Abstract
Background
The identification of smoking-related lung disease in current and former smokers with normal FEV1 is complex, leading to debate regarding using a ratio of forced expiratory volume in 1 s to forced vital capacity (FEV1/FVC) of less than 0.70 versus the predicted lower limit of normal (LLN) for diagnosis of airflow obstruction. We hypothesized that the discordant group of ever-smokers with FEV1/FVC between the LLN and 0.70 is heterogeneous, and aimed to characterize the burden of smoking-related lung disease in this group.
Methods
We compared spirometry, chest CT characteristics, and symptoms between 161 ever-smokers in the discordant group and 940 ever-smokers and 190 never-smokers with normal FEV1 and FEV1/FVC > 0.70 in the SPIROMICS cohort. We also estimated sensitivity and specificity for diagnosing objective radiographic evidence of chronic obstructive pulmonary disease (COPD) using different FEV1/FVC criteria thresholds.
Results
The discordant group had more CT defined emphysema and non-emphysematous gas trapping, lower post-bronchodilator FEV1 and FEF25–75, and higher respiratory medication use compared with the other two groups. Within the discordant group, 44% had radiographic CT evidence of either emphysema or non-emphysematous gas trapping; an FEV1/FVC threshold of 0.70 has greater sensitivity but lower specificity compared with LLN for identifying individuals with CT abnormality.
Conclusions
Ever-smokers with normal FEV1 and FEV1/FVC < 0.70 but > LLN are a heterogeneous group that includes significant numbers of individuals with and without radiographic evidence of smoking-related lung disease. These findings emphasize the limitations of diagnosing COPD based on spirometric criteria alone.
Similar content being viewed by others
Background
Airflow obstruction is a hallmark of chronic obstructive pulmonary disease (COPD), and by current recommendations [1] is confirmed by a reduced ratio of forced expiratory volume in 1 s (FEV1) to forced vital capacity (FVC). To simplify the diagnosis of airflow obstruction, a fixed cut-off ratio of FEV1/FVC (FEV1/FVC < 0.70) is often used instead of predicted lower limit of normal (LLN) (FEV1/FVC < LLN), defined as the lower fifth percentile of a reference population. [2, 3]
Because the predicted normal FEV1/FVC declines with age, a fixed cut-off ratio of FEV1/FVC < 0.70 has the potential for misclassification and over diagnosis in the elderly, [4,5,6,7,8,9,10] while using predicted LLN may better predict adverse clinical outcomes [11] and more accurately predict all-cause mortality. [4] Although there is a group of younger individuals for whom LLN is > 0.7, a particular challenge is presented by subjects who fall in a “discordant” group with FEV1/FVC ratio > LLN but < 0.7. Compared to subjects with FEV1/FVC > 0.70, the individuals in this discordant group have been found to have greater emphysema, airway wall thickening, and gas trapping, as well as greater risk for chronic obstructive pulmonary disease (COPD)-related hospitalization, emergency department visits, and mortality [12,13,14,15,16]. There has been recent interest in characterizing patients with mild smoking-related lung disease as evidenced by symptoms and radiographic abnormalities despite normal spirometry, [17, 18] highlighting the limitations of using spirometric criteria alone for diagnosis of COPD. We hypothesized that the discordant group of ever-smokers with FEV1/FVC between the LLN and 0.70 is heterogeneous, containing some individuals with smoking-related lung disease and some with changes in lung function related to aging. We address this hypothesis by characterizing clinical, radiographic and physiologic features of ever-smokers in this discordant group and comparing them with the group of individuals with FEV1/FVC > 0.70.Footnote 1
Methods
SPIROMICS study methods
SPIROMICS is a multicenter prospective cohort study that has enrolled 2981 participants including never-smokers, smokers without airway obstruction and smokers with mild, moderate and severe COPD, with the goals of identifying new COPD subgroups and intermediate markers of disease progression [19]. Participants were 40–80 years old at baseline. “Smokers” were defined as current or former smokers with lifetime smoking history of greater than 20 pack-years. The study design and exclusion criteria have previously been described [19]. The research protocol was approved by the institutional review boards of all participating institutions and all participants gave written informed consent.
Subjects and measure of exposure
We analyzed data for three groups of subjects included in SPIROMICS: Group 1) current or former smokers (ever-smokers) with normal post-bronchodilator FEV1 and FEV1/FVC > LLN but < 0.70 (discordant group, n = 161); Group 2) ever-smokers with normal FEV1 and FEV1/FVC > 0.70 (n = 940); and Group 3) never-smokers with normal FEV1 and FEV1/FVC > 0.70 (n = 190). In a supplementary analysis we also compared outcomes with a Group 4) patients with FEV1/FVC in the 75% quartile of those less than LLN (n = 379).
