Abstract
Background
Cough strength is associated with short-term outcome in patients with scheduled extubation who successfully complete a spontaneous breathing trial (SBT). However, the long-term outcome is unclear.
Methods
This was a prospective observational study performed in a respiratory ICU of a teaching hospital. COPD patients who successfully completed a SBT were candidates. We enrolled the case who assessed the cough strength by cough peak flow (CPF) or semiquantitative cough strength score (SCSS, ranging from 0 = weak to 5 = strong). Patients were followed up to two years by phone every 3 months.
Results
A total of 215 patients were enrolled in current study. Among them, CPF and SCSS were measured in 214 and 208 cases, respectively. Strong cough was associated with a 16% decrease in the risk of two-year mortality (adjusted hazard ratio [HR] 0.84, 95%CI: 0.78–0.91) per 10 L/min increment of CPF. When it was tested by SCSS, decrease in the risk of two-year mortality per unit increment was 27% (adjusted HR 0.73, 95%CI: 0.62–0.86). Similar results were confirmed in the discharged patients. In all patients, the two-year mortality was 75%, 53%, and 38% in patients with CPF < 60, 60–90, and > 90 L/min; and 85%, 70%, and 40% in patients with SCSS of 0–1, 2–3, and 4–5, respectively. Similar trend was found among the discharged patients whether it was assessed by CPF or SCSS.
Conclusions
In COPD patients, weak cough is associated with increased two-year mortality after a scheduled extubation. It provides objective information to caregivers to improve decision-making process during hospitalization and after discharge.
Similar content being viewed by others
Background
Endotracheal intubation is the main interface for invasive mechanical ventilation. When the respiratory failure is reversed, weaning from mechanical ventilation is considered. Spontaneous breathing trial (SBT) has been recommended to access whether the patient has the ability to maintain spontaneous breath without ventilator support [1,2,3]. After a successful SBT, endotracheal intubation has been removed. Many studies have reported the short-term outcome among patients with a successful SBT [4,5,6,7]. However, few studies focused on the long-term outcome.
Weak cough is a strong predictor of extubation failure in patients with a successful SBT [8]. It can be measured by cough peak flow (CPF) when the staff coaches the patient to cough through the endotracheal tube as strong as possible [9]. It also can be measured by a semiquantitative cough strength scale (SCSS) [10]. However, the long-term outcome is unclear among the patients with weak cough. Here, we aimed to explore the risk of death within two years after a successful SBT in COPD patients with various cough strength assessed by CPF and SCSS.
Methods
This was a prospective observational study performed in a respiratory intensive care unit (ICU) of a teaching hospital. Patients with acute exacerbation of chronic obstructive pulmonary disease (COPD) were candidates. We enrolled those who completed a successful SBT and was ready for extubation. However, patients who had a tracheotomy or were lost to follow up were also excluded. The study protocol was approved by our local ethics committee and institutional review board (approval date: May 13th, 2011 and November 10th, 2016). As the observational nature, informed consent was waived.
Patients were managed according to current guidelines and our hospital’s protocols [2, 3, 9, 11,12,13]. We used propofol, midazolam, dexmedetomidine, fentanyl and/or morphine to manage sedation and analgesia. The target was to maintain a Ramsay score of 3 to 4 or a Richmond agitation sedation scale of − 2 to 0. Strategies such as early exercise and mobilization to speed extubation, subglottic suctioning, elevation of the head of the bed, and hand hygiene were used to prevent ventilator-associated pneumonia. Tube feeding was given according to the energy consumption. Fluid resuscitation was given to hypotensive patients. And fluid limitation was given to the patients with excessive extravascular lung water.
The respiratory therapists and physicians screened the patients every morning to identify the candidates who would initiate the weaning process. The criteria for initiation of weaning process were as follows: improvement in the underlying condition that led to acute respiratory failure; PaO2/FiO2 > 150 mm Hg in patients with chronic hypoxia or > 200 mm Hg in those with acute hypoxia; requirement of FiO2 ≤ 0.5; pH ≥ 7.35; positive-end expiratory pressure (PEEP) < 8 cmH2O; temperature < 38 °C; systolic blood pressure between 90 and 180 mm Hg (without vasopressor therapy or with only a low-dose vasopressor such as dopamine or dobutamine < 5 ug/kg/min); heart rate < 140 beats/min; and breathing frequency < 30 breaths/min.
