Abstract
Glucocorticoids (GCs) and β2-adrenergic receptor (β2AR) agonists improve asthma outcomes in most patients. GCs also modulate gene expression in human airway smooth muscle (HASM), thereby attenuating airway inflammation and airway hyperresponsiveness that define asthma. Our previous studies showed that the pro-fibrotic cytokine, transforming growth factor- β1 (TGF-β1) increases phosphodiesterase 4D (PDE4D) expression that attenuates agonist-induced levels of intracellular cAMP. Decreased cAMP levels then diminishes β2 agonist-induced airway relaxation. In the current study, we investigated whether glucocorticoids reverse TGF-β1-effects on β2-agonist-induced bronchodilation and modulate pde4d gene expression in HASM. Dexamethasone (DEX) reversed TGF-β1 effects on cAMP levels induced by isoproterenol (ISO). TGF-β1 also attenuated G protein-dependent responses to cholera toxin (CTX), a Gαs stimulator downstream from the β2AR receptor. Previously, we demonstrated that TGF-β1 treatment increased β2AR phosphorylation to induce hyporesponsiveness to a β2 agonist. Our current data shows that expression of grk2/3, kinases associated with attenuation of β2AR function, are not altered with TGF-β1 stimulation. Interestingly, DEX also attenuated TGF-β1-induced pde4d gene expression. These data suggest that steroids may be an effective therapy for treatment of asthma patients whose disease is primarily driven by elevated TGF-β1 levels.
Similar content being viewed by others
Clinical relevance
TGF-β1 has been identifed as pivotal in mediating airway remodeling and irreversible airway obstruction. Inhaled GCs and β2AR agonists are commonly used to treat asthma. Our study, however, demonstrates a protective effect of dexamethasone on TGF-β1-mediated attenuation of β2-agonist and Gαs-dependent relaxation of airway smooth muscle.
Background
Asthma, a chronic inflammatory disease of the lungs manifests by several hallmarks: airway hyperresponsiveness, remodeling, and inflammation [1]. Airway smooth muscle (ASM) cells play an integral role in regulating bronchomotor tone in the asthma diatheses and are a direct target of β2-agonists, a common therapy that promotes bronchodilation [2]. While β2-agonists evoke ASM relaxation, β2-agonists are not effective in all patients [3]. Patients who fall into the “severe” category of asthma are frequently hyporesponsive to bronchodilators [4]. Studies show that β2AR tolerance or desensitization occurs after repeated bronchodilator use that diminishes drug efficacy [3, 5,6,7,8,9].
TGF-β1, a profibrotic cytokine whose levels are elevated in patients with asthma, augments human airway smooth muscle (HASM) cell stiffness and significantly increases myosin light chain (MLC) phosphorylation via Smad3 [10] that enhance contractile agonist-induced cell shortening and hyperresponsiveness. In addition to amplifying bronchoconstriction, we also demonstrated that TGF-β1 blunts intracellular cAMP by upregulating pde4d expression that decreases β2-agonist-induced cAMP levels [11].
Signaling downstream of seven transmembrane G protein-coupled receptors (GPCRs) involves Gαβγ trimer dissociation following receptor activation [12]. The Gα subunit family is comprised of Gαi, Gαq, and Gαs, playing fundamental roles in regulating HASM relaxation and contraction [13]. Cholera toxin (CTX) catalyzes the ADP-ribosylation of Gαs that elicits adenylyl cyclase (AC) activation, causing the accumulation of intracellular cAMP and further activation of PKA to induce HASM relaxation [14]. CTX, and the β2−agonist isoproterenol (ISO), induce actin depolymerization in HASM in PKA-independent and -dependent pathways integrating activation of Src protein tyrosine kinases and Gαs protein [15], to promote smooth muscle relaxation. Whether TGF-β1 directly modulates Gαs protein activation remains unknown.
Glucocorticoids (GCs) remain a cornerstone in the management of asthma. GC treatment alters gene expression in HASM, thereby modulating inflammation and airway reactivity. Others have demonstrated that dexamethasone, a glucocorticoid, can directly inhibit Smad3 activity [16, 17]. Our previous study showed that TGF-β1 blunted the effects of β2 agonist-induced reversal of carbachol-mediated phosphorylation of myosin light chain, a process which was Smad3-dependent [11]. Given this information, we posited that GC treatment would reverse TGF-β1-induced hyporesponsiveness to β2-agonist. Our data demonstrate that dexamethasone (DEX) reverses TGF-β1-induced attenuation of β2AR-induced signaling, rescuing β2-agonist- and Gαs-activator-mediated cAMP production by attenuating pde4d expression.