Pulmonary function methods
Pulmonary function testing was performed and interpreted according to the 2005 ATS/ERS guidelines; post-bronchodilator spirometric measurements were used for analysis [20, 21]. NHANES III spirometric references values were used to calculate percent predicted values and LLN [22].
Outcomes
We compared respiratory symptoms, quality of life, medication use, CT metrics, FEV1% predicted, forced expiratory flow rate between 25 and 75% of FVC or maximum mid-expiratory flow (FEF25–75), 6 min walk distance (6MWD), and two prospective variables: annual FEV1 change and exacerbation rate, between the three groups. Chronic bronchitis was defined as patient reported cough with sputum for at least 3 months for ≥2 years. Dyspnea was defined by the modified Medical Research Council (mMRC) dyspnea score, [23] stratified into two groups as mMRC ≥2 (moderate or severe dyspnea) vs mMRC 0–1 (mild or no dyspnea). Respiratory symptoms were also measured by the COPD Assessment Test (CAT) [24]. Quality of life was measured by the St. George’s Respiratory Questionnaire (SGRQ) [25]. Medication use was defined as patient-reported regular use of inhaled bronchodilators and/or inhaled steroid. Annual FEV1 change was defined using a regression model that incorporated the total number of study visits and spirometry measurements available for each participant. Each participant had a minimum of two spirometry measurements at least 200 days apart, with follow up ranging from 266 to 1749 days. Exacerbation rate was measured as the number of patient-reported events requiring health care utilization in the first year after study enrollment.
Multidetector-row computed tomography (MDCT) scans at full inspiration and full expiration were performed at the SPIROMICS baseline visit. Emphysema was defined using a threshold of <− 950 Hounsfield Units on full inspiration. Airway wall thickening was defined as the square root of the airway wall area for a standardized airway with an internal perimeter of 10 mm (Pi10) [26]. Parametric Response Mapping (PRM) was used to define functional small airways disease (fSAD), a measure of non-emphysematous gas trapping, as the percent of voxels with CT attenuation values > − 950 HU on the inspiratory exam and < − 856 HU on the expiratory scan, as previously described [27] using Imbio Lung Density software (Imbio, Minneapolis, MN).
Statistical analysis
Comparisons of categorical predictors across groups 1, 2 and 3 used chi-squared tests. For continuous variables, ANOVA was used to test for overall differences between the 3 groups; pairwise comparisons of continuous outcomes between any two groups were based on t-tests. [28] Multivariable linear regression was used to compare continuous measures (emphysema, fSAD, CT metrics, 6MWD, CAT score, quality of life, FEV1, and FEF25–75) between groups, adjusted for age, sex, race, smoking history (pack-years) and current smoking (yes/no). Multivariable logistic regression was used to compare binary clinical outcomes (emphysema present, fSAD present, chronic bronchitis, mMRC Dyspnea, and medication use) adjusted for the same patient characteristics described above.
Quantile regression models [29] applied to healthy never -smokers estimated the 95th percentile for PRM emphysema and, separately, the 95th percentile for PRM fSAD for a normal patient based on their age, sex, BMI and the scanner used. Hereafter, these estimated 95th percentiles will be used to define the upper limit of normal (ULN) for these PRM measures according to patient/scanner characteristics. Presence of emphysema or fSAD was defined when an individual’s observed PRM emphysema percent or PRM fSAD percent was greater than the estimated ULN for a normal patient with similar patient/scanner characteristics. Sensitivity and specificity of each FEV1/FVC cut-off for identifying individuals with radiographic CT evidence of smoking-related lung disease manifest as either emphysema and/or fSAD were estimated.
Results
We compared the discordant group (Group 1) with ever-smokers with normal spirometry (Group 2) and never-smokers (Group 3). The characteristics of the three groups are shown in Table 1. The discordant group had more male and white participants and was older than the other two groups.
Compared with ever-smokers with FEV1/FVC > 0.70 (Group 2), the discordant individuals (Group 1) had lower post-bronchodilator FEV1% predicted (92.1% vs 97.5%, p < 0.001) and reduced FEF25–75% predicted (61.2% vs 102.3%, p < 0.001) (Table 2, Fig. 1). The two groups of ever-smokers did not differ significantly in 6MWD (437.5 vs 437.2 m, p = 0.97), SGRQ (22.5 vs 24.2, p = 0.28), or CAT score (10.7 vs 11.3, p = 0.36). More smokers in the discordant group reported regular use of either inhaled corticosteroids and/or bronchodilators than either ever-smokers with FEV1/FVC > 0.70, Group 2, (34.4% vs. 25.1%, p = 0.01) or never-smokers, Group 3 (3.9%, p < 0.001). Groups 1 and 2 did not differ in the reported incidence of chronic bronchitis or moderate or severe dyspnea indicated by mMRC score ≥ 2. Nor did they differ with respect to FEV1 decline per year, or exacerbations per year (Table 2).
