Abstract
Background
Exercise ventilatory inefficiency is usually defined as high ventilation (\( \dot{\mathrm{V}}\mathrm{E} \)) versus low CO2 output (\( \dot{\mathrm{V}}\mathrm{CO}2 \)). The inefficiency may be lowered when airflow obstruction is severe because \( \dot{\mathrm{V}}\mathrm{E} \) cannot be adequately increased in response to exercise. However, the ventilatory inefficiency-airflow obstruction relationship differs to a varying degree. This has been hypothesized to be affected by increased dead space fraction of tidal volume (VD/VT), acidity, hypoxemia, and hypercapnia.
Methods
A total of 120 male patients with chronic obstructive pulmonary disease were enrolled. Lung function and incremental exercise tests were conducted, and \( \dot{\mathrm{V}}\mathrm{E} \) versus \( \dot{\mathrm{V}}\mathrm{CO}2 \) slope (\( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \)) and intercept (\( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \)) were obtained by linear regression. Arterial blood gas analysis was also performed in 47 of the participants during exercise tests. VD/VT and lactate level were measured.
Results
VD/VTpeak was moderately positively related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) (r = 0.41) and negatively related to forced expired volume in 1 sec % predicted (FEV1%) (r = − 0.27), and hence the FEV1%- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) relationship was paradoxical. The higher the \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \), the higher the pH and PaO2, and the lower the PaCO2 and exercise capacity. \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) was marginally related to VD/VTrest. The higher the \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \), the higher the inspiratory airflow, work rate, and end-tidal PCO2peak.
Conclusion
1) Dead space ventilation perturbs the airflow- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) relationship, 2) increasing ventilation thereby increases \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) to maintain biological homeostasis, and 3) the physiology- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \)- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) relationships are inconsistent in the current and previous studies.
Trial Registration
Similar content being viewed by others
Background
High ventilatory equivalents for oxygen and CO2 (\( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{O}2 \) and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2 \)) have been shown to be indexes of uneven alveolar ventilation-perfusion ratio (\( \dot{\mathrm{V}}\mathrm{A}/\dot{\mathrm{Q}} \)) [1] and markers of ventilation inefficiency caused by both heart and lung diseases [2]. The \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2 \) slope (\( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \)) is elevated in dyspneic patients and can differentiate congestive heart failure (CHF) from chronic obstructive pulmonary disease (COPD) with exercise impairment [3]. \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) has also been shown to be a marker of the severity and prognosis of CHF [4, 5] and an indicator of treatment response [6, 7], even though it cannot reflect the treatment effect in patients with CHF of different severity [8].
Compared to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2 \) ratio (\( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{R} \)) in COPD, \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2 \) intercept (\( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \)) i.e. dead space ventilation [9, 10], has been shown to be a better indicator of exertional ventilatory inefficiency and unfavorable patient outcomes i.e. mechanical constraint, pulmonary gas exchange, exertional dyspnea, and exercise intolerance [11]. In patients with COPD, \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) is negatively related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) and decreases when airflow obstruction [11] and emphysema are severe [12]. However, in patients with COPD, the relationship between \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and forced expired volume in one s % predicted (FEV1%) is weak [3, 11, 13], although it is slightly better when Global Initiative for Chronic Lung Disease (GOLD) staging is used to grade the severity [11]. Similarly, in patients with CHF the slope is increased, however it decreases when the patients have airflow limitation [12] or when an external dead space is large enough to hamper \( \dot{\mathrm{V}}\mathrm{E} \) compensation for hypercapnia [9].
Several mechanisms to explain overlapping \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) values across GOLD stage I to IV have been proposed [11]. These mechanisms include various afferent information from working limbs [14], peripheral chemoreceptors [15], pulmonary artery pressure, and VD/VT. However, no data or references have been reported for the last two factors [11].
In COPD, the lower the FEV1%, the lower the \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) [11, 13], and the lower the FEV1%, the larger the VD/VT [16, 17]. In contrast, the larger the VD/VT, the higher the \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) [1, 18]. In this context, \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) may be high or low at a given FEV1%. Hence, we hypothesized that the positive but weak relationship between \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and FEV1% may be influenced by VD/VT. We also evaluated other factors that may influence the relationship including hypoxemia and/or metabolic and/or respiratory acidity. This study aimed to elucidate the mechanisms underpinning the unclear relationship between FEV1% and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and between \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and exercise biological homeostasis.
