;54(1):283–94.
Du Y, Cui X, Sidorenkov G, Groen HJM, Vliegenthart R, Heuvelmans MA, et al. Lung cancer occurrence attributable to passive smoking among never smokers in China: a systematic review and meta-analysis. Transl Lung Cancer Res. 2020;9(2):204–17.
Acknowledgements
We would like to thank all patients that participated in the study.
Funding
This study was supported by Xiamen Municipal Bureau of Science and Technology (No. 3502Z20214ZD3011), Natural Science Foundation of Fujian Province (No.2021J05284), Department of Science and technology of Xiamen (No. 3502z20189007), Department of Science and technology of Fujian Province (2018-CXB-23), China, Deutsche Forschungsgemeinschaft (DFG) single project YU 142/1–3 (No. 272606465), and German Federal Ministry of Education and Research (BMBF) via German Center for Lung Research (DZL), Airway Research Center North (ARCN).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Study design: XH.Y., Data aquisition: L.S., X.Z., Z.W., S.F., J.L., J.W., N.L., H.L., X.Z., Y.F., J.L., J.P., G.Y., XY.Y., J.J. and G.G. .Data analysis and interpretation: L.Z., XH.Y and A.T. Writing: XH.Y., T.G., F.P., A.T. and AK.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards, and the approval was obtained from the Ethic of Committees of the First Affiliated Hospital of Xiamen University. Since this retrospective study contains anonymous patient information, informed consent was waived by the institutional review board (IRB).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Longyu Shan, Liang Zhang and Xiaolei Zhu contributed to the manuscript equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shan, L., Zhang, L., Zhu, X. et al. Chinese never smokers with adenocarcinoma of the lung are younger and have fewer lymph node metastases than smokers. Respir Res 23, 293 (2022). https://doi.org/10.1186/s12931-022-02199-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02199-z