Methods
HASM cell culture
HASM cells from the National Disease Research Interchange (Philadelphia, PA, USA) and the International Institute for the Advancement of Medicine (Edison, NJ, USA) were derived from trachea obtained from donors without chronic illness. All tissue was obtained from de-identified donors and was deemed non-human subjects research by the Rutgers University Institutional Review Board. Cells were cultured in Ham’s F12 medium with 10% fetal bovine serum. The cells were incubated and grown at 37 °C in 5% CO2. We have shown that isolated airway smooth muscle cells retain their phenotypic properties [18]. Primary HASM cells between passages 3–4 were used in all experiments. Donor demographics for the cell lines utilized in these studies are detailed in Table S1.
Western blot analysis
Primary HASM cells were serum deprived for 24 h prior to treatment. HASM cells were lysed with 0.6 N HClO4, scraped, collected, and pelleted as previously described [19]. The membrane was blocked with ready-to-use Odyssey Blocking Buffer (LI-COR BioSciences) containing 0.1% sodium azide and probed for phospho-Smad3, pMLC, total MLC, and GAPDH.
Measurement of intracellular cAMP levels
Grown to 90% confluency on 24-well plates, HASM cells were stimulated and lysed using the Applied Biosystems cAMP-Screen Immunoassay System following the manufacturer instructions as previously described [11]. The cells were lysed and incubated for 30 min in 5% CO2 and 37 °C. Conjugate Dilution Buffer, cAMP-AP Conjugate, anti-cAMP antibody, and the samples were added to pre-coated assay plate to incubate for 1 h on plate shaker. Plate was measured on luminometer after a 30-min incubation period with CSPD®/Sapphire-II RTU Substrate. Data was derived from standard curves and cAMP levels reported after using standard dilutions.
Quantification of pde4d, grk2, and grk3 expression (RNA isolation and qPCR)
Following treatment with TGF-β1 ± dexamethasone, cells were suspended in TRIzol reagent, and total RNA were isolated following the manufacturer protocol. RNA was isolated and purified from HASM cells using the RNeasy Mini Kit and cDNA was created using SuperScript IV First-Strand Synthesis System. All reactions were performed in 20 μL reaction volume in triplicate. For mRNA cDNA, PCR amplification consisted of 10 min of an initial denaturation step at 95 °C, followed by 40 cycles at 95 °C for 15 s, 60 °C for 60 s. Relative cDNA quantification was performed using TaqMan quantitative RT-PCR (Thermo Fisher Scientific) and the ΔΔCt method, and pde4d, grk2, and grk3 expression were normalized to expression of endogenous β-actin.
Statistical analysis
Graphs were created and statistical analyses were conducted using GraphPad Prism 5.01 h software (La Jolla, Ca, USA) to determine statistical significance evaluated using two-tailed Student’s paired t-test for two groups. P values of < 0.05 were considered significant. All results were confirmed by experiments in at least three unique cell lines. Data was fit to a normal distribution, and appropriate tests run to determine significance. For comparison of multiple conditions, one-way ANOVA was used with Bonferroni’s post-test. For the pde4d expression results, the differential expression analysis was performed under a negative binomial distribution model with DESeq2 (v.1.18.1), and the adjusted p values are noted.
Materials
Compounds were purchased from the following vendors: R&D Systems (TGF-β1; SB-431542), Sigma-Aldrich (albuterol [Alb], carbachol [Cch], cholera toxin [CTX], dexamethasone [DEX], isoproterenol [ISO]), Fisher BioReagents (Forskolin, [FSK]). Immunoblot antibodies were purchased from Abcam (phospho-Smad3; ab52903), Cell Signaling Technologies (phosphorylated myosin light chain pMLC, 3674S; GAPDH, 2118S; Tubulin, 3873S), and EMD Millipore (total myosin light chain [MLC, MABT180]). The following Taqman primer sets were purchased from Thermo Fisher Scientific: ACTB, actin beta, Hs01060665_g1; GRK2, G protein-coupled receptor kinase 2 Hs00176395_m1; GRK3, G protein-coupled receptor kinase 3, Hs00178266_m1; PDE4D, phosphodiesterase 4D, Hs01579625_m1.
