Abstract
Background
Several immune mechanisms activate in COVID-19 pathogenesis. Usually, coronavirus infection is characterized by dysregulated host immune responses, interleukine-6 increase, hyper-activation of cytotoxic CD8 T lymphocytes. Interestingly, Vitamin D deficiency has been often associated with altered immune responses and infections. In the present study, we evaluated Vitamin D plasma levels in patients affected with different lung involvement during COVID-19 infection.
Methods
Lymphocyte phenotypes were assessed by flow cytometry. Thoracic CT scan involvement was obtained by an image analysis program.
Results
Vitamin D levels were deficient in (80%) of patients, insufficient in (6.5%) and normal in (13.5%). Patients with very low Vitamin D plasma levels had more elevated D-Dimer values, a more elevated B lymphocyte cell count, a reduction of CD8 + T lymphocytes with a low CD4/CD8 ratio, more compromised clinical findings (measured by LIPI and SOFA scores) and thoracic CT scan involvement.
Conclusions
Vitamin D deficiency is associated with compromised inflammatory responses and higher pulmonary involvement in COVID-19 affected patients. Vitamin D assessment, during COVID-19 infection, could be a useful analysis for possible therapeutic interventions.
Trial registration: 'retrospectively registered'.
Similar content being viewed by others
Background
COVID-19 infection is still an open challenge to date. Although the clinical features following the penetration of the virus into our respiratory system are known, the pathobiology and the mechanisms that regulate this entry and the reasons behind the multivarious clinical pictures observed are still unknown. Unfortunately, about 20% of infected patients developed a severe respiratory disease characterized by diffuse pulmonary infiltrates and damage of alveolar type II pneumocytes, which undergoes apoptosis and death [1]. The involved alveolar units appear to be peripheral and subpleural. Furthermore, a viral driven hyperinflammation has been reported [2]. An early overproduction of pro-inflammatory cytokines has been described and defined cytokine storm [2, 3]. Among them, elevated IL-6 plasma levels were included as predictor of mortality [4]. Recent observations have shown that Vitamin D (VitD) is not a mere micronutrient involved in calcium metabolism and bone healthy but it also plays an important role as a pluripotent hormone in several immunological mechanisms [5].
It is known that enzymes catalyzing its activation and the VitD receptors (VDR), that mediates the actions of the vitamin itself, are widely distributed on the whole cell bodies and in particular in pulmonary alveolar epithelium and immune system. Although the in-vivo effects of VitD are not completely understood, a number of observations underline the VitD role in lung infection and in lung diseases’ development [5, 6]. VitD insufficiency has been related to viral infections of the lower respiratory tract [7] and to exacerbation in chronic obstructive lung diseases and asthma [5, 6].
Normally, VitD ingested or endogenously produced is hydroxylated to 25-hydroxy VitD (25HOD) by the liver. This metabolite is measured to assess VitD status. Its hormonal activity is reached after renal 1a-hydroxilase’s activation to 1,25OH2D. Without any supplementation, VitD status is strictly dependent by endogenous production that may be influenced by genetic variants of VitD-binding proteins, seasons, latitude, skin and lifestyle [8, 9]. More interestingly, studies concerning VitD effects on human adaptive immune responses, demonstrated the expression of the nuclear VitD receptor and VitD activating enzymes within immune cells [10]. In both activated T and B lymphocytes an up-regulation of VitD receptors was demonstrated [10, 11]. Recent studies underline an indirect, through helper T lymphocytes and direct role of VitD on B lymphocytes homeostasis. T lymphocytes are important targets of the immunomodulatory effects of VitD.
Notably, they influence the secretion of a variety of proinflammatory cytokines [12]. Th1 lymphocytes (IL-2, INF-gamma, TNF-alpha) and VitD plasma levels are inversely related to plasma levels of IL-6 [13]. VitD down-regulates IL-6 mRNA levels [13]. On the other hand, IL-6 synthesis has been correlated with immune cell differentiation and maturation, and cytokine production [14]. In T lymphocytes cultures, VitD seems to facilitate the development of a tolerogenic phenotype and the increase of genes involved as regulatory T lymphocytes (Tregs) [15].