Methods
Study design
We conducted an observational cross-sectional study on incremental maximal exercise in subjects with COPD at our institution. To obtain invasive measurement data, arterial catheterization was established for blood gas sampling in a subgroup of the participants. Each subject signed informed consent before entering the study. The local Institutional Review Board of our institutions (CS19014) approved this study. This study was conducted in compliance with the Declaration of Helsinki.
Subjects
We enrolled subjects aged ≥40 years with COPD but without any chronic diseases including uncontrolled diabetes mellitus, uncontrolled hypertension, anemia (hemoglobin < 13 g·dL− 1 in males), and no acute illnesses in the recent 1 month. The FEV1/forced vital capacity (FVC) was < 0.7 [19]. The diagnosis of COPD was made by pulmonologists according to the GOLD criteria [19]. All of the participants had to be able and willing to perform the study protocol including a maximal or symptom-limited cardiopulmonary exercise test (CPET). All of the participants were regularly followed-up at our pulmonary outpatient clinics and received optimized and individually tailored drug treatment, and they all had a stable clinical condition for at least 1 month.
We excluded subjects with a body mass index ≤18 kg·m− 2 or ≥ 32 kg·m− 2 and those with laboratory findings of hematological, metabolic or neuromuscular diseases, as these factors may confound exercise performance. Subjects with coexisting heart failure with/without documented pulmonary embolism, primary valvular heart disease, pulmonary artery hypertension, pericardial disease, exercise-induced angina, ST changes, and severe arrhythmias were also excluded. As few female subjects meet the criteria of COPD in Taiwan [20], they were not included in this study. We also excluded those who had contraindications to perform the exercise test and those who were participating in exercise training. However, recreational activity was allowed.
Measurements
Demographic and anthropometric data
Age, height, weight, body mass index, and cigarette consumption were recorded.
Functional daily activity
The oxygen-cost diagram (OCD) was used to evaluate the participants’ functional activity. The participants were asked to indicate a point on an OCD, a 10-cm long vertical line with everyday activities listed alongside the line, above which breathlessness limited them [21]. The distance from zero was measured and scored.
Pulmonary function testing
Cigarette smoking, drinking coffee, tea, or alcohol, and taking medications were not permitted 24 h before any test. Bronchodilators were not administered within 3 h for short-acting beta agonists and 12 h for long-acting beta agonists before the tests [22, 23]. FEV1, FVC, total lung capacity (TLC), residual volume (RV), and diffusing capacity for carbon monoxide (DLCO) were measured using spirometry, body plethysmography and the single-breath technique (MasterScreen™ Body, Carefusion, Wuerzburg, Germany), respectively in accordance with the currently recommended standards [24, 25]. The best of three technically satisfactory readings was used [24, 26, 27]. All of the spirometry data were obtained before and after inhaling 400 μg of fenoterol HCl. Post-dose measurements were performed 15 min after inhalation. Static lung volume data and DLCO data were obtained before inhaling fenoterol. For details, please refer to reference [22].
CPET
Each subject completed pulmonary gas exchange measured at rest and during exercise on the different days within 1 month after lung function test. Short-acting and long-acting beta bronchodilators were withheld 4–6 h and ≥ 12 h before the test, respectively. Gas exchange equipment including a face mask connected to a turbine pneumotachograph was used to measured \( \dot{\mathrm{V}}\mathrm{O}2 \) (mL/min), CO2 output (\( \dot{\mathrm{V}}\mathrm{CO}2 \)) (mL/min), minute ventilation (\( \dot{\mathrm{V}}\mathrm{E} \)) (L/min), tidal volume (VT) (L), breathing frequency (b/min), and end-tidal PCO2 (PETCO2) (mm Hg) breath-by-breath (MasterScreen CPX™, Carefusion, Wuerzburg, Germany), and then the data were averaged and reported at 15-s intervals of each stage using a computer. For each test, 12-lead electrocardiograms were recorded, pulse oximetry was used to record arterial oxyhemoglobin saturation (SPO2, %), and a sphygmomanometer was used to measure blood pressure every 2 min. An electromagnetically braked cycle ergometer (Lode, Groningen, the Netherlands) was used to adjust workload via a computer. The exercise test protocol was a 2-min period of rest followed by 2-min period of unloaded exercise, followed by ramp-pattern loaded exercise with a workload per stage selected according to the oxygen-cost diagram so that the loaded exercise could be completed within 10 ± 2 min of each participant reaching the limit of symptoms [28]. During each test, a pedaling frequency of 60 rpm was maintained with the aid of a visual pedal rate indicator. Calibrations of the turbine pneumotachograph were performed using a 3-L syringe before each test. The O2 and CO2 analyzers were calibrated with standard gases.