Results
TGF-β1 attenuates Gαs-mediated cAMP production in HASM
Upon activation of β2AR, ADP-ribosylation of the α subunit of stimulatory G protein (Gαs), stimulates adenylyl cyclase to increase intracellular cAMP [15, 19]. To further understand mechanisms underlying TGF-β1-mediated hyporesponsiveness to a β2-agonist, intracellular cAMP levels were measured in TGF-β1-treated HASM cells following stimulation with cholera toxin (CTX), a Gαs activator. Intracellular cAMP activity increased in a time-dependent manner following exposure to CTX, with the maximum level elicited at 45 min (Fig. 1a). Interestingly at 60 and 75 min, the CTX-induced cAMP levels extinguished. In TGF-β1-treated cells, CTX-induced cAMP levels were completely abrogated compared to that of the diluent control (Fig. 1b).
Cholera toxin (CTX) increases cAMP levels in HASM, which is blunted by overnight treatment with TGF-β1. A time course of CTX-, a Gαs activator, induced cAMP production in HASM (0.25 μg/ml, 0–75 min) was performed in the presence and absence of TGF-β1 (10 ng/ml, 18 h). Data is expressed fold change over vehicle control as mean ± SEM for n = 3 separate cell lines, three additional donors were added to the 45 min to confirm appropriate time point. *p < 0.05 as assessed by one-way ANOVA, and comparisons between two conditions assessed by Student’s t-test
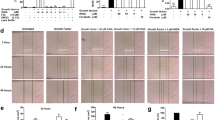
We previously demonstrated that isoproterenol (ISO) decreased carbachol (Cch)- and TGF-β1-induced phosphorylation of myosin light chain (pMLC); TGF-β1, however, decreased the ability of a β2-agonist to abrogate Cch-induced pMLC [11]. Cch-induced pMLC was inhibited by ISO, forskolin (an adenylyl cyclase activator) and CTX to comparable levels, as shown in Fig. 2. Interestingly, TGF-β1-induced pMLC was also decreased with CTX, forskolin, ISO and SB-431542, a TGF-β1 receptor antagonist. However, ISO and CTX-induced inhibition was less effective as compared with that of SB-431542 or forskolin in blocking TGF-β-induced pMLC (Fig. 2, upper right). Phosphorylation of SMAD3 (pSMAD3) induced by TGF-β1 confirmed engagement of TGF-β1 receptors and activation of downstream signaling pathways (Fig. 2, lower panel). Collectively, these data suggest that TGF-β1 inhibits the ability of β2AR or Gαs activation, but not forskolin, to increase cAMP levels and diminish pMLC.
Overnight TGF-β1 treatment impairs CTX-induced MLC dephosphorylation in HASM. Phosphorylation of myosin light chain (pMLC) following Cch (20 μM, 13 min) or TGF-β1 (10 ng/ml, 18 h) or was assessed following ISO (1 μM, 10 min), FSK (10 μM, 15 min), or CTX (0.25 μg/ml, 45 min) treatment. SB-431542 (5 μM, 1 h prior to TGF-β1 treatment), a TGF-β1 receptor inhibitor, was used as a control. All treatments (ISO/FSK/SB/CTX) significantly attenuated TGF-β1-induced pMLC (p < 0.05). Phosphorylation of MLC was normalized to total MLC for each experiment. *p < 0.05 as assessed by one-way ANOVA, and comparisons between two conditions assessed by Student’s t-test. Data is representative of n = 5–6 distinct HASM cell lines
TGF-β1 attenuates grk2 and grk3 expression in HASM
One mechanism by which inflammatory mediators can attenuate signaling downstream of the β2AR is through phosphorylation of the receptor [20,21,22,23]. Evidence suggests that GRK2 and 3 are associated with the β2AR, mediating desensitization through phosphorylation of intracellular portions of the receptor [24, 25]. We next examined the effect of TGF-β1 on expression of GRK2 and 3. As shown in Fig. 3, we show that TGF-β1 attenuates, rather than augments, grk2 and grk3 expression. Despite our previous findings showing that β2AR phosphorylation is increased following TGF-β1 exposure [26], we determined that the increase in TGF-β1-induced β2AR phosphorylation is not due to increased expression of GRK2/3.