In subject with VitD deficiency, its supplementation is able to reduce the risk to develop different viral infections [16]. Furthermore, subjects with low levels of VitD at the time of COVID-19 testing were at higher risk to be positive for COVID-19 compared to those subjects with sufficient VitD status [17].
Therefore, our study aimed to assess whether VitD deficiency was as a risk factor to develop more severe clinical pictures and more serious lung involvement in patients suffering from coronavirus infection admitted to our hospital during COVID-19 pandemic.
Materials and methods
Patients
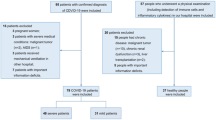
52 hospitalized patients affected with Covid-19 infection and with different degree of lung involvement, diagnosed by nasopharyngeal and oropharyngeal swabs (One-step RT-PCR Kit Qiagen, detection kit, Milan Italy) (TaqMan Fast Virus 1-Step Master Mix, Thermo Fisher, Milan, Italy), positive for the presence of the SARS-CoV-2 virus were enrolled. The research has been conducted at the Sant’Andrea Hospital, designated by the Regione Lazio as one of the COVID-19 referral Hospitals in Rome, Italy. No signs of malnutrition were reported. Comorbidities and patients’ characteristics were indicated in Table 1.
Exclusion criterions were recent cardiovascular accidents, diagnosis of cancer, autoimmune diseases. None of the patients enrolled in this study have had BCG vaccination, in Italy anti-tuberculosis vaccine prophylaxis is mandatory only for healthcare professionals exposed to a high risk of contagion and for those who have clinical contraindications to the use of anti-tuberculosis specific drugs.
In all patients, VitD serum levels were dosed during the acute phase of the disease. The dosage of the VitD was performed at the time of admission into the hospital, before starting any kind of therapy. At the same time, different markers of inflammation (high-sensitive C Reactive Protein hs-CRP, Procalcitonin PCT), cellular damage (hypersensitive troponin I, creatin kinase miocardial band, lactate deidrogenase) and coagulation (prothrombin time, fibrinogen and D-dimer) were assessed.
Flow cytometry was performed in the whole patients’ cohort by the automated AQUIOS CL® "load & go" flow cytometer (Beckman Coulter, Life Sciences Division, Indianapolis, USA), to define peripheral lymphocyte cell type count.
The interleukin-6 (IL-6) was measured using a dedicated kit (IL-6 Human SimpleStep Elisa Kit, Thermo Fisher, Milan, Italy).
CT acquisition
Chest CT scans were performed on all patients with COVID-19 infection using two 16-row multi-slice CT scanners. Patients were placed in a supine position and advanced head, continuous spiral scanning was performed from the lung top to the lung bottom. For CT acquisition, the tube voltage was 120 kVp with automatic tube current modulation and pitch 0.99–1.45 mm. From the raw data, CT images were reconstructed on a matrix of 512 × 512 and a field of view 350 × 350 mm as axial images (section thickness of 10 mm) The thickness of the axial reconstruction layer was 1.0 mm, the window width was 1000–2000 HU, and the window level was 700–500 HU. Quantitative results were individually assessed by two senior radiologists, and discussions were used to resolve differences in data interpretation.
Quantification of lung CT lesions by PII
The results of lung computed tomography (CT) scans were quantified using the pulmonary inflammation index (PII). This modified semi-quantitative scoring system (Lung Quantitative Software -Siemens) was used to quantitatively assess pulmonary involvement in all patients according to lung lesion distribution and size. A total of 20 lung segments (in both left and right lungs) were assessed. The lesion size score was based on the occupation major or minor than 50% of the lung segment, by the lesion. The score was 1 for ≥ 50% involvement and zero for < 50%, with a maximum total score of 20. Higher the value, the more severe inflammatory load. PII = lesion distribution score + lesion size score/total score (40 points) × 100%.
We arbitrarily divided patients with CT scan evaluation into two groups on the basis of lung involvement: TSS ≤ 7 and TSS > 7.