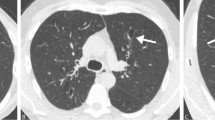
The discordant group had a modest but significantly greater percentage of lung with CT scan-defined emphysema than Group 2 (2.1% vs 0.7%, p < 0.001) or Group 3 (0.3%, p < 0.001). Individuals in the discordant group also had significantly increased PRM fSAD as compared to Groups 2 and 3 (18.0% vs. 9.1% and 7.1%, respectively, p < 0.001), without detectable differences in airway thickness (Table 3, Fig. 1). Density plots illustrating the distribution of emphysema and small airways disease are presented in supplementary material (Additional file 1: Figure S1 and Additional file 2: Figure S2).
Using age-adjusted ULN for % emphysema, more individuals in the discordant group met CT criteria for the presence of emphysema compared with Groups 2 and 3 (38.7% vs. 17.4% (p < 0.001) and 8.2% (p < 0.001), respectively). Similarly, using age-adjusted ULN for PRM fSAD, more individuals in the discordant group also met CT criteria for the presence of fSAD compared with Groups 2 and 3 (15.3% vs. 7.8% (p = 0.003) and 2.9% (p < 0.001), respectively). In the discordant group, 44% of subjects had CT evidence of smoking-related lung disease, manifest as either emphysema or fSAD, compared with 20.7% (p < 0.001) of Group 2 and 9.4% (p < 0.001) of Group 3 subjects. Conversely, 56% of individuals in the discordant group had CT scans without radiographic evidence of smoking-related lung disease (Table 3, Fig. 2 , Additional file 3: Figure S3).
Within the discordant group, those with CT evidence of smoking-related lung disease did not have significantly greater respiratory symptoms, FEV1 decline, exacerbations, or lower FEF25–75 compared with those without emphysema or fSAD (Table 4).
When compared to a fourth group of smokers with FEV1/FVC in the 75% quartile of those less than LLN, the discordant group had higher FEV1 and FEF25–75%, fewer respiratory symptoms and exacerbations, less airway wall thickness and fewer % of people with fSAD, however did not differ in the amount of emphysema or FEV1 decline (Additional file 4: Tables S1, S2 and S3).
A history of smoking had a significant association with symptoms in groups defined by either FEV1/FVC threshold compared to never-smokers. When compared with never-smokers without airflow obstruction, both groups of ever-smokers had more chronic bronchitis, dyspnea, respiratory symptoms as measured by CAT, and lower quality of life by SGRQ (Table 2).
Finally, we evaluated the sensitivity and specificity of the two thresholds for FEV1/FVC for identification of individuals with radiographic evidence of smoking-related lung disease (emphysema > age-adjusted ULN and/or PRM fSAD > age-adjusted ULN). A threshold of FEV1/FVC < 0.7 had a sensitivity of 0.85 and specificity of 0.72 for identifying any radiographic abnormality. The FEV1/FVC < LLN threshold had lower calculated sensitivity (0.78) and higher specificity (0.81) (Table 5). Thus the absolute ratio is more sensitive, while the LLN is more specific.
Discussion
In the SPIROMICS cohort, current or former smokers with normal FEV1 who are diagnosed with COPD based on GOLD spirometric criteria, but who do not have airflow obstruction based on the LLN threshold, have more emphysema and functional small airways disease by CT, increased use of inhaled medications, and lower mid-expiratory flow compared with current or former smokers without airway obstruction, defined by FEV1/FVC > 0.70. Almost half of individuals in this discordant group have CT evidence of smoking-related lung disease. Nevertheless, the discordant group did not have increased respiratory symptoms (chronic bronchitis, dyspnea, or CAT) or decreased exercise tolerance when compared with individuals with FEV1/FVC ratio > 0.7.
We have focused this analysis on individuals in this discordant group for three reasons. First, in reference populations the ratio of FEV1/FVC decreases with advancing age, suggesting that use of a fixed threshold of 0.7 may inappropriately classify some individuals. Second, studies of this population may help elucidate the boundaries of normal aging in the setting of cigarette smoking. Finally, there has been recent interest in the clinical picture of smokers who may have smoking-related lung disease in the setting of little or no airflow obstruction [17, 18]. This study contributes to the discussion in each of these three areas.
