Abstract
Background
Tidal expiratory flow limitation (EFLT) promotes intrinsic PEEP (PEEPi) in patients with chronic obstructive pulmonary disease (COPD). Applying non-invasive ventilation (NIV) with an expiratory positive airway pressure (EPAP) matching PEEPi improves gas exchange, reduces work of breathing and ineffective efforts. We aimed to evaluate the effects of a novel NIV mode that continuously adjusts EPAP to the minimum level that abolishes EFLT.
Methods
This prospective, cross-over, open-label study randomized patients to one night of fixed-EPAP and one night of EFLT-abolishing-EPAP. The primary outcome was transcutaneous carbon dioxide pressure (PtcCO2). Secondary outcomes were: peripheral oxygen saturation (SpO2), frequency of ineffective efforts, breathing patterns and oscillatory mechanics.
Results
We screened 36 patients and included 12 in the analysis (age 72 ± 8 years, FEV1 38 ± 14%Pred). The median EPAP did not differ between the EFLT-abolishing-EPAP and the fixed-EPAP night (median (IQR) = 7.0 (6.0, 8.8) cmH2O during night vs 7.5 (6.5, 10.5) cmH2O, p = 0.365). We found no differences in mean PtcCO2 (44.9 (41.6, 57.2) mmHg vs 54.5 (51.1, 59.0), p = 0.365), the percentage of night time with PtcCO2 > 45 mm Hg was lower (62(8,100)% vs 98(94,100)%, p = 0.031) and ineffective efforts were fewer (126(93,205) vs 261(205,351) events/hour, p = 0.003) during the EFLT-abolishing-EPAP than during the fixed-EPAP night. We found no differences in oxygen saturation and lung mechanics between nights.
Conclusion
An adaptive ventilation mode targeted to abolish EFLT has the potential to reduce hypercapnia and ineffective efforts in stable COPD patients receiving nocturnal NIV.
Trial registration: ClicalTrials.gov, NCT04497090. Registered 29 July 2020—Retrospectively registered, https://clinicaltrials.gov/ct2/show/NCT04497090.
Similar content being viewed by others
Background
We have recently introduced a novel automatic ventilation mode that continuously titrates expiratory positive airway pressure (EPAP) to the lowest value that abolishes tidal expiratory flow limitation (EFLT) [1]. This method, which uses the difference between inspiratory and expiratory reactance (ΔXrs) measured by the forced oscillation technique (FOT) [2, 3] to assess the presence of EFLT, minimizes the neural respiratory drive and trans-diaphragmatic pressure swings in COPD patients receiving non-invasive ventilation (NIV) [4].
We hypothesized that this adaptive ventilation mode would reduce hypercapnia during sleep in COPD patients with chronic hypercapnic respiratory failure. Moreover, since EFLT is associated with the development of intrinsic positive end-expiratory pressure (PEEPi)—which acts as an inspiratory threshold for the generation of inspiratory flows and can produce ineffective breath triggering [5]—we further hypothesized that the EFLT-abolishing respiratory support mode would reduce the triggering load imposed by PEEPi reducing the probability of ineffective efforts.
This pilot study aimed to evaluate the effects of a novel ventilation mode that automatically adjusts EPAP at the minimum level able to abolish EFLT compared to the standard fixed-EPAP mode in stable COPD patients receiving nocturnal NIV.
Materials and methods
Study design
In this prospective, randomized, cross-over, open-label pilot study, patients were studied in the hospital over two non-consecutive nights while using either fixed-EPAP or EFLT-abolishing-EPAP.
Population
We enrolled moderate to severe COPD patients [6], with FEV1 ≤ 50%Pred, a history of more than 3 exacerbations per year or more than 1 hospitalization per year. Patients were well established on nocturnal NIV for chronic hypercapnic respiratory failure for longer than 6 months. Inclusion criteria were age below 85 years and presence of EFLT in the supine position at EPAP = 4 cmH2O [1]. Exclusion criteria were COPD exacerbation within the past two months, acute illness, or clinical instability.
Outcomes
The primary outcome was PtcCO2, expressed as mean overnight value and the percentage of night time spent in hypercapnia. Secondary outcomes were oxygen saturation, ineffective efforts, breathing pattern, and oscillatory mechanics. We hypothesized that mean PtcCO2 and the percentage of the night spent in hypercapnia would be lower during the EFLT-abolishing-EPAP than during the fixed-EPAP night.