SOFA score
The Sequential Organ Failure Assessment (SOFA) score is a mortality prediction score based on the degree of dysfunction of six organ systems (Respiratory system, Nervous system, Cardiovascular system, Liver, Coagulation, Kidneys). It depends on the following variables: PaO2/FiO2, Platelets count in × 10 ^3/µL, Glasgow Coma Scale from grade 0 to + 4, bilirubin value measured in mg/dL (μmol/L), mean arterial pressure measured in mmHg, creatinine, mg/dL (μmol/L) [18]. Epidemiological, anamnestic and clinical data regarding patients are listed in Table 1 (Table 1).
Analyzing SOFA score results, we arbitrarily classified patients into two groups. The first group resulted in SOFA < 2, the latter in SOFA > 2.
Lung immune prognostic index score
The Lung Immune Prognosis Index (LIPI) score was obtained combining baseline dNLR and LDH. It is usually considered as a prognostic value, irrespective of treatment modality, in several human malignancies. By LIPI SCORE we categorized 3 groups: good (dNLR < 3 + LDH 3 + LDH < upper limit of normal (ULN), intermediate (dNLR > 3 or LDH > ULN), poor risk (dNLR > 3 + LDH > ULN) [19]. Analyzing LIPI score, we identified two main groups: the first had LIPI 0–1 values, the second had LIPI = 2.
Statistical analysis
Continuous variables (quantitative) are described by mean and standard deviation (SD) while categorical variables by frequency or percentage. The comparison of quantitative variables (inflammatory markers, markers of cellular damage, and coagulation) between groups was performed using the Student's t test and by the Mann‐Whitney‐Wilcoxon text for independent samples. The chi-square test or Fisher’s exact test was used to examine categorical data (SOFA; LIPI and CT score). The correlation between VitD and other parameters was assessed using Spearman’s correlation test.
Descriptive statistical analysis was performed on raw data where applicable. Results were expressed as means ± SD. A two-tailed P value of 0.05 or less was used as a criterion to indicate statistical significance. NS = not significant. Data were statistically analyzed using the GraphPad software (version) (GraphPad Software, San Diego, CA).
Ethical approval
A written informed consent was obtained by participants to the study. The study was approved by our Institutional Ethical Committee (University La Sapienza of Rome, Italy) (Prot.# 52SA_2020, RIF. CE 5773_2020), on the basis that it complied with the declaration of Helsinki and that the protocol followed existing good clinical practice guidelines.
Results
Fifty-two patients affected with COVID-19 infection with lung involvement were enrolled in the study (27 female and 25 male, the median age was 68,4 years, ranging from 29 to 94 years) (Patient’s characteristics reported in Table 1). The mean age of the patients with very low VitD plasma levels (under 10 ng/ml) and patients with VitD plasma levels over 10 was 77.5 + 16 and 68,9 + 18 respectively. Therefore, patients were classified into two subgroups, according to VitD plasma levels. The former “under 10 ng/mL”, named Group 1 (mean value 5.65 + 2.43 ng/mL), and the latter, “over 10 ng/mL” named Group 2 (21,54 + 8,81 ng/dL). Data regarding flow cytometry are listed in Table 2.
Although any significant difference in lymphocyte cell count has been found between the two groups, by flow cytometry, a moderate correlation was demonstrated comparing VitD plasma levels to TCD8 + cytotoxic lymphocytes and CD4 + /CD8 + ratio in both groups (Fig. 1a, b). In particular, a statistically lower TCD8 + cell count was observed in Group 1 in comparison to Group 2 and (Fig. 2b), consequently, CD4 + /CD8 + ratio resulted increased (Fig. 2a). Furthermore, a robust correlation was identified comparing VitD and Neutrophils cell count, Neutrophils and Lymphocytes ratio (NLR) and derived Neutrophils/ Leucocytes-Neutrophils ratio (dNLR) (Fig. 1c–e). A statistically significant increase in NLR, Neutrophils cell count and dNLR was detected in Group 1, if compared to Group 2 (Table 2 and Fig. 2c, d).