Calculation of \( \dot{V}E/\dot{V} CO2S \) and \( \dot{V}E/\dot{V} CO2R \)
Linear regression was used to quantify the relationship between \( \dot{\mathrm{V}}\mathrm{E} \) and \( \dot{\mathrm{V}}\mathrm{CO}2 \) to obtain \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \). For linear regression, data of the entire loaded exercise [5] were used if the respiratory or ventilatory compensation point (RCP or VCP) [1, 29] were not identified by PETCO2 curve; data below the RCP were used if the RCP or VCP was identified. PETCO2 curve reveals slow increase from start of exercise to anaerobic threshold and is then relatively stable during isocapneic buffering period. After the period, PETCO2 starts to decrease where RCP is defined. To be noted, RCP was reported in four of 16 subjects with pulmonary emphysema in a previous study [12]. \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{R} \) was directly calculated. \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2 \) nadir (\( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{N} \)) was the lowest value of \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{R} \) during loaded exercise period [30].
VD/VT measurement
Brachial artery catheterization was established and blood samples were drawn and heparinized in a subgroup of the participants at rest and at the last 15 s of every minute during loaded exercise and at peak exercise. The sample was immediately placed on ice and then analyzed for pH, PCO2, and PO2 with body temperature correction (model 278, CIBA-Corning, Medfield, MA, USA). The VD/VT was calculated using a standard formula as follows [31].
where P \( \overline{\mathrm{E}} \) CO2 = \( \dot{\mathrm{V}}\mathrm{CO}2/\dot{\mathrm{V}}\mathrm{E} \) × (PB - 47 mmHg) and PB was barometric pressure measured daily and VDm was the dead space of mouth piece and pneumotachograph as the manufacture reported.
Statistical analysis
Data were summarized as mean ± standard deviation. Comparisons between two groups were performed using two-sample t test. Pearson’s or Spearman’s correlation coefficients were used when appropriate for quantifying the pair-wise relationships among the interested continuous variables. Statistical significance was set at p ≤ 0.05.
Marginal statistical significance was set at 0.05 < p < 0.1.
Results
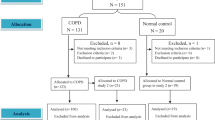
A total of 120 male subjects with COPD aged 67.0 ± 6.8 years were enrolled after excluding nine subjects aged ≥80 years (Fig. 1 and Table 1). Most of the participants had moderate to severe disease severity. Overall, 118 subjects completed the exercise test after excluding two who had poor motivation (Table 1). In the entire group and its subgroup of patients who underwent blood gas sampling, \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) were moderately negatively related (Table 2, r = − 0.40 - − 0.44, p < 0.001 - < 0.0001). The relationships between \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and the pulmonary physiology variables of interest were similar to some extent between the entire group and the subgroup of patients who underwent blood gas sampling (Table 2).
\( \dot{V}E/\dot{V} CO2S \) versus Pulmonary Physiology and Exercise Capacity. \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) was related to a varying degree to expiratory flow (r = 0.20–0.42, p < 0.05 - < 0.01), and marginally related to inspiratory flow. \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) was not related to any of the volume excursion variables at peak exercise except for VT/FEV1 in the subgroup analysis (Table 2, r = − 0.32, p < 0.05). \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) was positively related to an increase in SPO2 (r = 0.32–0.50). \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) was mildly negatively related to \( \dot{\mathrm{V}}\mathrm{O}2 \)peak% (r = − 0.27 - -0.33). In the subgroup of patients who underwent blood gas sampling, at peak exercise, \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) was moderately positively related to pH and PaO2 (Table 3, r = 0.40–0.53), and strongly negatively related to PaCO2 and PETCO2 (Tables 2 and 3, r = − 0.60 - -0.62).
In the subgroup of patients who underwent blood gas sampling, with regards to pulmonary physiology variables, VD/VTpeak was moderately positively related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \), and marginally negatively related to FEV1% (Table 2 and Fig. 2, r = − 0.27, p = 0.08).
