Abstract
Respiratory diseases including asthma and chronic obstructive pulmonary disease (COPD) are characterised by excessive and persistent inflammation. Current treatments are often inadequate for symptom and disease control, and hence new therapies are warranted. Recent emerging research has implicated dyslipidaemia in pulmonary inflammation. Three ATP-binding cassette (ABC) transporters are found in the mammalian lung – ABCA1, ABCG1 and ABCA3 – that are involved in movement of cholesterol and phospholipids from lung cells. The aim of this review is to corroborate the current evidence for the role of ABC lipid transporters in pulmonary lipid homeostasis and inflammation. Here, we summarise results from murine knockout studies, human diseases associated with ABC transporter mutations, and in vitro studies. Disruption to ABC transporter activity results in lipid accumulation and elevated levels of inflammatory cytokines in lung tissue. Furthermore, these ABC-knockout mice exhibit signs of respiratory distress. ABC lipid transporters appear to have a crucial and protective role in the lung. However, our knowledge of the underlying molecular mechanisms for these benefits requires further attention. Understanding the relationship between cholesterol and inflammation in the lung, and the role that ABC transporters play in this may illuminate new pathways to target for the treatment of inflammatory lung diseases.
Similar content being viewed by others
Background
Asthma and chronic obstructive pulmonary disease (COPD) affect over 500 million people worldwide, contributing to significant morbidity and mortality [1, 2]. These chronic lung diseases share many features, including airway obstruction, persistent airway inflammation, and presence of multiple inflammatory mediators. However, the underlying pathophysiological processes and responses to therapy for each disease are distinct [3]. The pathogenesis of asthma involves secretion of pro-inflammatory cytokines from airway epithelial cells and macrophages [4], resulting in airway infiltration of CD4+ T-lymphocytes, mast cells and eosinophils. Contrastingly, CD8+ T-lymphocytes, macrophages and neutrophils are the more predominant cell-types found in lung tissue of patients with COPD [5]. Currently, bronchodilators including beta-agonists and anti-cholinergics, and anti-inflammatory corticosteroids are the mainstays of treatment. However, lack of disease-modifying effects of and poor responses to these therapies mean that alternate therapeutic targets and drugs are needed.
Inflammation is a complex response of the immune system to tissue injury or foreign bodies. Whilst this is considered a beneficial protective mechanism in assisting restoration of normal tissue function, persistent and excessive inflammatory responses may eventuate in disease. Recently, studies have implicated dyslipidaemia in pulmonary inflammation and various lung pathologies [6] and alongside, statins (3-hydroxymethyl-3-glutaryl coenzyme A (HMG-CoA) reductase inhibitors) have been tried as therapies for asthma and COPD with varying results [7–9].
Reverse cholesterol transport (RCT) is a protective mechanism for regulating lipid homeostasis, acting to shuttle excess cholesterol to the liver for subsequent excretion from the body. Essential to this mechanism is the interplay between ATP-binding cassette (ABC) transporters and extracellular lipid acceptors such as high-density lipoproteins (HDL) [10, 11]. Currently, 48 ABC transporters have been identified that facilitate the movement of a diverse range of substrates across cellular membranes [12]. Three ABC lipid transporters, ABCA1, ABCG1 and ABCA3, are expressed in mammalian lung cells. The first two contribute to RCT by mediating cellular cholesterol and phospholipid efflux and are transcriptionally regulated by liver X receptors (LXRs) [10, 11, 13]. ABCA3, a phospholipid exporter, is specifically involved in pulmonary surfactant production. Surfactants in the lung contain approximately 90% phospholipids and 10% surfactant proteins and are essential to prevent alveolar collapse by reducing surface tension at the air-liquid interface [14, 15]. Studies in lungs of knockout mice have established that absence of ABC transporters result in disrupted lipid homeostasis, diminished surfactant production, impaired respiratory physiology and increased expression of inflammatory cytokines in lung cells [10, 15, 16]. Furthermore, mutations to these transporter genes are associated with human lung diseases such as alveolar proteinosis and respiratory distress syndrome [14, 17, 18]. Given that hyperlipidaemia has been demonstrated to accelerate inflammation in murine bronchoalveolar lavages [19], the role of ABC transporters in the pathogenesis of inflammatory lung diseases is an exciting and promising avenue to explore.