The histograms indicate that patients belonging to Group 1 had a statistically significant difference with Group2. The a indicate that patients in Group 1 with low Vit D levels had more elevated CD4/CD8 ratio (a), NLR (c) and dNLR (d). Contrarily, CD8 + cell count was lower in Group 1 than Group 2 (b). The comparison of quantitative variables between groups was performed using the Student's t test and by the Mann‐Whitney‐Wilcoxon text for independent samples
Assessing IL-6 plasma levels, the key element in cytokines’ inflammatory scenario, high variability of values was found among patients. However, a significant difference was documented in Group 1, despite Group 2 (Table 2).
In patients belonging to Group 1, D-Dimer plasma levels were statistically increased in comparison to Group 2 (Table 2), and a statistically significant difference was found.
A correspondence between VitD and platelets count has been documented, although not statistically significant. Pro-calcitonin levels were also investigated and no difference between the two groups was demonstrated.
Other inflammatory markers (hs-CRP), cellular damage markers (such as hypersensitive Troponin, creatin kinase myocardial band, lactate dehydrogenase), coagulation markers (fibrinogen and prothrombin time) studied did not demonstrated statistically significant differences between the two groups.
We detected lower SOFA score, LIPI score and TSS values in patients with higher VitD levels. These patients were mainly included in the Group 2. Obviously, patients with higher SOFA and LIPI score, and higher TSS values displayed lower VitD levels, belonging to the Group 1 (Fig. 3a–c). Moreover, assessing LIPI score we documented that 62.5% were LIPI 2 (poor prognosis), 33.4% were LIPI 1 (intermediate prognosis), and 4.1% were LIPI 0 (good prognosis).
Although the number of patients was not particularly high, such as to allow a clear statistical evaluation, Group 1 showed a higher mortality rate than Group 2. In fact, three patient died in Group 1 but none in Group 2.
Discussion
The present study compared, for the first time, VitD plasma levels with different health related scores, inflammatory markers, markers of cellular damage and coagulation and radiological findings during COVID-19 illness. Interestingly, patients with low VitD plasma levels had compromised biochemical and clinical findings reflected by the profound immunological involvement. Vitamin D insufficiency and deficiency were common in COVID-19 affected patients. About 8% of the study cohort had normal VitD plasma levels. Patients with more severe COVID-19 disease had lower Vit D plasma levels regardless of age.
VitD plasma levels are the most accurate markers for defining VitD state. 25(OH)D is the major form of circulating of VitD and it is the one measured. In general, in population VitD levels lower than 20 ng/mL (50 nmol/L) are commonly considered as deficiency status. Moreover, levels ranging 20 to 29 ng/mL (52–72 nmol/L) should define insufficiency, while levels above 30 (75 nmol/L) sufficiency [20]. Moreover, these levels are strictly related to VitD effects on bone metabolism, while VitD levels useful for investigation of other aspects of human health are under investigation [10, 20]. For a long time, 10 ng/ml has been considered the VitD level for defining deficiency [21, 22]. Although the Endocrine Society has defined 20 ng/ml VitD plasma levels, in terms of 25(OH)D, as the threshold to define the deficiency status [23] there are insufficient evidences to clarify the optimal plasma VitD concentration necessary for the global well-being [23,24,25].
From a general point of view, VitD activity also seems essential in the regulation of oxidative stress and survival mechanisms [26]. Furthermore, a broad number of studies suggests the role of VitD not only as a simple micronutrient, linked to calcium homeostasis, but as a pluripotent hormone which has extensive immuno-modulatory functions [27]. The respiratory alveolar epithelium represents the first line of defense able to counteract and prevent the entry of inhaled pathogens. It represents one of the main actor of the innate immunity including alveolar macrophages and dendritic cells. If stimulated, these cells activate a variety of intracellular signaling pathways for specific antimicrobial defenses, release of inflammatory mediators and adaptive immune responses [28]. Adaptive immune response is strictly related to the ability of T and B lymphocytes to secrete cytokines and produce immunoglobulins respectively [29]. VitD deficiency has been correlated with increasing levels of IL-6[30], while VitD supplementation down regulates IL-6 levels in several studies [31]. IL-6 is elevated in COVID-19 patients with severe disease and it is also considered a relevant prognostic marker. It has been reported that mortality is higher in patients with elevated levels of IL-6. Therefore, IL-6 and IL-6R are receiving more attention as potential therapeutic targets for the treatment of COVID-19. In our COVID-19 infected patient group we have documented elevated IL-6 levels, upper the normal limits, in almost all the patients. Unfortunately, large variability among them was documented. Therefore, no correlation was detectable between VitD and IL-6 values in this cohort of patients. Moreover, in patients with very low VitD levels, the IL-6 values was slightly, but not significantly, more elevated in comparison with patients with higher VitD levels. This result may be attributed to sample size.