TGF-β1 attenuates expression of grk2 and grk3. HASM were treated with TGF-β1 (10 ng/ml, 18 h), total RNA was isolated, and gene expression was assessed by TaqMan qPCR. Expression of grk2 and grk3 was normalized to endogenous β-actin. Data is representative of n = 5–6 different donors as mean ± SEM, *p < 0.05 by Student’s t-test
Dexamethasone rescues TGF-β1-induced decreases in cAMP levels induced by ISO or CTX
Glucocorticoids remain a cornerstone in the management of asthma by decreasing airway inflammation and reversing β2AR desensitization [27, 28]. To address whether the steroids also modulate TGF-β1 effects on cAMP levels, HASM cells were pretreated with dexamethasone (DEX) in the presence and absence of TGF-β1 and then treated with either ISO or CTX. As shown in Fig. 4a, TGF-β1 treatment attenuated ISO-induced cAMP by 47.3% and DEX pretreatment significantly rescued TGF-β1’s effect by 35.7%. Similarly, TGF-β1 treatment attenuated CTX-induced cAMP by 61.7%, and DEX pretreatment significantly reversed TGF-β1’s effect by 43.8% (Fig. 4b). These data demonstrate partial steroid responsiveness of TGF-β1-mediated hyporesponsiveness to β2AR-induced cAMP levels in HASM.
Dexamethasone (DEX) rescues TGF-β1-mediated attenuation of ISO- and CTX-induced cAMP production in HASM. HASM were pretreated with (a, b) DEX (10–100 nM, 30 min) prior to TGF-β1 (10 ng/ml, 18 h) stimulation. HASM were subsequently stimulated with (a) ISO (1 μM, 5 min) or (b) CTX (0.25 μg/ml, 45 min) and assessed for cAMP generation. Data is expressed % of max cAMP produced either by ISO (a) or CTX (b). Data is representative of (a) n = 6–13 (b) or n = 4–8 separate cell lines as mean ± SEM, *p < 0.05 comparing control/ISO/CTX to TGF-β1/ISO/CTX, and TGF-β1/ISO/CTX to TGF-β1/ISO/CTX ± DEX using a one-way ANOVA, and comparisons between two conditions assessed by Student’s t-test
Dexamethasone attenuates TGF-β1-induced pde4d expression in HASM
Previously, we determined that the blunted cAMP response to ISO induced by TGF-β1 was dependent upon increased pde4d expression [11]. Given that DEX rescued TGF-β1-induced attenuation of ISO- and CTX-induced cAMP production, we posited that DEX pretreatment would attenuate TGF-β1-mediated pde4d expression. In a dose-dependent manner, DEX pretreatment significantly attenuated TGF-β1-induced pde4d expression in HASM as shown in Fig. 5.
DEX inhibits TGF-β1-induced pde4d expression in HASM. HASM were pretreated with DEX (1–100 nM, 30 min) prior to stimulation with TGF-β1 (10 ng/ml, 18 h). mRNA was extracted, reverse transcribed, and assessed for pde4d and β-actin expression by TaqMan qPCR analysis. Data is represented as % of pde4d expression induced by TGF-β1, with pde4d expression normalized to β-actin. Data is representative of n = 4–6 separate cell lines as mean ± SEM, *p < 0.05 using a one-way ANOVA, and comparisons between two conditions assessed by Student’s t-test. TGF-β1 vs TGF-β1+ DEX (30 nM), p = 0.08
Discussion
Insensitivity to current therapeutics occurs in some patients with severe asthma. Evidence suggests that β2AR dysfunction can manifest in an inflammatory milieu due to inflammatory cytokines associated with severe asthma and airway remodeling such as TGF-β1, IL-13, and TNF-α [11, 19, 29]. Additionally, TGF-β1 stimulation has been shown to attenuate β adrenergic receptor signaling in other cell types [30, 31] in addition to HASM. We examined whether generation of cAMP levels in HASM cells after CTX-treatment occurred in the presence and absence of TGF-β1 (Fig. 1b). As we had previously described, β2-agonist-induced responses were blunted following TGF-β1 treatment. To characterize mechanisms by which TGF-β1 diminishes β2AR responses, the effects of TGF-β1 on Gαs-induced reversal of agonist-induced HASM cell contractile signaling was examined. We extended our previous findings that phosphorylation of MLC, an important element of agonist-induced HASM contraction, induced by Cch or TGF-β1 was reversed by activation of the β2AR and Gαs (Fig. 2). Given these data and our previously published results, these data suggest that the effects of TGF-β1 are more prominent at the receptor and Gαs level rather than downstream at the level of adenylyl cyclase, as evidenced by the lack of effect of TGF-β1 on forskolin-induced increases in cAMP levels.