Flow chart showing the deductive mechanism of exercise ventilatory inefficiency and biological homeostasis. VD/VT: dead space fraction of tidal volume, \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \): minute ventilation versus CO2 output slope, FEV1: forced expired volume in one s, SPO2peak: oxyhemoglobin saturation measured by pulse oximetry at peak exercise, PaO2: arterial partial pressure of O2, PaCO2: arterial partial pressure of CO2. Solid line with two-direction arrowheads: positive correlation, dashed line with two-direction arrowheads: negative correlation. Solid line with a single direction arrowhead: positively inducing, dashed line with a single direction arrowhead: negatively inducing
\( \dot{V}E/\dot{V} CO2I \) versus Pulmonary Physiology and Exercise Capacity. \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) was mildly related to inspiratory flow (r = 0.22–0.30, p < 0.05), marginally to mildly related to \( \dot{\mathrm{V}}\mathrm{O}2 \)peak% (Table 2, r = 0.27–0.28) and mildly to moderately related to Workpeak% (Table 2, r = 0.30–0.43), but not to expiratory flow or all volume excursion variables.
In the subgroup of patients who underwent blood gas sampling, \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) was moderately related to an increase in PETCO2 (Table 2, r = 0.53) and marginally related to VD/VTrest (r = 0.28, p = 0.08), but not to VD/VTpeak.
Discussion
The main findings of this study confirm that in male subjects with COPD, \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) was correlated to a varying degree with FEV1% and GOLD stage. We further found that VD/VTpeak was the main cause of the relationships (Fig. 2). A high \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) improved arterial pH, PO2, and PCO2, but was not caused by these factors. The findings support our hypothesis. Additionally, \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) was marginally related to dead space at rest and \( \dot{\mathrm{V}}\mathrm{O}2\mathrm{peak} \) and significantly related to increases in inspiratory airflow, PaCO2, and work rate.
\( \dot{V}E/\dot{V} CO2S \) versus Pulmonary Physiology of COPD. The results revealed that expiratory airflow graded by FEV1%, GOLD stage, and FEV1/VC was related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) to a varying degree (Fig. 2 and Table 2, |r| = 0.20–0.44). This is in line with previous reports that in patients with heart and lung diseases, severe airflow impairment may limit \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) to compensate for metabolic acidosis during heavy exercise [3, 9, 11, 12]. However, this notion is not consistent with the study by Teopompi et al., who reported that \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and FEV1% were not related (Supplementary Table) [13], although the role of inspiratory muscles was not considered. With regards to the tension time index of ventilatory muscle mechanics in normal healthy people and those with a disease, the inspiratory muscles may adapt to a level below or within the critical zone to sustain breathing in various conditions [32, 33]. As the mechanical load increases to a level which the inspiratory muscles can no longer tolerate, alveolar hypoventilation develops and the PaCO2 point may be reset [34]. However, in the current study, mean inspiratory airflow was marginally related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) in the entire group and not significantly related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) in the subgroup, suggesting that mean inspiratory airflow was not sensitive enough to be related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \).
However, expiratory airflow was related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) to a varying degree, which may be explained by VD/VT. In the current study, VD/VTpeak was positively related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \), similar to previous reports which used \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{R} \) ranging from 31 to 40 in parallel with a VD/VT ratio ranging from 0.37 to 0.49 [16]. Combining the positive VD/VTpeak- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) relationship with the positive FEV1%- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) relationship, it can be deduced that a high VD/VTpeak and a high FEV1% together may synergistically amplify \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) (Fig. 2). However, FEV1% and VD/VTpeak were negatively related in this study (r = − 0.27) and in a previous report (r = − 0.377) [17]. As a result, the relationship between FEV1% and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) was perturbed [3, 11, 13]. Hence, the relationship between VD/VTpeak and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) may also have been perturbed (Fig. 2 and Table 3).
Nevertheless, the high VD/VT was also biphasic, i.e. it caused an increase or decrease in \( \dot{\mathrm{V}}\mathrm{E} \) at a given level of metabolism. An appropriately high VD/VT may increase \( \dot{\mathrm{V}}\mathrm{E} \) to maintain arterial isocapnia. However, Poon and Tin [35] and Gargiuro et al. [9] reported that excessive mechanical constraints may occur in patients with CHF when external dead space volume is loaded to an inappropriate extent. The biphasic effect of high VD/VTpeak on \( \dot{\mathrm{V}}\mathrm{E} \) may further modify the \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \)-FEV1% relationship.