Elevated neutrophil count predicts ongoing inflammation and decreased lymphocyte count is considered an indicator of poor prognosis. A combination of these two measures, and the derived NLR ratio dNLR, are considered predictive of an inflammatory status. In acute Covid-19 disease, the more severe status is often associated with increased neutrophil cell count and a reduction of lymphocytes. Both CD4 T-helper and CD8 T-cytotoxic lymphocytes may be affected, with a CD4 more severe reduction associated with more severe disease and worst prognosis. In our patients, a moderate correlation was observed between CD4 + /CD8 + ratio, CD8 + count and VitD plasma levels. In addition, a statistically significant difference was detected between patients with low VitD plasma levels in comparison with those with more elevated VitD levels in both CD4 + /CD8 + and CD8 cell count. More elevated CD4/CD8 ratio was detected in patients with low VitD plasma levels. T-lymphocytes are essential in coordinating several immune functions, helping macrophages and B lymphocytes to counteract the development of the disease. The disease-induced loss of lymphocyte activity markedly weakens the immunological responses. Although, the causes of peripheral blood lymphocyte (PBMC) suppression were under investigation, no viral gene expression was detected in PBMC and no COVID-19 virus infection was demonstrated in these cells [32]. Their reduction may be the result of migration/compartmentalization to the site of damaged tissue [33]. In fact, the T CD8 + cell expansion into the lung of mild symptomatic COVID patients, assessed by bronchoalveolar lavage has been described [33].
Categorizing patients as a function of TC Score analyzing lung findings obtained by high resolution computer tomography, we demonstrated that lower plasma levels of Vit D are strictly related with an increase lung involvement characterized by more diffuse ground glass opacities within the lung. This condition reflects more severe disease linked to the redundant and dysfunctional immunological responses described. The same is true describing the sequential organ failure assessment (SOFA) score. It allows us to assess the performance of different organ systems into the body, returning the risk of mortality, based on the relationship between organ failure and mortality. The relationship observed in LIPI and SOFA scores allow us to hypothesize that very low levels of VitD are associated with worst prognosis in COVID-19 patients.
None of the patients studied have had BCG vaccination, patients over the age of 65 had undergone anti-flue vaccination. Although a no-specific protection of vaccination, in particular with BCG, from different viral infections has been reported [34] the protective role of BCG or other vaccination to protect against COVID-19 infection should be interpreted with caution [35]. Furthermore, no significant difference in COVID-19 spread rate was detected between countries with or without current BCG vaccination policy [36].
Conclusions
Although an inverse correlation between plasma VitD and all causes of mortality in healthy or general medical clinic cohorts has been described in particular in the lowest quantile (0–9 ng/ml), the effect of VitD deficiency in COVID-19 progression or disease severity is far to be assessed. Our data underline a relationship between VitD plasma levels and different serum markers of disease. At the moment it is difficult to argue if VitD supplementation can play a role in fighting the severity of the disease as well as reducing its mortality, but it may be a useful as well as a safe recommendation for almost all patients.
Availability of data and materials
The data that support the plots within this paper and other finding of this study are available from the corresponding author upon reasonable request.
Abbreviations
- VitD:
-
Vitamin D
- CT:
-
Computed tomography
- PII:
-
Pulmonary inflammation index
- SOFA:
-
Sequential Organ Failure Assessment
- LIPI:
-
Lung Immune Prognosis Index
- PBMC:
-
Peripheral blood lymphocyte
- NLR:
-
Neutrophils and Lymphocytes ratio
- dNLR:
-
Derived Neutrophils/ Leucocytes-Neutrophils ratio
- TSS:
-
Total severity score
References
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–8. https://doi.org/10.1016/S0140-6736(20)30937-5.