Our findings support the presence of early/mild disease among individuals in this discordant group and thus provide potential pathophysiologic explanation for previous studies demonstrating increased risk for COPD-related health effects in this group, including increased adjusted risk of death, COPD-related emergency department visits and hospitalizations [13, 15]. These studies suggest that the LLN threshold lacks sensitivity, failing to identify a number of individuals with clinically significant disease.
However, because the predicted FEV1/FVC may decline with normal age, using a fixed cut-off ratio of FEV1/FVC < 0.70 increases diagnosis of obstruction in the elderly, and in very old adults has the potential to classify changes associated with aging as COPD [4,5,6,7,8,9]. In a cohort of adults 80 years and older, airflow obstruction defined by FEV1/FVC < LLN, but not FEV1/FVC between LLN and 0.70, was associated with increased mortality [4]. Similarly, small amounts of emphysema may occur due to aging-related changes rather than as a consequence of early smoking-related disease. In the multiethnic MESA cohort, full-lung CT scans of healthy nonsmokers revealed a small percent of emphysema (median 1.1%) that was increased in men and with age. [30] CT-defined functional small airway abnormality also increases with age [31]. Therefore, the predicted “normal” amount of emphysema and small airways disease increases with aging even in the absence of smoking exposure. An important question is how to distinguish between early/mild COPD and normal aging. In our study we used data from normal individuals to create age-adjusted upper limits of normal for both emphysema and PRM fSAD, suggesting that the CT abnormality we have identified in the discordant group is beyond that associated with normal aging. Our study extends previous findings by including innovative imaging parameters of small airways disease and comparisons with normal lung density [16].
We found significantly reduced FEF25–75% and CT scan evidence of non-emphysematous air trapping in the discordant group. Reduction in mid-expiratory flow is generally assumed to be an indication of small airways disease [32,33,34]. We did not identify differences in airway wall thickness, manifest as path specific Pi10, associated with our discordant group. However, changes in lumen dimension may mask changes in wall thickening/thinning by this parameter [35]. CT air trapping is also thought to reflect small airways disease and has been associated with lower lung function and accelerated lung function decline [36, 37]. The functional small airways disease measurement using PRM helps to distinguish non-emphysematous air trapping from emphysema on CT [27]. Thus physiologic and CT scan data both point to subtle but potentially clinically important small airways abnormalities in this discordant group. Several studies have suggested that in the natural history of COPD, small airways may become narrowed or lost prior to the onset of emphysema [34, 38,39,40] and thus these abnormalities may be an earlier indication of smoking-related COPD. We evaluated two prospective variables: exacerbations in the first year after enrollment, and FEV1 decline over a period of up to 4 years. Though we did not detect more FEV1 decline or exacerbations in the discordant group or those with radiographic emphysema or fSAD, exacerbation rate was overall low in these patients with mild smoking-related lung disease. Longer follow up time will enhance our understanding of the significance of these mild radiographic and physiologic abnormalities as predictors of progression to COPD.
The choice of a threshold of FEV1/FVC for diagnosing airflow obstruction may depend on the goals of testing and whether a more sensitive or specific test is preferred. In the SPIROMICS cohort, using a FEV1/FVC threshold of 0.70 is more sensitive but less specific for identifying individuals with radiographic manifestations of COPD, while using LLN is more specific but less sensitive. As a screening test for early/mild disease in ever-smokers, a more sensitive test may be preferred. However, identifying airflow obstruction using either FEV1/FVC threshold will incorrectly classify individuals.
There are several important features of this study. SPIROMICS is a large multi-center cohort whose subjects are extensively characterized for symptoms, physiology and radiology. MDCT scans performed at baseline allowed detailed assessment of emphysema, air trapping, and airway wall thickness and image analysis by PRM allowed differentiation of non-emphysematous air trapping from emphysema on CT. We recognize several limitations to our study. FEF25–75% is an effort-dependent measurement like FEV1 and we cannot exclude a confounding effect of limited effort or frailty. However, the subjects described in this report all had studies that met ATS criteria and had normal FVC. The specificity statistic is biased because the analysis data set is not a random sample. Additionally, never-smokers with FEV1/FVC < 0.7 were not included in SPIROMICS and thus could not be compared in this analysis.