This review will explore current available knowledge regarding the roles of ABCA1, ABCG1 and ABCA3 in maintaining lipid homeostasis and controlling inflammation in the lung. Clarifying this relationship between pulmonary lipidosis and inflammation will help guide relevant future research studies that could ultimately identify an alternative therapeutic angle for the treatment of asthma, COPD and other related conditions.
ABCA1
ABCA1 is widely expressed throughout the body and contributes to RCT by exporting cholesterol and phospholipids out of cells to extracellular acceptors [20, 21]. In the periphery, the lipid-poor HDL protein component apolipoprotein (apo)A-I, is the predominant extracellular acceptor, and binding of apoA-I to cells expressing ABCA1 leads to the generation of small nascent HDL particles [22, 23]. Homozygous and heterozygous mutations to the ABCA1 alleles result in Tangier disease and familial hypoalphalipoproteinaemia respectively, with only around 100 Tangier patients diagnosed worldwide [24]. Phenotypically, these patients have markedly reduced HDL levels, resulting in lipid accumulation in numerous tissues and subsequent increased risks of atherosclerosis and cardiovascular complications [25]. Furthermore, it was recently shown that ABCA1-mutation carriers exhibit signs of systemic inflammation as indicated by elevated levels of circulating inflammatory cytokines [24]. Over 73 mutations, including single nucleotide polymorphisms have also been reported in the ABCA1 gene [26], raising the possibility of more widespread cholesterol metabolic disruptions across populations. The contributions of such mutations to development of asthma and COPD are yet to be explored.
ABCA1 tissue expression is second highest in the lungs, after the liver, suggesting a critical role for the transporter and lipid homeostasis in pulmonary function [27]. Immunohistochemical data have identified the presence of ABCA1 in alveolar type I (ATI) and type II (ATII) pneumocytes and alveolar macrophages [28, 29] (see Table 1 and Fig. 1). Cholesterol loading and treatment with LXR-agonists, both separately and conjointly, were also able to induce ABCA1 expression and function in human airway smooth muscle (ASM) cells [30].
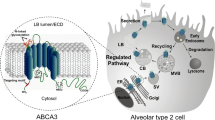
ABC transporters expressed in various cell types of the alveolus. Three ABC transporters, namely ABCA1, ABCG1 and ABCA3, are expressed in lung cells present in the alveolus, notably the alveolar epithelial type I and II cells (ATI and ATII respectively) that line the alveoli or air sacs, and alveolar macrophages that are phagocytes of the pulmonary immune system. LB – lamellar bodies, where ABCA3 contributes lipids that eventually are secreted as surfactants (represented via the black arrow)
Phenotype of ABCA1-knockout mouse models
The critical role of ABCA1 in lung inflammation is evident from murine knockout models. Abca1-knockout mice exhibit interrupted lipid export, and a 70% reduction in plasma cholesterol and phospholipids due to virtually undetectable HDL and apoA-I levels, compared to wild-type [10]. Phenotypically, these mice at 4 months of age exhibit massive cholesterol and phospholipid accumulation in alveolar macrophages and ATII pneumocytes, as compared with wild-type littermates [10]. Furthermore, oil red-O staining of Abca1 −/− lungs revealed pulmonary focal lesions, and microscopic analysis revealed enlarged foamy alveolar macrophages and ATII cell hyperplasia, indicating alveolar proteinosis [10]. Physiologically, these mice experienced reduced tidal volume and hyperventilation, although it was unclear whether this respiratory distress was directly attributable to abnormalities in the lung or in other organs [10]. Nevertheless, these findings, coupled with similar but progressively severe observations in 7-, 12- and 18-month-old mice [31], suggest that absence of ABCA1 results in age-dependent progressive pulmonary disease. Supporting the importance of this ABCA1-mediated pathway in normal lung physiology is the finding that apoA-I−/− mice have elevated inflammatory cell infiltration (particularly neutrophils and leukocytes), collagen deposition and airway hyper-responsiveness, and impaired pulmonary vasodilatation [32]. These observations strongly implicate a role of ABCA1-mediated RCT in inflammatory lung diseases.