If the patients reached the criteria for initiation of the weaning process, a SBT was performed for 30 to 120 min. The method of SBT was low level of pressure support ventilation (6–8 cm H2O). If the patient experienced an unsuccessful SBT, the pervious ventilation parameters were used and weaning attempt was assessed next day. The criteria for an unsuccessful SBT were as follows: respiratory rate > 35 breaths/min; rapid shallow breathing index (f/Vt) > 105; SpO2 < 90% with a FiO2 > 0.5; heart rate > 140 or < 50 beats/min; systolic blood pressure > 180 or < 90 mm Hg; pH < 7.3; diminishing consciousness or diaphoresis; and clinical signs indicating respiratory muscle fatigue, labored breathing, or both.
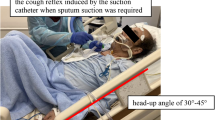
Extubation was performed when the patient successfully completed a SBT. Before extubation, cough strength was assessed. Before measurement, the head of the bed was elevated at 30–45°, and secretions were removed by suction. An external flowmeter (Chestgraph HI-101, Chest MI, Tokyo, Japan) or a built-in ventilator flowmeter (PB840, Covidien, Mansfield, Massachusetts) were used to measure CPF [9]. For external flowmeter, we disconnected the ventilator, connected the flowmeter to the endotracheal tube, and coached the patient to cough with as much effort as possible. For built-in ventilator flowmeter, the parameters of the ventilator were the same as those in a SBT. We also coached the patient to cough with as much effort as possible. At the same time, we froze the waveform of the flow velocity. Then we visually picked the peak of the flow velocity from the graph and kept the number to single digits. The best of 3 values was recorded.
To measure SCSS, we coached the patient to cough with as much effort as possible when the ventilator was disconnected. The SCSS ranged from 0 to 5: 0 = no cough on command, 1 = audible movement of air through the endotracheal tube but no audible cough, 2 = weakly (barely) audible cough, 3 = clearly audible cough, 4 = stronger cough and 5 = multiple sequential strong coughs [10]. After extubation, prophylactic use of noninvasive ventilation (NIV) or high flow nasal cannula (HFNC) was performed in patients at high risk for extubation failure [14,15,16]. All the patients with a scheduled extubation were followed up every 3 months to two years after extubation.
Statistical analysis
Patients were classified to low, moderate and strong cough, respectively, if the CPF < 60, 60–90 and > 90 L/min, or SCSS of 0–1, 2–3 and 4–5 [17, 18]. Qualitative and categorical variables were reported as numbers and percentages, and differences between groups were compared with the χ2 test or Fisher’s exact test, as appropriate. Continuous variables were reported as mean values and standard deviations or median values and interquartile ranges, as appropriate. Differences between groups were compared with one-way ANOVA or the Kruskal–Wallis H test. Cox regression was used to analyze the risk for two-year mortality. Directed acyclic graphs (DAGs) were used to select variables that were introduced in the multivariable model as potential confounders [19, 20]. Browser-based software, DAGitty (http://www.dagitty.net), was used to create DAGs. The cumulative two-year survival probability was analyzed by creating Kaplan–Meier curves. The results were presented as hazard ratios (HR) with 95% confidence interval (CI). Statistical significance was defined as P ≤ 0.05.
Results
From February 2011 to November 2019, we screened 224 COPD patients with scheduled extubation (Fig. 1). However, we excluded 8 patients due to failure to measure cough strength and one patient due to lost during two-year follow up. Therefore, 215 patients were enrolled in current study. Among them, CPF were measured in 214 cases (212 for external flowmeter and 2 for built-in ventilator flowmeter). SCSS was measured in 208 cases. There were 92 (43%), 85 (40%) and 37 (17%) patients with CPF < 60, 60–90 and > 90 L/min, respectively. Among patients who measured SCSS, 26 (13%), 102 (49%), 80 (38%) cases had SCSS of 0–1, 2–3, and 4–5, respectively.