At peak exercise, the more severe the airflow obstruction and emphysema, the lower the \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) [3, 11, 12]. Although Paolotti et al. [12] agreed with this notion, they proposed another two hypotheses: (1) an improvement in ventilatory efficiency during exercise due to reduced physiological dead space; (2) a higher arterial CO2 (PaCO2) set-point, as they found that the hypercapnia was related to emphysema. In this study, the increase in \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) at peak exercise was related to an increase in VD/VT but not to a decrease in VD/VT. A higher PaCO2 point was not reset; instead, a lower PaCO2 level developed. Notably, only 10 subjects had arterial blood gas data during exercise in their study, and the formula for VD/VT did not subtract apparatus VD [12], which was addressed by Wasserman et al. and Sun et al. [2, 30]. A high FEV1% is associated with a high \( \dot{\mathrm{V}}\mathrm{E} \); a high \( \dot{\mathrm{V}}\mathrm{E} \) is associated with a high \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \); a high \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) is associated with a high pH and PaO2, and a low PaCO2 (Fig. 2). In other words, this also suggests that mechanical constraints may limit the increase in \( \dot{\mathrm{V}}\mathrm{E} \) during exercise with a negative influence on gas exchange values at peak exercise (i.e. PaO2 and SPO2 decrease, PaCO2 increase).
Interestingly, \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) was highly negatively related to emphysema (r = − 0.77, p < 0.001) [12] in Paolotti et al’s study and in the current study as represented by VTpeak/FEV1 as the emphysema factor [13] (Table 2), whereas it was moderately positively related to VD/VTpeak in the current study and in another report [16]. In this context, it can be deduced that emphysema may be inversely related to VD/VTpeak. However, Paoletti et al. reported that when emphysema was measured by high resolution computed tomography, the FEV1% and VD/VTpeak-rest were weakly related to the emphysema extent [12, 36]. When emphysema was evaluated by pathology, the feature of loss of alveolar attachments was related to high \( \dot{\mathrm{V}}\mathrm{D} \) and VD/VT [37] and low FEV1% [17].
Volume excursion at peak exercise i.e. VT/IC and VT/VC and VT/FEV1 (emphysema factor) [13] and dynamic hyperinflation (DH) as represented by EELVpeak /TLC [11] have been reported to be mildly to moderately negatively related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) in the literature (Supplementary Table, r = − 0.31 - -0.35 and − 0.48 - -0.60). However, in the current study, even though none of the markers of volume excursion and DH as represented by VT/TLC [38, 39] were related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \), the emphysema factor was mildly negatively related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) (r = − 0.32).
\( \dot{V}E/\dot{V} CO2I \) versus Pulmonary Physiology. In patients with heart failure and normal subjects with or without external VD at rest and during exercise, \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) is assumed to be \( \dot{\mathrm{V}}\mathrm{D} \) when \( \dot{\mathrm{V}}\mathrm{CO}2 \) is zero [9, 40]. However, our findings may challenge this notion, as \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) was not significantly related to VD/VTrest or VD/VTpeak (Table 2). Other studies have also not supported that \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) is an index of \( \dot{\mathrm{V}}\mathrm{D} \). The \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) has been reported to be ≤0 L in more than 10% of subjects in previous reports [3, 29] even though other studies have reported no patients with ≤0 L (0.9–9.9 L) [13]. In normal subjects, Sun et al. reported a \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) value of 11.7 L/min [30]. In patients with heart failure, Gargiulo et al. reported that the average of VD and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) at rest was 0.3–0.5 L ± 0.2 L, with a VT of 0.38 ± 0.08 L [9]. These values are too large to be biological plausible for VD and \( \dot{\mathrm{V}}\mathrm{D} \) in their study [9]. Nevertheless, the apparatus VD was also not subtracted from the physiological VD when calculating VD/VT [9]. In this context, despite an increase in PETCO2 being moderately related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) in the current study and to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) in Paoletti et al’s report [12], whether or not \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) reflects \( \dot{\mathrm{V}}\mathrm{D} \) remains unclear.
On the other hand, in the current study, we found that \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) was mildly related to inspiratory flow rather than FEV1% (Table 2). The loss of alveolar attachments is a feature of emphysema with high \( \dot{\mathrm{V}}\mathrm{D} \) and VD/VT [37] and is usually measured in fully inflated lungs so that expiratory flow obstruction cannot sufficiently reflect the condition, and thus its severity can be underestimated [41]. However, Teopompi et al. reported that \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) was moderately negatively related to FEV1% and diffusing capacity [13]. Moreover, they reported that the inconsistence in the \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \)-FEV1% relationship was attributed to volume excursion constraint which developed during exercise [13], whereas volume excursion constraint was not related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) or \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) in the current study.