Ventilation strategy
Pressure support NIV was delivered using a non-commercial version of BiPAP Synchrony Ventilator (Philips-Respironics) via an unvented facial mask (AMARA, Philips-Respironics). The ventilator measured EFLT by FOT [1, 2, 7] and, in EFLT-abolishing-EPAP mode, it continuously adjusted EPAP to the minimum level able to abolish EFLT [1], with a minimum EPAP of 4 cmH2O and keeping the pressure support (∆P) constant.
Measurements
Full laboratory polysomnography (Alice5, Philips-Respironics) was performed according to the American Academy of Sleep Medicine recommendations [8]. During each study night, we recorded PtcCO2 and oxygen saturation (SpO2) (TOSCA, Radiometer) continuously. Airway opening pressure, flow and volume tracings were exported from the ventilator for offline analysis. We calculated the following parameters: mean PtcCO2 and SpO2; the percentage of night time spent in hypercapnia (PtcCO2 > 45 mm Hg) and with SpO2 < 90% (T90); mean tidal volume (VT), respiratory rate (RR), minute ventilation (VE), inspiratory resistance and reactance (RINSP and XINSP, respectively), ΔXrs, and the number of ineffective efforts (IE) per hour. We identified ineffective efforts by the presence of a positive deflection in expiratory flow without a concomitant breath delivered by the ventilator, as previously described [9]. At the end of the night, we asked the patients to report about their comfort on the ventilator.
Statistical analysis
We compared parameters from the two nights using Wilcoxon signed-rank test. p-Values < 0.05 were considered statistically significant. Statistical analyses were performed using SigmaPlot v11 (Systat Software, Inc., San Jose, CA, USA).
Results
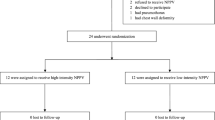
We screened thirty-six patients from April 2015 to April 2017. Of these patients, 19 did not satisfy the inclusion criteria as they did not present EFLT, one withdrew consent after the screening, two did not perform the second night trial because they got acutely sick, one was excluded from the analysis because of poor data quality, and 12 were included in the analysis (Fig. 1). Table 1 reports the characteristics of the patients included in the analysis.
Some patients acknowledged the presence of the oscillations, but they got acclimated after just few minutes. No patients reported discomfort during the EFLT-abolishing-EPAP night. We observed large within-night fluctuations in EPAP during the EFLT-abolishing-EPAP night: the minimum within-night IQR was 1.8 cmH2O, the maximum within-night IQR was 8.8 cmH2O. Figure 2 shows the airway pressure and PtcCO2 of a representative patient during both nights.
The EPAP applied by the EFLT-abolishing mode was not significantly different from the prescribed EPAP (median (IQR) 7.0 (6.0, 8.8) cmH2O during the EFLT-abolishing-EPAP night vs 7.5 (6.5, 10.5) cmH2O during the fixed-EPAP night, p = 0.365), despite its larger within-night variability. ΔXrs values clustered around the EFLT threshold during the EFLT-abolishing-EPAP night (Fig. 3).
EPAP and tidal expiratory flow limitation index during the night with EFLT-abolishing-EPAP vs. fixed-EPAP. Data are reported as individual values (open symbols dotted lines) and as mean ± SD of all subjects (closed symbols). Dashed-line: tidal expiratory flow limitation threshold. EPAP: expiratory positive airway pressure. ΔXrs: difference between mean inspiratory and expiratory reactance (tidal expiratory flow limitation index). ΔXrs data for 11 subjects
Figure 4 shows gas exchange parameters during the EFLT-abolishing-EPAP and the fixed-EPAP nights. The percentage of time spent in hypercapnia was lower (median (IQR) = 62 (8, 100)% vs. 98 (94, 100)%, p = 0.031) during the EFLT-abolishing-EPAP than during the fixed-EPAP night. We found no differences in mean PtcCO2 between the EFLT-abolishing-EPAP and the fixed-EPAP night (44.9 (41.6, 57.2) mmHg vs 54.5 (51.1, 59.0), respectively; p = 0.365). Mean SpO2 and T90 did not differ between nights.
Gas exchange during the night with EFLT-abolishing-EPAP vs. fixed-EPAP. Data are reported as individual values (open symbols dotted lines) and as mean ± SD of all subjects (closed symbols). SpO2: peripheral oxygen saturation. T90: percentage of time spent with oxygen saturation SpO2 below 90%. PtcCO2: transcutaneous partial pressure of carbon dioxide. T hypercapnia: percentage of time spent with a PtcCO2 > 45 mm Hg. T hypercapnia and ΔXrs data for 11 subjects. *p < 0.05 between nights
The IE were fewer (126 (93, 205) vs. 261 (205, 351) events/hour, p = 0.003) during the EFLT-abolishing-EPAP than during the fixed-EPAP night. Additionally, mean VT was lower (p = 0.029), and RR was higher (p = 0.035) during the EFLT-abolishing-EPAP than during the fixed-EPAP night. VE, RINSP, and XINSP did not differ between nights, even if the statistical power was too low to exclude an effect of the ventilation mode on these variables (Table 2).