Patients with weak cough were older than those with strong cough (Table 1). They also had higher Charlson comorbidity index, higher proportion of difficult and prolonged weaning, longer duration of mechanical ventilation before extubation, higher proportion of reintubation at 72 h after extubation, and higher duration of hospital stay after extubation than the cases with strong cough.
Two-year mortality was 59.8% and 60.1% in scheduled extubation patients who measured CPF and SCSS, respectively. In patients with CPF < 60, 60–90, and > 90 L/min, it was 75%, 53% and 38%, respectively (Table 2). Among the patients who measured SCSS, two-year mortality was 85%, 70%, and 40% in the cases with SCSS of 0–1, 2–3, and 4–5, respectively. There were 173 discharged patients tested CPF and two-year mortality was 50.2%. For the 167 discharged patients measured SCSS, two-year mortality was 50.3%. The two-year mortality was 62%, 49%, and 32% in discharged patients with CPF < 60, 60–90, and > 90 L/min, and 67%, 62%, and 34% in discharged patients with SCSS of 0–1, 2–3 and 4–5, respectively.
Confounders were identified by DAGs to explore the association between weak cough and two-year mortality (Fig. 2). In the overall cohort, the crude HR of two-year mortality was 0.81 (95%CI: 0.76–0.87) per 10 L/min increment of CPF. When it was adjusted by confounders, the HR was 0.84 (95%CI: 0.78–0.91) (Table 3). Compared with patients with CPF > 90 L/min, the HR of two-year mortality was 1.60 (95%CI: 0.88–2.91) and 3.14 (1.77–5.59) in patients with CPF 60–90 and > 90 L/min, respectively (Fig. 3). Similar results were found in the discharged patients.
Confounders were identified by the directed acyclic graphs to explore the association between weak cough and death. The red nodes above represent confounders and have been used to adjust the hazard ratio between weak cough and death. The blue nodes below represent mediators and cannot be used to adjust the hazard ratio. MV mechanical ventilation, NIV noninvasive ventilation, HFNC high-flow nasal cannula
Among the patients who measured SCSS, the crude and adjusted HR of two-year mortality was 0.68 (95%CI: 0.59–0.78) and 0.73 (0.62–0.86) per unit increment, respectively. Compared with patients with SCSS of 4–5, the HR of 2 year mortality was 2.22 (95%CI: 1.46–3.37) and 4.83 (2.79–8.36) in patients with SCSS 2–3 and 0–1, respectively (Fig. 4). The results were confirmed in the discharged patients.
Discussion
Current study shows that 60% of COPD patients with a scheduled extubation after a successful SBT died within two years after extubation. Cough strength measured by CPF or SCSS is strongly associated with two-year mortality. Weaker cough indicates higher risk for two-year mortality.
CPF is most commonly used and SCSS is secondary used to measure cough strength for prediction of extubation failure [8]. Weaker cough is associated with higher risk of extubation failure. In our study, we confirmed the results that patients with weaker cough had higher risk of reintubation. For the high risk patients, preventive use of NIV or HFNC is an alternative strategy to avoid reintubation [14,15,16].
Two-year mortality is 51% in general ICU survivors ≥ 65 years who received mechanical ventilation [21]. In our study, the two-year mortality was 60% in COPD patients with scheduled extubation and 50% in the discharged ones. Due to chronic lung disease, the two-year mortality was higher than the previous study [21]. In addition, increased age, prolonged mechanical ventilation and co-morbidities are associated with long-term outcomes [22,23,24]. We counted these factors as confounders. After adjustment of these confounders, we still found weak cough was strongly associated with two-year mortality. Therefore, the assessment of cough strength is important. For patients with weak cough, more attention should be paid such as aspiration. For the studies focused on prognosis, cough strength should be considered as a confounder to adjust the risk of interesting events (e.g. reintubation and mortality).
Cough strength is positively correlated with maximal inspiratory pressure (MIP) [25]. One-year mortality is 31% in scheduled extubation patients with low MIP (< 30 cmH2O) and 7% in those with high MIP (≥ 30 cmH2O) [26]. This is one reason for higher two-year mortality in patients with lower cough strength. In addition, weak cough also increases the risk of pneumonia within one year and aspiration is the most probable reason [27,28,29]. Moreover, persistent sputum production is a common feature in COPD patients [30, 31]. Weak cough diminishes the ability of airway clearance, which increases the risk for plugging, atelectasis and apnea. These reasons can further explain the association between weak cough and increased two-year mortality.