Conclusions
Ever-smokers who have normal FEV1 and FEV1/FVC < 0.70 but > LLN (discordant group) have on average more emphysema and small airways disease, and increased respiratory medication use compared with those with FEV1/FVC > 0.70. This is a heterogeneous group that includes a large number of individuals with CT evidence of either emphysema or non-emphysematous gas trapping, as well as many individuals without radiographic evidence of early smoking-related lung disease for whom it is likely that normal aging accounts for the apparent spirometric abnormality. The diagnosis of early/mild COPD requires a more sophisticated approach that goes beyond currently accepted spirometric criteria.
Abbreviations
- 6MWD:
-
6 min walk distance
- CAT:
-
COPD Assessment Test
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- FEF25–75 :
-
Forced expiratory flow rate between 25 and 75% of FVC
- FEV1 :
-
Forced expiratory volume in 1 s
- fSAD:
-
Functional small airways disease
- FVC:
-
Forced vital capacity
- LLN:
-
Lower limit of normal
- mMRC:
-
Modified Medical Research Council
- Pi10:
-
Square root of the airway wall area for a standardized airway with an internal perimeter of 10 mm
- PRM:
-
Parametric Response Mapping
- SGRQ:
-
St. George’s Respiratory Questionnaire
- ULN:
-
Upper limit of normal
References
Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–65.
Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981;123:659–64.
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005:948–68.
Turkeshi E, Vaes B, Andreeva E, Matheï C, Adriaensen W, Van Pottelbergh G, Degryse J-M. Airflow limitation by the global lungs initiative equations in a cohort of very old adults. Eur Respir J. 2015;46(1):123–32.
Vollmer WM, Gíslason T, Burney P, Enright PL, Gulsvik A, Kocabas A, Buist AS. Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J. 2009;34:588–97.
Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, Miller MR, Jensen RL, Falaschetti E, Schouten JP, Hankinson JL, Stocks J, Quanjer PH. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–51.
Vaz Fragoso CA, Concato J, McAvay G. Chronic obstructive pulmonary disease in older persons: a comparison of two spirometric definitions. Respiratory. 2010;104(8):1189–96.
Hansen JE, Sun X-G, Wasserman K. Spirometric criteria for airway obstruction: use percentage of FEV1/FVC ratio below the fifth percentile, not. CHEST. 2007;131:349–55.
Quanjer PH. Correctly defining criteria for diagnosing chronic obstructive pulmonary disease matters. Am J Respir Crit Care Med. 2014;189:230.
Vaz Fragoso CA, McAvay G, Van Ness PH, Casaburi R, Jensen RL, MacIntyre N, Gill TM, Yaggi HK, Concato J. Phenotype of Normal spirometry in an aging population. Am J Respir Crit Care Med. 2015;192:817–25.
van Dijk W, Tan W, Li P, Guo B, Li S, Benedetti A, Bourbeau J, CanCOLD study group. Clinical relevance of fixed ratio vs lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med. 2015;13:41–8.
Bhatt SP, Sieren JC, Dransfield MT, Washko GR, Newell JD, Stinson DS, Zamba GKD, Hoffman EA, COPDGene Investigators. Comparison of spirometric thresholds in diagnosing smoking-related airflow obstruction. Thorax. 2014;69:409–14.
Mannino DM, Sonia Buist A, Vollmer WM. Chronic obstructive pulmonary disease in the older adult: what defines abnormal lung function? Thorax. 2007;62:237–41.
Mohamed Hoesein FAA, Zanen P, Lammers J-WJ. Lower limit of normal or FEV1/FVC. Respir Med. 2011;105:907–15.
Izquierdo Alonso JL, De Lucas Ramos P, Rodríguez Glez-Moro JM. The use of the lower limit of Normal as a criterion for COPD excludes patients with increased morbidity and high consumption of health-care resources. Archivos de Bronconeumología (English Edition). 2012;48:223–8.
Hoesein FAAM, de Jong PA, Lammers J-WJ, Mali WP, Schmidt M, de Koning HJ, van der Aalst C, Oudkerk M, Vliegenthart R, van Ginneken B, van Rikxoort EM, Zanen P. Computed tomography structural lung changes in discordant airflow limitation. PLoS One. 2013;8:e65177.
Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, Gouskova NA, Hansel NN, Hoffman EA, Kanner RE, Kleerup E, Lazarus SC, Martinez FJ, Paine R, Rennard S, Tashkin DP, Han MK, SPIROMICS research group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–21.
Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JHM, Grenier PA, Kauczor H-U, Bailey WC, DeMeo DL, Casaburi RH, Friedman P, Van Beek EJR, Hokanson JE, Bowler RP, Beaty TH, Washko GR, Han MK, Kim V, Kim SS, Yagihashi K, Washington L, McEvoy CE, Tanner C, Mannino DM, Make BJ, Silverman EK, Crapo JD. Clinical and radiologic disease in smokers with Normal spirometry. JAMA Intern Med. 2015;175:1539.
Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, Rennard S, SPIROMICS research group. Design of the Subpopulations and Intermediate Outcomes in COPD study (SPIROMICS). Thorax. 2014;69:491–4.
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. ATS/ERS task force: standardisation of spirometry. Eur Respir J. 2005;26(2):319–38.
Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. ATS/ERS task force: general considerations for lung function testing. Eur Respir J. 2005;26:153–61.
Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87.
Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–6.
Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–54.
Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's respiratory questionnaire. Am Rev Respir Dis. 1992;145:1321–7.
Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, Elliott WM, Hogg JC, Paré PD. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–6.
Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, Galbán S, Rehemtulla A, Kazerooni EA, Martinez FJ, Ross BD. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–5.
Welch BL. The generalization of `student“s” problem when several different population variances are involved. Biometrika. 1947;34:28.
Koenker R (2012). Package ‘quantreg’. http://cran.r-project.org/web/packages/quantreg/quantreg.pdf, accessed 15 Aug 2018.
Hoffman EA, Ahmed FS, Baumhauer H, Budoff M, Carr JJ, Kronmal R, Reddy S, Barr RG. Variation in the percent of emphysema-like lung in a healthy, nonsmoking multiethnic sample. The MESA lung study. Ann Am Thorac Soc. 2014;11:898–907.
Martinez CH, Diaz AA, Meldrum C, Curtis JL, Cooper CB, Pirozzi C, Kanner RE, Paine R, Woodruff PG, Bleecker ER, Hansel NN, Barr RG, Marchetti N, Criner GJ, Kazerooni EA, Hoffman EA, Ross BD, Galbán CJ, Cigolle CT, Martinez FJ, Han MK, SPIROMICS Investigators. Age and small airway imaging abnormalities in subjects with and without airflow obstruction in SPIROMICS. Am J Respir Crit Care Med. 2017;195:464–72.
McFadden ER Jr, Linden DA. A reduction in maximum mid-expiratory flow rate. A spirographic manifestation of small airway disease. Am J Med. 1972;52(6):725–37.
Gelb AF, Zamel N. Simplified diagnosis of small-airway obstruction. N Engl J Med. 1973;288:395–8.
van den Berge M, ten NHT H, Cohen J, Douma WR, Postma DS. Small airway disease in asthma and COPD: clinical implications. CHEST. 2011;139:412–23.
Smith BM, Hoffman EA, Rabinowitz D, Bleecker E, Christenson S, Couper D, Donohue KM, Han MK, Hansel NN, Kanner RE, Kleerup E, Rennard S, Barr RG. Comparison of spatially matched airways reveals thinner airway walls in COPD. The multi-ethnic study of atherosclerosis (MESA) COPD study and the Subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax. 2014;69:987–96.
Mets OM, de Jong PA, van Ginneken B, Kruitwagen CLJJ, Prokop M, Oudkerk M, Lammers J-WJ, Zanen P. CT air trapping is independently associated with lung function reduction over time. PLoS One. 2013;8:e61783.
Bommart S, Marin G, Bourdin A, Molinari N, Klein F, Hayot M, Vachier I, Chanez P, Mercier J, Vernhet-Kovacsik H. Relationship between CT air trapping criteria and lung function in small airway impairment quantification. BMC Pulm Med. 2014;14:29.
McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, Wright AC, Gefter WB, Litzky L, Coxson HO, Paré PD, Sin DD, Pierce RA, Woods JC, McWilliams AM, Mayo JR, Lam SC, Cooper JD, Hogg JC. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–75.
Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Paré PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53.
Verbanck S, Schuermans D, Meysman M, Paiva M, Vincken W. Noninvasive assessment of airway alterations in smokers. Am J Respir Crit Care Med. 2004;170:414–9.
Pirozzi CS, Paine R, Tashkin DP, Kleerup EC, Woodruff PG, Han MK, Quibrera PM, Carretta E, Kanner R: Evidence of Clinical COPD in Smokers with Airway Obstruction Diagnosed with FEV1/FVC Ratio of Less than 0.70 but Not Less than Predicted Lower Limit of Normal. In American Thoracic Society International Conference Abstracts. American Thoracic Society; 2015:A4473–A4473.