Discussion
This is the first report of the nocturnal application of an adaptive NIV mode that continuously adjusts EPAP to the minimum level that abolishes EFLT in hypercapnic COPD patients. This ventilation mode was well tolerated, reduced the frequency of ineffective efforts and the percentage of night time spent in hypercapnia. We found no differences in RINSP, XINSP, and ΔXrs between the two modes. The median EPAP did not significantly differ between nights; however, on an individual basis, some patients received significantly different (either higher or lower) EPAP levels during the EFLT-abolishing-EPAP and the fixed-EPAP nights. During the EFLT-abolishing-EPAP night, the EPAP presented large fluctuations, suggesting that the ventilator automatically adapted the EPAP level to the changes in lung mechanics associated with changes in posture and sleep stage.
The individual responses to abolishing EFLT were highly heterogeneous, and this heterogeneity may have contributed to the lack of statistically significant differences in gas exchange between nights. In several subjects, we noticed a clinically relevant improvement in either PtcCO2 or SpO2 during the EFLT-abolishing-EPAP night. One patient presented a markedly higher mean PtcCO2 during the EFLT-abolishing-EPAP than during the fixed-EPAP night. This patient was very flow-limited, received a median EPAP of 12 cmH2O during the EFLT-abolishing-EPAP night vs 6 cmH2O during the fixed-EPAP night, and presented a much higher VE during the EFLT-abolishing-EPAP than during the fixed-EPAP night. We did not identify any parameter able to predict the gas exchange response of a given patient to the EFLT-abolishing ventilation mode. Larger studies are needed to draw conclusions about the clinical benefits of this novel adaptive mode and to identify phenotypes that may better benefit from it.
NIV is used in stable COPD patients with hypercapnic respiratory failure to reduce arterial partial pressure of CO2 [10]. In our study improvements in PtcCO2 and in the percentage of night time spent in hypercapnia were not associated with increases in pressure support or VE, highlighting the relevant role of EPAP in the control of hypercapnia in COPD patients. Titrating EPAP to abolish EFLT may reduce CO2 via several mechanisms [5, 11, 12]: (1) reducing work of breathing by improving patient-ventilator synchronization, (2) unloading the inspiratory muscles by counteracting the intrinsic PEEP, (3) reducing the ventilation-perfusion mismatch by eliminating choke-points. Moreover, EFLT is highly variable within the same patient, e.g. it changes with body posture [1] and sleep stage. Therefore, an adaptive ventilation mode that continuously adjusts EPAP based on patient respiratory mechanics increases the time spent with the optimal EPAP compared with a fixed-EPAP mode, even if the average EPAP applied by the two ventilation modes is similar.
This study has several limitations. Since it was a short-term study, we could not assess long-term effectiveness and safety. Moreover, this was a pilot study on a small number of patients. Ten to fifteen patients is the typical sample size for pilot studies. This number is not calculated on statistical bases, but it is appropriate to assess the feasibility of a new method, inform possible improvements and collect preliminary data for larger clinical trials. The most reliable method for the assessment of ineffective efforts is to identify tidal swings in trans-diaphragmatic pressure (measured by gastric and oesophageal probes) that are not followed by an assisted breath. Our definition of ineffective efforts may have missed the efforts that did not generate any deflation in the flow signal, underestimating the actual number of events. However, we preferred not to measure the trans-diaphragmatic pressure because it could have disrupted sleep. We used PtcCO2 and pulse oximetry as indicators of gas exchange. Arterial blood gas measurements would have been more precise; however, in the ordinary setting of polysomnography, it is not possible to have an invasive continuous measurement of arterial blood gasses. On the other hand, single blood gas measurements are not representative of the gas exchange during sleep, and multiple overnight assessments usually determine sleep disruption.