In the current study, the relationships between \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and \( \dot{\mathrm{V}}\mathrm{O}2 \)peak% and Workpeak% were negative to a varying extent, which is consistent with the previous reports (Table 2 and Supplementary Table) [3, 11, 13]. However, the relationship between \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) and \( \dot{\mathrm{V}}\mathrm{O}2 \)peak% in the current study was different to a previous report [11] (Table 2 and Supplementary Table). The reason is unclear. In the current study, VD/VTpeak was simultaneously the opposite of \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) and \( \dot{\mathrm{V}}\mathrm{O}2 \)peak% (r = − 0.23 and − 0.62, respectively) and VT/TIpeak was simultaneously consistent with \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) and \( \dot{\mathrm{V}}\mathrm{O}2 \)peak% (r = 0.22–0.30 and 0.59, respectively). The heterogeneity of the population of this study may also have contributed to the inconsistencies. Further studies are warranted to clarify this issue.
Lastly, an interesting finding was the difference between \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{R} \) and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) in combination with \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \). \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) have consistently been negatively related to a varying degree both in the current study and in previous studies (Table 2, r = − 0.25 - -0.74) [11, 13]. The sum of \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) was reported to be close to or closely related to \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{R} \) in a previous report [11]. In the current study, the sum of the two variables and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{R} \) were similar (39.5 ± 7.5 versus 38.6 ± 7.8, p = 0.52). The relationship between the sum of \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{R} \) has been reported to be mathematical [1, 2]. Further mathematical simulation studies on this issue are warranted.
Study limitations
There are several limitations to this study. First, correlation studies allow researchers to study the relationships between one variable and others, and may not be appropriate to infer a cause and effect. However, it is reasonable to consider that a high VD/VT may induce \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) rather than to consider that a high \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) induces a high VD/VT. Similarly, a high FEV1% may induce a high \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) rather than a high \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) induces a high FEV1%. Second, the number of cases in this subgroup study was small, and this may have caused insufficient power when performing correlation coefficient analysis on VD/VT and the other variables of interest. However, the sample size of 46 achieved a power of 80% to detect a difference between a correlation of 0.4 and the null (no correlation) using a two-sided test with a significance level of 0.05. As the power is related to type II error, a non-significant test results should be interpreted more conservatively. Third, all of the participants in this study were male, so the results cannot be applied to females. As only 4% of patients with COPD are female in Taiwan [20], and as breathing pattern and dead space are different between men and women [42], it would be difficult to enroll a sufficient number of female subjects with COPD to compare the differences between male and female patients with COPD. To calculate \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \), the methodology to identify VCP or RCP [1, 9, 29] and whether to use the entire loaded exercise data [5] or data below VCP/RCP [2, 3, 11,12,13] are inconsistent in the literature. Further studies are warranted to clarify these issues.
Clinical implication
Although airflow obstruction may attenuate the increase in \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) during incremental exercise, an increase in dead space ventilation may amplify \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and thus perturb the \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) - FEV1% relationship. Nevertheless, airflow obstruction is usually accompanied with increased dead space ventilation. Hence, this study reveals the paradoxical relationship among the three factors (i.e. \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \), airflow obstruction and dead space ventilation). The role of \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) as a marker of ventilatory insufficiency in COPD is also questionable. Further studies are warranted to study the clinical applications and importance of exercise \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) and \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) in patients with COPD.
Conclusions
Using VD/VT measurements, we found that dead space ventilation perturbs the airflow- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) relationship. Increasing ventilation thereby increasing \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) may be the cause rather than the effect of maintaining biological homeostasis. The pulmonary physiology- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \)- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) relationship is inconsistent between the current study and previous studies.
Availability of data and materials
Uploaded.
Abbreviations
- \( \dot{\mathrm{V}}\mathrm{E} \) :
-
Ventilation
- \( \dot{\mathrm{V}}\mathrm{CO}2 \) :
-
CO2 output
- VD/VT :
-
Dead space fraction of tidal volume
- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \) :
-
\( \dot{\mathrm{V}}\mathrm{E} \) versus \( \dot{\mathrm{V}}\mathrm{CO}2 \) slope
- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \) :
-
\( \dot{\mathrm{V}}\mathrm{E} \) versus \( \dot{\mathrm{V}}\mathrm{CO}2 \) intercept
- FEV1%:
-
Forced expired volume in one s % predicted
- CHF:
-
Congestive heart failure
- COPD:
-
Chronic obstructive pulmonary disease
- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{R} \) :
-
\( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2 \) ratio
- GOLD:
-
Global Initiative for Chronic Lung Disease
- FVC:
-
Forced vital capacity
- CPET:
-
Cardiopulmonary exercise test
- OCD:
-
Oxygen-cost diagram
- TLC:
-
Total lung capacity
- RV:
-
Residual volume
- DLCO:
-
Diffusing capacity for carbon monoxide
- PETCO2 :
-
End-tidal PCO2
- SPO2 :
-
Arterial oxyhemoglobin saturation was measured by pulse oximetry
- RCP or VCP:
-
Respiratory or ventilatory compensation point
- \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{N} \) :
-
\( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2 \) nadir
References
Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Measurements during integrative cardiopulmonary exercise testing. In: Wasserman K, editor. Principles of exercise testing and interpretation. 4th ed. Philadelphia: Lippincot Williams & Wilkins; 2005. p. 76–110.
Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Normal values. In: Wasserman K, editor. Principles of exercise testing and interpretation. 4th ed. Philadelphia: Lippicott Williams & Wilkins; 2005. p. 160–82.
Teopompi E, Tzani P, Aiello M, et al. Ventilatory response to carbon dioxide output in subjects with congestive heart failure and in patients with COPD with comparable exercise capacity. Respir Care. 2014;59(7):1034–41.
Arena R, Myers J, Abella J, et al. Influence of heart failure etiology on the prognostic value of peak oxygen consumption and minute ventilation/carbon dioxide production slope. Circulation. 2007;115(18):2410–7.
Tabet JY, Beauvais F, Thabut G, Tartière JM, Logeart D, Cohen-Solal A. A critical appraisal of the prognostic value of the VE/VCO2 slope in chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2003;10(4):267–72.
Kim V, Kretschman DM, Sternberg AL, DeCamp MMJ, Criner GJ. National Emphysema Treatment Trial Research Group. Weight gain after lung reduction surgery is related to improved lung function and ventilatory efficiency. Am J Respir Crit Care Med. 2012;186(11):1109–16.
Metra M, Raccagni D, Carini G, et al. Ventilatory and arterial blood gas changes during exercise in heart failure. In: Wasserman K, editor. Exercise gas exchange in heart failure. 1st ed. Armonk: Futura; 1996. p. 125–43.
Koike A, Hiroe M, Marumo F. Increased ventilatory response to exercise as related to functional capacity. In: Wasserman K, editor. Exercise gas exchange in heart disease. 1st ed. Armonk, Futura; 1996. p. 109–23.
Gargiulo P, Apostolo A, Perrone-Filardi P, Sciomer S, Palange P, Agostoni P. A non invasive estimate of dead space ventilation from exercise measurements. PLoS One. 2014;9(1):e87395.
Whipp BJ, Ward SA, Wasserman K. Ventilatory control to exercise and their control in man. Am Rev Respir Dis. 1984;129(2 Pt 2):S17–20.
Neder JA, Arbex FF, Alencar MC, et al. Exercise ventilatory inefficiency in mild to end-stage COPD. Eur Respir J. 2015;45(2):377–87.
Paoletti P, De Filippis F, Fraioli F, et al. Cardiopulmonary exercise testing (CPET) in pulmonary emphysema. Respir Physiol Neurobiol. 2011;179(2–3):167–73.
Teopompi E, Tzani P, Aiello M, Gioia MR, Marangio E, Chetta A. Excess ventilation and ventilatory constraints during exercise in patients with chronic obstructive pulmonary disease. Respir Physiol Neurobiol. 2014;197:9–14.
Gagnon P, Bussières JS, Ribeiro F, et al. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(7):606–15.
Peters MM, Webb KA, O'Donnell DE. Combined physiological effects of bronchodilators and hyperoxia on exertional dyspnoea in normoxic COPD. Thorax. 2006;61(7):559–67.
Jones NL, Berman LB. Gas exchange in chronic air-flow obstruction. Am Rev Respir Dis. 1984;129(2 Pt 2):S81–S3.
Wright JL, Cagle P, Churg A, Colby TV, Myers J. Diseases of the small airways. Am Rev Respir Dis. 1992;146(1):240–62.
Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Physiology of exercise. In: Wasserman K, editor. Principles of exercise testing and interpretation. 4th ed. Philadelphia: Lippicott Williams & Wilkins; 2005. p. 10–65.
GOLD Committees. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (revised 2015). Disclosure forms for GOLD Committees are posted on the GOLD Website, wwwgoldcopdorg 2017.
Huang TH, Hsiue TR, Lin SH, Liao XM, Su PL, Chen CZ. Comparison of different staging methods for COPD in predicting outcomes. Eur Respir J. 2018;51(3).
Chuang ML, Lin IF, Lee CY. Clinical assessment tests in evaluating patients with chronic obstructive pulmonary disease - a cross-sectional study. Medicine (Baltimore). 2016;95(47):e5471.