Pirozzi CS, Gu T, Quibrera P, Carretta E, Han MK, Murray S, Cooper CB, Tashkin DP, Kleerup EC, Hoffman EA, Martinez C, Christenson S, Hansel NN, Barr RG, Bleecker ER, Ortega VE, Martinez FJ, Kanner RE, Paine R: Heterogeneous Burden of Emphysema and Functional Small Airway Abnormalities in Smokers with FEV1/FVC Ratio Above Lower Limit of Normal but Below 0.7. In American Thoracic Society International Conference Abstracts. Am J Respir Crit Care Med 2018;197:A6397
Acknowledgements
The authors thank the SPIROMICS participants and participating physicians, investigators and staff for making this research possible. More information about the study and how to access SPIROMICS data is at www.spiromics.org. We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E Alexis, PhD; Wayne H Anderson, PhD; R Graham Barr, MD, DrPH; Eugene R Bleecker, MD; Richard C Boucher, MD; Russell P Bowler, MD, PhD; Elizabeth E Carretta, MPH; Stephanie A Christenson, MD; Alejandro P Comellas, MD; Christopher B Cooper, MD, PhD; David J Couper, PhD; Gerard J Criner, MD; Ronald G Crystal, MD; Jeffrey L Curtis, MD; Claire M Doerschuk, MD; Mark T Dransfield, MD; Christine M Freeman, PhD; MeiLan K Han, MD, MS; Nadia N Hansel, MD, MPH; Annette T Hastie, PhD; Eric A Hoffman, PhD; Robert J Kaner, MD; Richard E Kanner, MD; Eric C Kleerup, MD; Jerry A Krishnan, MD, PhD; Lisa M LaVange, PhD; Stephen C Lazarus, MD; Fernando J Martinez, MD, MS; Deborah A Meyers, PhD; John D Newell Jr., MD; Elizabeth C Oelsner, MD, MPH; Wanda K O’Neal, PhD; Robert Paine, III, MD; Nirupama Putcha, MD, MHS; Stephen I. Rennard, MD; Donald P Tashkin, MD; Mary Beth Scholand, MD; J Michael Wells, MD; Robert A Wise, MD; and Prescott G Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Thomas Croxton, PhD, MD.
Notation of prior abstract publication/presentation
Evidence of Clinical COPD in Smokers with Airway Obstruction Diagnosed with FEV1/FVC Ratio of Less than 0.70 but Not Less than Predicted Lower Limit of Normal. Presented at the American Thoracic Society International Conference, May 19, 2015, Denver, CO, USA.
Funding
SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C HHSN268200900019C, HHSN268200900020C), which were supplemented by contributions made through the Foundation for the NIH from AstraZeneca; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici SpA; Forest Research Institute, Inc.; GSK; Grifols Therapeutics, Inc.; Ikaria, Inc.; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; Regeneron Pharmaceuticals, Inc.; and Sanofi. This analysis was also supported by R01 HL122438 and K24 HL138188.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the SPIROMICS GIC coordinating center at the University of North Carolina at Chapel Hill on reasonable request.
Author information
Authors and Affiliations
Consortia
Contributions
CP, RP, and RK conceived and supervised the overall study. TG, PQ, EC, MKH, and SM participated in the statistical analysis. CP wrote the first draft of the manuscript. RK, MKH, CC, DT, EK, IB, EH, CM, SC, NH, GB, EB, VO, FM, TG, SM, PQ, EC, and RP participated in writing of the manuscript, provided important intellectual content, and read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research protocol was approved by the institutional review boards of all participating institutions and all participants gave written informed consent. Institutional review board approval reference numbers for each clinical site are available as Additional file 5.