Attenuation of β2AR signaling occurs after phosphorylation of the receptor, promoting internalization and desensitization of the β2AR [32,33,34]. Activation of GRK2 and GRK3, members of the GRK (G protein-coupled receptor kinase) family, provide a mechanism by which β2AR desensitization occurs. We previously demonstrated that TGF-β1 stimulation induces β2AR phosphorylation that is consistent with desensitization of the receptor [26]. To assess a potential role for GRKs in attenuating β2-agonist-induced relaxation of HASM, grk2 and grk3 expression were assessed with overnight TGF-β1 treatment. In Fig. 3, we demonstrate that TGF-β1 treatment decreased grk2 and grk3 expression. Given these data, it is highly unlikely that upregulation of GRK2/3 expression contributes the hyporesponsiveness to bronchodilators induced by TGF-β1. Current evidence and our previous studies suggest that TGF-β1 likely modulates activity of these types of kinases, rather than modulating GRK expression to induce hyporesponsiveness to β2-agonists.
Currently, glucocorticoids like dexamethasone are used to attenuate inflammation associated with asthma as well as restore β2AR responsiveness [3, 19, 35], and reverse the effects of inflammatory mediator-induced β2AR dysfunction. In osteoblasts, it has been demonstrated that dexamethasone suppresses the transcriptional activity of, but not expression, Smad3, attenuating TGF-β1-induced alkaline phosphatase activity and type I collagen expression [16]. Additionally, dexamethasone also repressed transcriptional activation of PAI-1 through inhibition of Smad3/4 by direct interaction between the glucocorticoid receptor and Smad3 [17]. We previously showed that TGF-β1-induced attenuation of ISO reversal of Cch-induced pMLC was Smad3-dependent in HASM. We also showed that TGF-β1 treatment increased expression of pde4d, suggesting a role for phosphodiesterases in TGF-β1-induced hyporesponsiveness to bronchodilators. Given our data and others’ observations concerning the effects of glucocorticoids on TGF-β1/Smad3-dependent signaling, we posited that dexamethasone would thereby reverse TGF-β1- and Smad3-induced attenuation of ISO-induced cAMP accumulation and pde4d expression in HASM. Figure 6 depicts a model of mechanisms underlying glucocorticoid-mediated rescue of TGF-β1-induced β2AR hyporesponsiveness. We demonstrate that TGF-β1-induced attenuation of β2-agonist- and Gαs-induced cAMP accumulation in HASM can be rescued by treatment with dexamethasone (Fig. 4a & b). Our data also show that DEX treatment reversed TGF-β1-induced pde4d expression (Fig. 5). Consistent with these data, we previously demonstrated that DEX (1 uM, 18 h) stimulation alone has little effect pde4d expression [36]. We recognize that changes in transcript expression do not necessarily translate to changes in protein expression or PDE4D activity. Previous work in HASM has demonstrated that PDE4D5 is expressed and, in part, controls cAMP production following β2 agonist stimulation [37, 38]. As in our study, Billington et al. demonstrated transcript expression, but not protein expression or enzymatic activity, of the specific PDE isoform. Interestingly, Niimi et al. demonstrated that transcript expression of PDE4D isoforms in HASM was altered, but not protein or enzymatic activity [39]. Extensive work by the Houslay group has shown the difficulty of isolating specific isoforms and splice variants of PDE enzymes, as well as the difficulty of enzymatic activity assessment [40]. Given the findings of Billington, Niimi, and Trian, and the complexities of the assays performed by the Houslay group, we recognize the limitations to our work, but feel that our results likely translate to functional outcomes affecting bronchomotor tone in the context of TGF-β1-induced hyposensitivity to β2 agonists and cAMP mobilizers.
A proposed model of GC-mediated rescue of TGF-β1-induced hyporesponsiveness to bronchodilators. We previously demonstrated that TGF-β1 induces Smad2/3 activation to increase pde4d expression, leading to increased cAMP hydrolysis and attenuating HASM cell relaxation responses. We posited that DEX binds to the glucocorticoid receptor (GR), inhibiting both increased pde4d expression and rescuing TGF-β1-induced attenuation of β2AR and Gαs-induced cAMP production. AC = Adenylyl Cyclase; β2AR = β2-adrenergic receptor; DEX = Dexamethasone; Gαs = Stimulatory Gα protein; GR = Glucocorticoid Receptor; PDE4D = Phosphodiesterase 4D; TBR-I/II = TGF-β receptor I/II; pMLC = phosphorylated myosin light chain
Despite the fact that we showed that TGF-β1 attenuates CTX-induced cAMP production, and that DEX rescues TGF-β1-mediated attenuation of both ISO- and CTX-induced cAMP production, it remains to be seen if these mechanisms are operative in vivo. Despite this limitation, we have shown effects of TGF-β1 on HASM to be recapitulated in small airways derived from human lungs [10]. Additionally, while it would be interesting to study this phenomenon in asthma-derived HASM, we and others have demonstrated that β2-agonist-induced cAMP production in these cells is already blunted due partially to increased PDE expression [26, 38]. Therefore, exposure of asthma-derived HASM to TGF-β1 will likely have little effect on modulating β2-agonist-induced cAMP production.