Chuang ML, Lin IF. Investigating the relationships among lung function variables in chronic obstructive pulmonary disease in men. Peer J. 2019;7:e7829.
Chuang ML, Lin IF, Wasserman K. The body weight-walking distance product as related to lung function, anaerobic threshold and peak VO2 in COPD patients. Respir Med. 2001;95(7):618–26.
Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38.
Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–22.
ATS/ERS S. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624.
Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–61.
Chuang ML, Lee CH, Lin IF. Using the oxygen-cost diagram in ramp-slope selection for dyspneic patients. Intern Med. 2010;49(14):1325–32.
American Thoracic Society, American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–77.
Sun XG, Hansen JE, Garatachea N, Storer TW, Wasserman K. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. 2002;166(11):1443–8.
Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Calculations, formulas, and examples. In: Wasserman K, editor. Principles of exercise testing and interpretation. 4th ed. Philadelphia: Lippincot Williams & Wilkins; 2005. p. 556–65.
Vassilakopoulos T, Zakynthinos S, Roussos C. Assessing muscular oxygenation during incremental exercise using near-infrared spectroscopy: comparison of three different methods. Am J Respir Crit Care Med. 1998;158(2):378–85.
Zocchi L, Fitting JW, Majani U, Fracchia C, Rampulla C, Grassino A. Effect of pressure and timing of contraction on human rib cage muscle fatigue. Am Rev Respir Dis. 1993;147(4):857–64.
Bégin P, Grassino A. Chronic Alveolar Hypoventilation Helps To Maintain the Inspiratory Muscle Effort of COPD Patients Within Sustainable Limits. Chest. 2000;117(5 Suppl 1):271S–3S.
Poon CS, Tin C. Mechanism of augmented exercise hyperpnea in chronic heart failure and dead space loading. Respir Physiol Neurobiol. 2013;186(1):114–30.
Kurashima K, Takayanagi N, Sato N, et al. High resolution CT and bronchial reversibility test for diagnosing COPD. Respirology. 2005;10(3):316–22.
Nici L. Mechanisms and measures of exercise intolerance in chronic obstructive pulmonary disease. Clin Chest Med. 2000;21(4):693–704.
Chuang ML. Combining dynamic hyperinflation with dead space volume during maximal exercise in patients with chronic obstructive pulmonary disease. J Clin Med. 2020;9(10):1127.
Chuang ML, Hsieh MJ, Lin IF. Developing a new marker of dynamic hyperinflation in patients with obstructive airway disease - an observational study. Sci Rep. 2019;9(1):7514.
Agostoni P, Apostolo A, Sciomer S. Evolution of the concept of ventilatory limitation during exercise. Combining the pneumologist and cardiologist point of view. Respir Physiol Neurobiol. 2011;179(2–3):127–8.
Thurlbeck WM. Pathophysiology of chronic obstructive pulmonary disease. Clin Chest Med. 1990;11(3):389–403.
Gläser S, Ittermann T, Koch B, et al. Influence of smoking and obesity on alveolar-arterial gas pressure differences and dead space ventilation at rest and peak exercise in healthy men and women. Respir Med. 2013;107(6):919–26.
Acknowledgements
I thank Professor Lin, I-Feng of National Yang Ming University, Institute of Public Health, for her comments on statistical analysis.
The name of the institution(s) review board: The Institutional Review Board of Chung Shan Medical University Hospital.
Funding
This study was supported in part by Chung Shang Medical University Hospital. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
This study was supported in part by the Minister of Science and Technology, Taiwan.
Author information
Authors and Affiliations
Contributions
MLC: initiated and designed the study, analyzed and interpreted the data, wrote the manuscript, and approved the version to be published.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Signed informed consent was obtained from each participant. The local Institutional Review Boards of the two institutions approved this study.
The name of the ethics committee: Chung Shan Medical University Hospital (CS19014).
Registered at this site: Chung Shan Medical University Hospital, Taichung, Taiwan.
Registration number: CSH-2019-C-030.
Studies involving animals: “Not applicable”.
Consent for publication
“Not applicable”.
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Supplementary Table
. Summary of correlation (r) of \( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2 \) slope (\( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{S} \)) and its intercept (\( \dot{\mathrm{V}}\mathrm{E}/\dot{\mathrm{V}}\mathrm{CO}2\mathrm{I} \)) with pulmonary physiology.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chuang, ML. Mechanisms affecting exercise ventilatory inefficiency-airflow obstruction relationship in male patients with chronic obstructive pulmonary disease. Respir Res 21, 206 (2020). https://doi.org/10.1186/s12931-020-01463-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-020-01463-4