Airway clearance technologies such as positive expiratory pressure (PEP) devices or directed huffing increase sputum expectoration [32]. This can reduce the risk for sputum plugging and pulmonary atelectasis. In addition, pulmonary rehabilitation and inspiratory muscle training can improve the MIP [33, 34]. Higher MIP indicates higher cough strength. Therefore, airway clearance technologies, pulmonary rehabilitation and inspiratory muscle training may benefit patients with weak cough.
Current study had several limitations. First, only COPD patients were enrolled in current study. The results cannot be extrapolated to other patients. Second, we only reported the association between cough strength and two-year mortality. The quality of life is unclear. It is encouraged to explore the association between cough strength and quality of life. Third, the weight, body mass index, delirium and fluid balance may influence the measurement of cough strength and extubation. Failure to collect these variables may skew the results. Fourth, we did not record the cause of death. It is unable to determine the mechanism of weak cough and death. Further study should explore the mechanism why weak cough increases two-year mortality. Fifth, the BODE index or ADO index, which was assessed in non-critically ill patients with COPD, was associated with poor prognosis [35, 36]. Current study was focused on critically ill patients with COPD. In ICUs, it was unable to measure pulmonary function test or 6-min walking test. So, we did not collect BODE index or ADO index. However, we have collected Charlson Comorbidity index and age as confounders, which can partly reflect the originally frail.
Conclusion
Sixty percent of COPD patients with a scheduled extubation after a successful SBT died within two years after extubation. Among the discharged patients, two-year mortality was still as high as 50%. Weak cough tested by CPF or SCSS was associated with increased two-year mortality.
Availability of data and materials
The datasets analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- SBT:
-
Spontaneous breathing trial
- CPF:
-
Cough peak flow
- SCSS:
-
Semiquantitative cough strength score
- PEEP:
-
Positive-end expiratory pressure
- AECOPD:
-
Acute exacerbation of chronic obstructive pulmonary disease
- MV:
-
Mechanical ventilation
- NIV:
-
Noninvasive ventilation
- HFNC:
-
High-flow nasal cannula
- ICU:
-
Intensive care unit
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Ouellette DR, Patel S, Girard TD, et al. Liberation from mechanical ventilation in critically ill adults: an official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline: inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest. 2017;151:166–80.
Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–56.
MacIntyre NR. Evidence-based ventilator weaning and discontinuation. Respir Care. 2004;49:830–6.
Funk GC, Anders S, Breyer MK, et al. Incidence and outcome of weaning from mechanical ventilation according to new categories. Eur Respir J. 2010;35:88–94.
Béduneau G, Pham T, Schortgen F, et al. Epidemiology of weaning outcome according to a new definition. The WIND study. Am J Respir Crit Care Med. 2017;195:772–83.
Pu L, Zhu B, Jiang L, et al. Weaning critically ill patients from mechanical ventilation: a prospective cohort study. J Crit Care. 2015;30(862):e867–e813.
Jeong BH, Ko MG, Nam J, et al. Differences in clinical outcomes according to weaning classifications in medical intensive care units. PLoS ONE. 2015;10: e0122810.
Duan J, Zhang X, Song J. Predictive power of extubation failure diagnosed by cough strength: a systematic review and meta-analysis. Crit Care. 2021;25:357.
Bai L, Duan J. Use of cough peak flow measured by a ventilator to predict re-intubation when a spirometer is unavailable. Respir Care. 2017;62:566–71.
Duan J, Zhou L, Xiao M, et al. Semiquantitative cough strength score for predicting reintubation after planned extubation. Am J Crit Care. 2015;24:e86-90.
MacIntyre NR, Cook DJ, Ely EW Jr, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120:375S-395S.
Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306.
Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005; 171:388–416.
Ferrer M, Valencia M, Nicolas JM, et al. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006;173:164–70.
Nava S, Gregoretti C, Fanfulla F, et al. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med. 2005;33:2465–70.
Hernández G, Vaquero C, Colinas L, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316:1565–74.