Ritchie AI, Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. 2020;395:1111. https://doi.org/10.1016/S0140-6736(20)30691-7.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. HLH across speciality collaboration, UK, COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. https://doi.org/10.1016/S0140-6736(20)30628-0.
Baune BT, Rothermundt M, Ladwig KH, Meisinger C, Berger K. Systemic inflammation (Interleukin 6) predicts all-cause mortality in men: results from a 9-year follow-up of the MEMO Study. Age (Dordr). 2011;33:209–17. https://doi.org/10.1007/s11357-010-9165-5.
Hejazi ME, Modarresi-Ghazani F, Entezari-Maleki T. A review of Vitamin D effects on common respiratory diseases: Asthma, chronic obstructive pulmonary disease, and tuberculosis. J Res Pharm Pract. 2016;5:7–15. https://doi.org/10.4103/2279-042X.176542.
Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158:20–5. https://doi.org/10.1111/j.1365-2249.2009.04001.x.
Teymoori-Rad M, Shokri F, Salimi V, Marashi SM. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29:e2032. https://doi.org/10.1002/rmv.2032.
Holick MF. Vitamin d Deficiency. N Engl J Med. 2007;357:266–81. https://doi.org/10.1056/NEJMra070553.
Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung C-L, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen A-L, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson J-O, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin M-R, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–8. https://doi.org/10.1016/S0140-6736(10)60588-0.
Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–21. https://doi.org/10.3390/nu5072502.
Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–96. https://doi.org/10.1016/j.coph.2010.04.001.
Aslam MM, John P, Bhatti A, Jahangir S, Kamboh MI. Vitamin D as a principal factor in mediating rheumatoid arthritis-derived immune response. Biomed Res Int. 2019. https://doi.org/10.1155/2019/3494937.
Zhang Y, Leung DYM, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–35. https://doi.org/10.4049/jimmunol.1102412.
Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, Malaguarnera L. Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol. 2012;280:36–43. https://doi.org/10.1016/j.cellimm.2012.10.009.
Navarro-Barriuso J, Mansilla MJ, Naranjo-Gómez M, Sánchez-Pla A, Quirant-Sánchez B, Teniente-Serra A, Ramo-Tello C, Martínez-Cáceres EM. Comparative transcriptomic profile of tolerogenic dendritic cells differentiated with vitamin D3, dexamethasone and rapamycin. Sci Rep. 2018;8:14985. https://doi.org/10.1038/s41598-018-33248-7.
Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S, Stelmach I, Kumar GT, Urashima M, Camargo CA. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017. https://doi.org/10.1136/bmj.i6583.
Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3:e2019722. https://doi.org/10.1001/jamanetworkopen.2020.19722.
Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37:1649–54. https://doi.org/10.1097/CCM.0b013e31819def97.
Kazandjian D, Gong Y, Keegan P, Pazdur R, Blumenthal GM. Prognostic value of the lung immune prognostic index for patients treated for metastatic non-small cell lung cancer. JAMA Oncol. 2019. https://doi.org/10.1001/jamaoncol.2019.1747.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA, The, . Report on dietary reference intakes for calcium and vitamin D from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(2011):53–8. https://doi.org/10.1210/jc.2010-2704.
Baeke F, van Etten E, Gysemans C, Overbergh L, Mathieu C. Vitamin D signaling in immune-mediated disorders: evolving insights and therapeutic opportunities. Mol Aspects Med. 2008;29:376–87. https://doi.org/10.1016/j.mam.2008.05.004.
Laaksi I, Ruohola J-P, Tuohimaa P, Auvinen A, Haataja R, Pihlajamäki H, Ylikomi T. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–7. https://doi.org/10.1093/ajcn/86.3.714.
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. https://doi.org/10.1210/jc.2011-0385.
Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, El-Hajj Fuleihan G, Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019. https://doi.org/10.1530/EJE-18-0736.