Conclusion
In conclusion, the use of a NIV mode that continuously auto-titrates EPAP to abolish EFLT during sleep has the potential to control hypercapnia better and reduce ineffective efforts compared with fixed-EPAP modes in COPD. Larger studies are needed to draw conclusions about the clinical benefits of this novel ventilation mode and to assess its long term effects.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- EPAP:
-
Expiratory positive airway pressure
- EFLT :
-
Tidal expiratory flow limitation
- FOT:
-
Forced oscillation technique
- IE:
-
Number of ineffective efforts per hour
- ΔXrs:
-
Difference between mean inspiratory and expiratory reactance
- NIV:
-
Non-invasive ventilation
- PtcCO2 :
-
Transcutaneous partial pressure of carbon dioxide
- RINSP :
-
Mean inspiratory resistance
- RR:
-
Respiratory rate
- SpO2 :
-
Peripheral oxygen saturation
- T90:
-
Percentage of night time with SpO2 < 90%
- VE :
-
Minute ventilation
- VT :
-
Tidal volume
- XINSP :
-
Inspiratory reactance
References
Milesi I, Porta R, Barbano L, Cacciatore S, Vitacca M, Dellacà RL. Automatic tailoring of the lowest PEEP to abolish tidal expiratory flow limitation in seated and supine COPD patients. Respir Med. 2019;155:13–8.
Dellacà RL, Santus P, Aliverti A, Stevenson N, Centanni S, Macklem PT, et al. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J. 2004;23:232–40.
Zannin E, Chakrabarti B, Govoni L, Pompilio PP, Romano R, Calverley PMA, et al. Detection of expiratory flow limitation by forced oscillations during noninvasive ventilation. Am J Respir Crit Care Med. 2019;200:1063–5.
Suh E-S, Pompilio P, Mandal S, Hill P, Kaltsakas G, Murphy PB, et al. Auto-titrating external positive end-expiratory airway pressure to abolish expiratory flow limitation during tidal breathing in patients with severe chronic obstructive pulmonary disease: a physiological study. Eur Respir J. 2020. https://doi.org/10.1183/13993003.02234-2019.
Fanfulla F, Taurino AE, Lupo NDA, Trentin R, D’Ambrosio C, Nava S. Effect of sleep on patient/ventilator asynchrony in patients undergoing chronic non-invasive mechanical ventilation. Respir Med Respir Med. 2007;101:1702–7.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Am J Respir Crit Care Med. 2017;199(5):557–82.
Dellacà RL, Rotger M, Aliverti A, Navajas D, Pedotti A, Farré R, et al. Noninvasive detection of expiratory flow limitation in COPD patients during nasal CPAP. Eur Respir J. 2006;27:983–91.
American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Darien, IL, USA: American Academy of Sleep Medicine; 2007.
Fanfulla F, Delmastro M, Berardinelli A, Lupo NDA, Nava S. Effects of different ventilator settings on sleep and inspiratory effort in patients with neuromuscular disease. Am J Respir Crit Care Med. 2005;172:619–24.
Ergan B, Oczkowski S, Rochwerg B, Carlucci A, Chatwin M, Clini E, et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur Respir J. 2019;54(3):1901003.
Appendini L, Purro A, Patessio A, Zanaboni S, Carone M, Spada E, et al. Partitioning of inspiratory muscle workload and pressure assistance in ventilator-dependent COPD patients. Am J Respir Crit Care Med. 1996;154(5):1301–9.
Elliott MW, Mulvey DA, Moxham J, Green M, Branthwaite MA. Inspiratory muscle effort during nasal intermittent positive pressure ventilation in patients with chronic obstructive airways disease. Anaesthesia. 2007;48:8–13.
Acknowledgements
We acknowledge Cristina Zanoni1 and Luca Gitti1 for their support in performing the measurements; we also acknowledge Bob Romano2, Jim McKenzie2 and Peter Hill2 for their technical support. 1 Pneumologia Riabilitativa, ICS S. Maugeri IRCCS, Lumezzane (Brescia), Italy; 2 Philips Respironics. Pittsburgh (PA), USA.
Funding
Politecnico di Milano University, Institution of EZ, IM, SC and RLD, owns a patent on the use of FOT for the detection of expiratory flow limitation licensed to Philips Respironics. Istituti Clinici Scientifici Maugeri received a free loan of part of the equipment used for this study and a research grant from Philips.
Author information
Authors and Affiliations
Contributions
EZ participated in data analysis and interpretation and prepared the first version of the manuscript. IM participated in the conception and design of the study, data collection, data analysis and interpretation. RP, LB and SC participated in the data collection and analysis. RT contributed to data analysis. FF participated in data analysis and interpretation and critically revised the manuscript. MV participated in the conception and design of the study, interpretation of the data and critically revised the manuscript. RLD participated in the conception and design of the study, data analysis, interpretation of the data and preparation of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of ICS Maugeri (No. 897 CEC/2013) and written informed consent was obtained from all patients.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zannin, E., Milesi, I., Porta, R. et al. Effect of nocturnal EPAP titration to abolish tidal expiratory flow limitation in COPD patients with chronic hypercapnia: a randomized, cross-over pilot study. Respir Res 21, 301 (2020). https://doi.org/10.1186/s12931-020-01567-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-020-01567-x