Conclusion
Regardless of evidence that steroids may not reverse the TGF-β1-induced remodeling effects [41] in asthma, our findings suggest that in asthma patients with high levels of TGF-β1, steroids may be an effective treatment to reverse β2AR hyporesponsiveness observed in these patients.
Availability of data and materials
All data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- TGF-β1:
-
Transforming growth factor β1
- GC:
-
Glucocorticoid
- β2AR:
-
Beta 2 adrenergic receptor
- HASM:
-
Human airway smooth muscle
- PDE4D:
-
Phosphodiesterase 4D
- DEX:
-
Dexamethasone
- ISO:
-
Isoproterenol
- CTX:
-
Cholera toxin
- GRK:
-
G protein receptor-coupled kinase
- ASM:
-
Airway smooth muscle
- Smad3:
-
Mothers against decapentaplegic homolog 3
- cAMP:
-
Adenosine 3′,5′-cyclic monophosphate
- GPCR:
-
G protein-coupled receptor
- ADP:
-
Adenosine monophosphate
- PKA:
-
Protein kinase A
- AC:
-
Adenylyl cyclase
- PBS:
-
Phosphate buffered saline
- pMLC:
-
Phosphorylated myosin light chain
- MLC:
-
Myosin light chain
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- cAMP-AP:
-
Adenosine 3′,5′-cyclic monophosphate-alkaline phosphatase
- CSPD:
-
Chemiluminescent alkaline phosphatase substrate
- RNA:
-
Ribonucleic acid
- qPCR:
-
Quantitative polymerase chain reaction
- mRNA:
-
Messenger ribonucleic acid
- cDNA:
-
Complementary deoxyribonucleic acid
- ANOVA:
-
Analysis of variance
- Alb:
-
Albuterol
- Cch:
-
Carbachol
- FSK:
-
Forskolin
- IL-13:
-
Interleukin 13
- TNFα:
-
Tumor necrosis factor α
References
Postma DS, Kerstjens HA. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:S187–92.
Morgan SJ, Deshpande DA, Tiegs BC, Misior AM, Yan H, Hershfeld AV, Rich TC, Panettieri RA, An SS, Penn RB. β-agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent. J Biol Chem. 2014;289:23065–74.
Newnham DM, Grove A, McDevitt DG, Lipworth BJ. Subsensitivity of bronchodilator and systemic beta 2 adrenoceptor responses after regular twice daily treatment with eformoterol dry powder in asthmatic patients. Thorax. 1995;50:497–504.
Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med. 2006;144:904–12.
Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64:450–504.
Haney S, Hancox RJ. Tolerance to bronchodilation during treatment with long-acting beta-agonists, a randomised controlled trial. Respir Res. 2005;6:107.
Wraight JM, Hancox RJ, Herbison GP, Cowan JO, Flannery EM, Taylor DR. Bronchodilator tolerance: the impact of increasing bronchoconstriction. Eur Respir J. 2003;21:810–5.
Yates DH, Worsdell M, Barnes PJ. Effect of regular salmeterol treatment on albuterol-induced bronchoprotection in mild asthma. Am J Respir Crit Care Med. 1997;156:988–91.
Yim RP, Koumbourlis AC. Tolerance & resistance to β2-agonist bronchodilators. Paediatr Respir Rev. 2013;14:195–8.
Ojiaku CA, Cao G, Zhu W, Yoo EJ, Shumyatcher M, Himes BE, An SS, Panettieri RA Jr. TGF-β1 evokes human airway smooth muscle cell shortening and Hyperresponsiveness via Smad3. Am J Respir Cell Mol Biol. 2018;58:575–84.
Ojiaku CA, Chung E, Parikh V, Williams JK, Schwab A, Fuentes AL, Corpuz ML, Lui V, Paek S, Bexiga NM, et al. Transforming growth factor-β1 decreases β(2)-agonist-induced relaxation in human airway smooth muscle. Am J Respir Cell Mol Biol. 2019;61:209–18.
Penn RB, Benovic JL. Regulation of heterotrimeric G protein signaling in airway smooth muscle. Proc Am Thorac Soc. 2008;5:47–57.