Xiao M, Duan J. Weaning attempts, cough strength and albumin are independent risk factors of reintubation in medical patients. Clin Respir J. 2018;12:1240–6.
Fan L, Zhao Q, Liu Y, et al. Semiquantitative cough strength score and associated outcomes in noninvasive positive pressure ventilation patients with acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2014;108:1801–7.
Lederer DJ, Bell SC, Branson RD, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16(1):22.
Etminan M, Collins GS, Mansournia MA. Using causal diagrams to improve the design and interpretation of medical research. Chest. 2020;158:S21–8.
Wunsch H, Guerra C, Barnato AE, et al. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–56.
Meynaar IA, Van Den Boogaard M, Tangkau PL, et al. Long-term survival after ICU treatment. Minerva Anestesiol. 2012;78:1324–32.
Demiselle J, Duval G, Hamel JF, et al. Determinants of hospital and one-year mortality among older patients admitted to intensive care units: results from the multicentric SENIOREA cohort. Ann Intensive Care. 2021;11:35.
Poukkanen M, Vaara ST, Reinikainen M, et al. Predicting one-year mortality of critically ill patients with early acute kidney injury: data from the prospective multicenter FINNAKI study. Crit Care. 2015;19:125.
Kang SW, Shin JC, Park CI, et al. Relationship between inspiratory muscle strength and cough capacity in cervical spinal cord injured patients. Spinal Cord. 2006;44:242–8.
Medrinal C, Prieur G, Frenoy É, et al. Respiratory weakness after mechanical ventilation is associated with one-year mortality—a prospective study. Crit Care. 2016;20:231.
Kulnik ST, Birring SS, Hodsoll J, et al. Higher cough flow is associated with lower risk of pneumonia in acute stroke. Thorax. 2016;71:474–5.
Niimi A, Matsumoto H, Ueda T, et al. Impaired cough reflex in patients with recurrent pneumonia. Thorax. 2003;58:152–3.
Sakai Y, Ohira M, Yokokawa Y. Cough strength is an indicator of aspiration risk when restarting food intake in elderly subjects with community-acquired pneumonia. Respir Care. 2020;65:169–76.
Khurana S, Ravi A, Sutula J, et al. Clinical characteristics and airway inflammation profile of COPD persistent sputum producers. Respir Med. 2014;108:1761–70.
Burgel PR, Nesme-Meyer P, Chanez P, et al. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009;135:975–82.
Westerdahl E, Osadnik C, Emtner M. Airway clearance techniques for patients with acute exacerbations of chronic obstructive pulmonary disease: physical therapy practice in Sweden. Chron Respir Dis. 2019;16:1479973119855868.
Sudo E, Ohga E, Matsuse T, et al. The effects of pulmonary rehabilitation combined with inspiratory muscle training on pulmonary function and inspiratory muscle strength in elderly patients with chronic obstructive pulmonary disease. Nihon Ronen Igakkai Zasshi. 1997;34:929–34.
Vieira F, Pereira DS, Costa TB, et al. Effects of a long-term pulmonary rehabilitation program on functional capacity and inflammatory profile of older patients with COPD. J Cardiopulm Rehabil Prev. 2018;38:E12–5.
Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12.
Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet. 2009;374:704–11.
Acknowledgements
We thank all the staffs in ICUs to participate in data collection.
Funding
This study was supported by the Science and Technology Research Program of Chongqing Municipal Education Commission (No. KJQN202100414) and Chongqing Health Commission (No. 2020FYYX138).
Author information
Authors and Affiliations
Contributions
JD conceived and managed the study. JD and YH joined in study design, data collection, data analysis, and manuscript preparation. YH followed up the patients. MD, WH, RZ, LJ and LB participated in patient recruitment and data collection, and revised the manuscript. All authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board of the First Affiliated Hospital of Chongqing Medical University approved the study. As the observational nature, informed consent was waived.
Consent for publication
Not applicable.
Competing interests
We declare that there is no competing interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hong, Y., Deng, M., Hu, W. et al. Weak cough is associated with increased mortality in COPD patients with scheduled extubation: a two-year follow-up study. Respir Res 23, 166 (2022). https://doi.org/10.1186/s12931-022-02084-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-022-02084-9