Consent for publication
Not applicable
Competing interests
Dr. Tashkin reports personal fees from Boehringer-Ingelheim, personal fees from AstraZeneca, personal fees from Sunovion, personal fees from Theravance/Innoviva, personal fees from Mylan, outside the submitted work. Dr. Kleerup reports grants from NIH, grants from Foundation for the NIH, during the conduct of the study; grants from Boehringer Ingelheim, grants from Novartis, grants from Pearl/AstraZeneca, grants from Sunovion/Sepracor, outside the submitted work. Boehringer Ingelheim and GlaxoSmithKline supplied inhalers for pulmonary function testing in this study. Dr. Han reports personal fees from GSK, personal fees from BI, personal fees from AZ, non-financial support from Sunovion, non-financial support from Novartis, outside the submitted work. Dr. Cooper reports grants from Equinox Health Clubs, personal fees from Equinox Health Clubs, grants from Amgen, personal fees from PulmonX, GlaxoSmithKline, outside the submitted work; and works with scientific engagement for the GlaxoSmithKline Global Respiratory Franchise. Dr. Barjaktarevic reports grants from NIH, during the conduct of the study; personal fees from Astra Zeneca, from Grifols, from CSL Behring, outside the submitted work. Dr. Hoffman is a founder and shareholder of VIDA Diagnostics, a company commercializing lung image analysis software developed, in part, at the University of Iowa. Dr. Christenson reports personal fees from AstraZeneca, non-financial support from Genentech, grants from MedImmune, outside the submitted work. Dr. Hansel reports grants and personal fees from AstraZeneca, grants and personal fees from GSK, grants from Boehringer Ingelheim, grants from NIH, grants from COPD Foundation, outside the submitted work. Dr. Bleecker has undertaken clinical trials through his employer, Wake Forest School of Medicine and University of Arizona, for AstraZeneca, MedImmune, Boehringer Ingelheim, Cephalon/Teva, Genentech, Johnson and Johnson (Janssen), Novartis, Regeneron, and Sanofi Genzyme, personal fees from ERB, has also served as a paid consultant for AztraZeneca, MedImmune, Boehringer Ingelheim, Glaxo Smith Kline, Novartis, Regeneron, and Sanofi Genzyme, outside the submitted work. Dr. Martinez reports grants from NHLBI, during the conduct of the study; grants from National Institutes of Health, personal fees from Continuing Education, personal fees from Forest Laboratories, other from Janssen, personal fees from GlaxoSmithKline, personal fees from Nycomed/Takeda, personal fees from AstraZeneca, personal fees from Boehringer Ingelheim, personal fees from Bellerophon (formerly Ikaria), personal fees from Genentech, personal fees from Novartis, personal fees from Pearl, personal fees from Roche, personal fees from Sunovion, personal fees from Theravance, personal fees from CME Incite, personal fees from Annenberg Center for Health Sciences at Eisenhower, personal fees from Integritas, personal fees from InThought, personal fees from National Association for Continuing Education, personal fees from Paradigm Medical Communications, LLC, personal fees from PeerVoice, personal fees from UpToDate, personal fees from Haymarket Communications, personal fees from Western Society of Allergy and Immunology, from Proterixbio (formerly Bioscale), personal fees from Unity Biotechnology, personal fees from ConCert Pharmaceuticals, personal fees from Lucid, personal fees from Methodist Hospital, personal fees from Columbia University, personal fees from Prime Healthcare Ltd., personal fees from WebMD, personal fees from PeerView Network, personal fees from California Society of Allergy and Immunology, personal fees from Chiesi, personal fees from Puerto Rico Thoracic Society, outside the submitted work. Ms. Carretta reports funding from the National Heart, Lung, and Blood Institute, the Foundation for the NIH, Genentech, and the COPD Foundation during the conduct of the study. Dr. Paine reports grants from National Heart Lung and Blood Institute, grants from COPD Foundation, during the conduct of the study; grants from Department of Veterans Affairs, outside the submitted work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Baseline characteristics for the four groups. Table S2. Comparison of physiologic and clinical variables between ever-smokers with normal FEV1 and FEV1/FVC > LLN but < 0.70 (“discordant” group, Group 1), ever-smokers with normal FEV1 and FEV1/FVC > 0.70 (Group 2), never-smokers with normal FEV1 and FEV1/FVC > 0.70 (Group 3) and ever-smokers with FEV1/FVC ≤ LLN and > 75th quartile (Group 4). Table S3. Comparison of CT variables between ever-smokers with normal FEV1 and FEV1/FVC > LLN but < 0.70 (“discordant” group, Group 1), ever-smokers with normal FEV1 and FEV1/FVC > 0.70 (Group 2), never-smokers with normal FEV1 and FEV1/FVC > 0.70 (Group 3) and ever-smokers with FEV1/FVC ≤ LLN and > 75th quartile (Group 4). (DOCX 100 kb)
Additional file 2:
Figure S1. Density plot of the distribution of emphysema. (PNG 58 kb)
Additional file 3:
Figure S2. Density plot of the distribution of functional small airways disease. (PNG 55 kb)
Additional file 4:
Figure S3. Venn diagram illustrating patients within the discordant group (ever-smokers with normal FEV1 and FEV1/FVC > LLN but < 0.70) who have emphysema, functional small airways disease (fSAD), and both on chest CT imaging. (PDF 34 kb)
Additional file 5:
Heterogeneous Burden of Lung Disease in Smokers with Borderline Airflow Obstruction. (DOCX 73 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Pirozzi, C.S., Gu, T., Quibrera, P.M. et al. Heterogeneous burden of lung disease in smokers with borderline airflow obstruction. Respir Res 19, 223 (2018). https://doi.org/10.1186/s12931-018-0911-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-018-0911-z