Luttrell LM. Transmembrane signaling by G protein-coupled receptors. Methods Mol Biol. 2006;332:3–49.
Bharati K, Ganguly NK. Cholera toxin: a paradigm of a multifunctional protein. Indian J Med Res. 2011;133:179–87.
Hirshman CA, Zhu D, Pertel T, Panettieri RA, Emala CW. Isoproterenol induces actin depolymerization in human airway smooth muscle cells via activation of an Src kinase and GS. Am J Physiol Lung Cell Mol Physiol. 2005;288:L924–31.
Iu MF, Kaji H, Sowa H, Naito J, Sugimoto T, Chihara K. Dexamethasone suppresses Smad3 pathway in osteoblastic cells. J Endocrinol. 2005;185:131–8.
Song CZ, Tian X, Gelehrter TD. Glucocorticoid receptor inhibits transforming growth factor-beta signaling by directly targeting the transcriptional activation function of Smad3. Proc Natl Acad Sci U S A. 1999;96:11776–81.
Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Phys. 1989;256:C329–35.
Koziol-White CJ, Yoo EJ, Cao G, Zhang J, Papanikolaou E, Pushkarsky I, Andrews A, Himes BE, Damoiseaux RD, Liggett SB, et al. Inhibition of PI3K promotes dilation of human small airways in a rho kinase-dependent manner. Br J Pharmacol. 2016;173:2726–38.
Albano GD, Zhao J, Etling EB, Park SY, Hu H, Trudeau JB, Profita M, Wenzel SE. IL-13 desensitizes β2-adrenergic receptors in human airway epithelial cells through a 15-lipoxygenase/G protein receptor kinase 2 mechanism. J Allergy Clin Immunol. 2015;135:1144–53 e1141–1149.
Amrani Y, Chen H, Panettieri RA Jr. Activation of tumor necrosis factor receptor 1 in airway smooth muscle: a potential pathway that modulates bronchial hyper-responsiveness in asthma? Respir Res. 2000;1:49–53.
Laporte JD, Moore PE, Panettieri RA, Moeller W, Heyder J, Shore SA. Prostanoids mediate IL-1beta-induced beta-adrenergic hyporesponsiveness in human airway smooth muscle cells. Am J Phys. 1998;275:L491–501.
Pang L, Holland E, Knox AJ. Role of cyclo-oxygenase-2 induction in interleukin-1beta induced attenuation of cultured human airway smooth muscle cell cyclic AMP generation in response to isoprenaline. Br J Pharmacol. 1998;125:1320–8.
Deshpande DA, Yan H, Kong KC, Tiegs BC, Morgan SJ, Pera T, Panettieri RA, Eckhart AD, Penn RB. Exploiting functional domains of GRK2/3 to alter the competitive balance of pro- and anticontractile signaling in airway smooth muscle. FASEB J. 2014;28:956–65.
Kong KC, Gandhi U, Martin TJ, Anz CB, Yan H, Misior AM, Pascual RM, Deshpande DA, Penn RB. Endogenous Gs-coupled receptors in smooth muscle exhibit differential susceptibility to GRK2/3-mediated desensitization. Biochemistry. 2008;47:9279–88.
Chachi L, Alzahrani A, Koziol-White C, Biddle M, Bagadood R, Panettieri RA Jr, Bradding P, Amrani Y. Increased β2-adrenoceptor phosphorylation in airway smooth muscle in severe asthma: possible role of mast cell-derived growth factors. Clin Exp Immunol. 2018;194:253–8.
Howarth PH, Knox AJ, Amrani Y, Tliba O, Panettieri RA Jr, Johnson M. Synthetic responses in airway smooth muscle. J Allergy Clin Immunol. 2004;114:S32–50.
Nino G, Hu A, Grunstein JS, Grunstein MM. Mechanism of glucocorticoid protection of airway smooth muscle from proasthmatic effects of long-acting beta2-adrenoceptor agonist exposure. J Allergy Clin Immunol. 2010;125:1020–7.
Hotta K, Emala CW, Hirshman CA. TNF-alpha upregulates Gialpha and Gqalpha protein expression and function in human airway smooth muscle cells. Am J Phys. 1999;276:L405–11.
Huntgeburth M, Tiemann K, Shahverdyan R, Schluter KD, Schreckenberg R, Gross ML, Modersheim S, Caglayan E, Muller-Ehmsen J, Ghanem A, et al. Transforming growth factor beta(1) oppositely regulates the hypertrophic and contractile response to beta-adrenergic stimulation in the heart. PLoS One. 2011;6:e26628.