SACN vitamin D and health report, GOV.UK. (n.d.). https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report. Accessed 6 Jul 2020.
Chirumbolo S, Bjørklund G, Sboarina A, Vella A. The role of Vitamin D in the immune system as a pro-survival molecule. Clin Ther. 2017;39:894–916. https://doi.org/10.1016/j.clinthera.2017.03.021.
Hansdottir S, Monick MM. Vitamin D effects on lung immunity and respiratory diseases. Vitam Horm. 2011;86:217–37. https://doi.org/10.1016/B978-0-12-386960-9.00009-5.
Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286:L887-892. https://doi.org/10.1152/ajplung.00323.2003.
Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–5. https://doi.org/10.1126/science.1183021.
Naghavi Gargari B, Behmanesh M, Shirvani Farsani Z, Pahlevan Kakhki M, Azimi AR. Vitamin D supplementation up-regulates IL-6 and IL-17A gene expression in multiple sclerosis patients. Int Immunopharmacol. 2015. https://doi.org/10.1016/j.intimp.2015.06.033.
Goncalves-Mendes N, Talvas J, Dualé C, Guttmann A, Corbin V, Marceau G, Sapin V, Brachet P, Evrard B, Laurichesse H, Vasson M-P. Impact of Vitamin D supplementation on influenza vaccine response and immune functions in deficient elderly persons: a randomized placebo-controlled trial. Front Immunol. 2019;10:65. https://doi.org/10.3389/fimmu.2019.00065.
Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, Guo D, Hu W, Yang J, Tang Z, Wu H, Lin Y, Zhang M, Zhang Q, Shi M, Liu Y, Zhou Y, Lan K, Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9:761–70. https://doi.org/10.1080/22221751.2020.1747363.
Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Chen L, Li J, Wang X, Wang F, Liu L, Zhang S, Zhang Z. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. MedRxiv. 2020. https://doi.org/10.1101/2020.02.23.20026690.
Moorlag SJCFM, Arts RJW, van Crevel R, Netea MG. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019. https://doi.org/10.1016/j.cmi.2019.04.020.
Connecting BCG Vaccination and COVID-19: Additional Data | medRxiv, (n.d.). https://www.medrxiv.org/content/10.1101/2020.04.07.20053272v2. Accessed 16 Jan 2021.
Hensel J, McAndrews KM, McGrail DJ, Dowlatshahi DP, LeBleu VS, Kalluri R. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci Rep. 2020;10:18377. https://doi.org/10.1038/s41598-020-75491-x.
Acknowledgements
We greatly appreciate the Sant'Andrea Hospital COVID-19 group for the assistance and the courtesy to provide hospitalized patients clinical data. In particular, we are gratefully to Prof De Biase Luciano, Prof Paolo Martelletti, Prof Antonio Aceti and Prof Monica Rocco, part of the members of the Study Group that have applied to the Ethic Committee.
Funding
Not received.
Author information
Authors and Affiliations
Contributions
The authors have made the following declaration about their contributions. Conceived and designed: AR, AP, MD, BS, SG. Development and methodology: CDV, RM, VS, PA, AM, Acquisition of data: MI, CDD, AL, EM. FF. Analysis and interpretation of the results: AR, AP, MD, MI. Writing, review and revision of the manuscript: AR, AP, MD, SS Study supervision: AR, MD. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by our Institutional Ethical Committee (Sapienza University of Rome, Italy) (Prot.# 52SA_2020, RIF. CE 5773_2020), on the basis that it complied with the declaration of Helsinki and that the protocol followed existing good clinical practice guidelines. A written informed consent to participate in the study was obtained from every participant. In case of adults with cognitive decline the consent to participate to the study was given by the legal guardian. An administrative permission to access the clinical/personal patient data used in our research was given by the Health Director of our Hospital, who is also a co-author of the manuscript.
Consent for publication
Not applicable.
Competing interests
The authors declare they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ricci, A., Pagliuca, A., D’Ascanio, M. et al. Circulating Vitamin D levels status and clinical prognostic indices in COVID-19 patients. Respir Res 22, 76 (2021). https://doi.org/10.1186/s12931-021-01666-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-021-01666-3