Wagener BM, Roux J, Carles M, Pittet J-F. Synergistic inhibition of β2-adrenergic receptor–mediated alveolar epithelial fluid transport by Interleukin-8 and transforming growth factor-β. Anesthesiol J Am Soc Anesthesiol. 2015;122:1084–92.
Krasel C, Bünemann M, Lorenz K, Lohse MJ. Beta-arrestin binding to the beta2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J Biol Chem. 2005;280:9528–35.
Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. Beta-arrestin-dependent, G protein-independent ERK1/2 activation by the beta2 adrenergic receptor. J Biol Chem. 2006;281:1261–73.
Song Q, Ji Q, Li Q. The role and mechanism of β-arrestins in cancer invasion and metastasis (review). Int J Mol Med. 2018;41:631–9.
Cooper PR, Panettieri RA Jr. Steroids completely reverse albuterol-induced beta(2)-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol. 2008;122:734–40.
Himes BE, Jiang X, Wagner P, Hu R, Wang Q, Klanderman B, Whitaker RM, Duan Q, Lasky-Su J, Nikolos C, et al. RNA-Seq transcriptome profiling identifies CRISPLD2 as a glucocorticoid responsive gene that modulates cytokine function in airway smooth muscle cells. PLoS One. 2014;9:e99625.
Billington CK, Le Jeune IR, Young KW, Hall IP. A major functional role for phosphodiesterase 4D5 in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:1–7.
Trian T, Burgess JK, Niimi K, Moir LM, Ge Q, Berger P, Liggett SB, Black JL, Oliver BG. β2-agonist induced cAMP is decreased in asthmatic airway smooth muscle due to increased PDE4D. PLoS One. 2011;6:e20000.
Niimi K, Ge Q, Moir LM, Ammit AJ, Trian T, Burgess JK, Black JL, Oliver BG: Beta2-agonists upregulate PDE4 mRNA but not protein or activity in human airway smooth muscle cells from asthmatic and nonasthmatic volunteers. Am J Physiol Lung Cell Mol Physiol 2012, 302:L334–L342.
MacKenzie SJ, Baillie GS, McPhee I, MacKenzie C, Seamons R, McSorley T, Millen J, Beard MB, van Heeke G, Houslay MD. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in upstream conserved region 1 (UCR1). Br J Pharmacol. 2002;136:421–33.
Bourke JE, Li X, Foster SR, Wee E, Dagher H, Ziogas J, Harris T, Bonacci JV, Stewart AG. Collagen remodelling by airway smooth muscle is resistant to steroids and β<sub>2</sub>−agonists. 2011;37:173–82.
Acknowledgements
None.
Funding
National Institutes of Health (NIH) grants P01-HL114471 and UL1TR003017.
Author information
Authors and Affiliations
Contributions
E. Chung – study design, data acquisition, data analysis and interpretation, drafting and editing of the manuscript, final approval of publication; C.A. Ojiaku - study design, data acquisition, data analysis and interpretation, drafting and editing of the manuscript, final approval of publication; G. Cao – study design, data acquisition, data analysis and interpretation, drafting and editing of the manuscript, final approval of publication; V. Parikh - data acquisition, data analysis and interpretation, drafting and editing of the manuscript, final approval of publication; B. Deeney - data acquisition, data analysis and interpretation, drafting and editing of the manuscript, final approval of publication; S. Xu –data analysis and interpretation, drafting and editing of the manuscript, final approval of publication; S. Wang - data acquisition, data analysis and interpretation, drafting and editing of the manuscript; R. Panettieri - drafting and editing of the manuscript, final approval of publication; C. Koziol-White - drafting and editing of the manuscript, final approval of publication
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Human tissue was acquired through the National Disease Research Interchange (NDRI) and the International Institute for the Advancement of Medicine (IIAM) and was obtained commercially from anonymous donors and according to the procedures approved by the Rutgers University IRB. As such, the human tissue is exempt from requiring IRB approval. Although information concerning the cause of death, gender, race, age, body weight & height of the donor is available, there are no unique identifiers that can link the subject’s identification to the tissue sample.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Donor demographics for cAMP and pde4d expression studies. All cells were derived from subjects with no history of chronic disease.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chung, E., Ojiaku, C.A., Cao, G. et al. Dexamethasone rescues TGF-β1-mediated β2-adrenergic receptor dysfunction and attenuates phosphodiesterase 4D expression in human airway smooth muscle cells. Respir Res 21, 256 (2020). https://doi.org/10.1186/s12931-020-01522-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-020-01522-w