Mechanisms of ABCA1-mediated RCT and role in pulmonary inflammation
The mechanism by which ABCA1 exports lipids has been predominantly studied in the context of its role in macrophage lipid homeostasis and the development of atherosclerosis. ABCA1 is thought to export lipids from the inner to the outer leaflet of the cell membrane via an ATP-dependent mechanism [12]. The subsequent method of transfer of these lipids to apoA-I is still open for debate. One model proposes that hydrolysis of ATP causes a conformational change in the transporter, enabling apoA-I to bind [33]; alternatively, apoA-I may bind and solubilise lipids in exovesiculated membrane lipid domains that arise from membrane asymmetry [34]. It is worth noting that evidence for the presence of apoA-I in the lung is currently limited. Bates and colleagues [28] were able to stain cryosections of murine lungs with anti-mouse apoA-I antibody, to verify that adequate levels of apoA-I exist for interaction with pneumocyte ABCA1. Meanwhile, Delvecchio and colleagues [30] performed in vitro experiments involving incubation of ASM cells with exogenous apoA-I, and were able to confirm the capacity of ASM ABCA1 to efflux lipids to apoA-I. Of further interest was the finding that apoA-I is reduced in sputum samples of patients with COPD compared to controls (which were smokers), with the authors suggesting that apoA-I could potentially be investigated as a biomarker [35]. Inhaled apoA-I mimetic peptides, which are under development as treatments for atherosclerosis, have been suggested as a potential treatment for inflammatory lung conditions [36].
Studies addressing the mechanisms by which ABCA1 activity affects inflammatory processes in the lung have investigated various pathways. Firstly, it has been proposed that excess cholesterol itself is pro-inflammatory and acts as the trigger for the cellular inflammatory response [37], and hence ABCA1 activity may simply be anti-inflammatory by removing excess cholesterol. Alternatively, other studies have investigated its transcriptional upregulation via LXR. In human alveolar macrophages, LXR-agonists that increased ABCA1 mRNA expression were shown to reduce LPS-induced airway inflammation and neutrophil production. However, explanations for the underlying mechanisms are conflicting. In one study, this was attributed to a NF-κB-dependent pathway [38], whereas another study found no involvement of either of the pro-inflammatory transcription factors NF-κB or AP-1 [29]. However, it is worthwhile noting that LXR also affects ABCG1 expression (covered in the next section), effects on which were not accounted for in the latter study.
Lastly, Dai and colleagues have investigated effects of ABCA1 overexpression in alveolar macrophages and showed that the anti-inflammatory effects of ABCA1 may occur via suppression of granulocyte-colony stimulating factor, which subsequently could reduce the involvement of inflammatory cells such as neutrophils [39]. Evidently, further research is required to clarify the mechanisms by which ABCA1 activity affects inflammatory pathways.
ABCA1 and asthma
The association between ABCA1 function and asthma is still a novel and underdeveloped area. Mice overexpressing human ABCA1 exhibited reduced neutrophil count, IgE levels, peri-bronchial inflammation and airway epithelial thickness in response to daily ovalbumin challenges when compared to wildtype mice [39]. Furthermore, treatment of house dust mite-challenged mice with intranasal apoA-I was able to minimise eosinophilia, neutrophilia, interleukin (IL)-17E, IL-33 and airway hyperresponsiveness [40]. The same authors also revealed that apoA-I levels in bronchoalveolar lavage fluid of asthmatic patients were significantly lower than that from healthy subjects [40]. These findings suggest that upregulation of ABCA1 and/or apoA-I activity could be beneficial in patients with inflammatory lung diseases.
In summary, ABCA1 plays crucial roles in the maintenance of lipid homeostasis and modulation of inflammatory responses in lung cells. However, further studies are necessary to elucidate the exact mechanisms by which these effects occur and whether therapeutic targeting of these pathways will be effective.
ABCG1
Like ABCA1, ABCG1 is also ubiquitously expressed in the body [41]. ABCG1 is considered a half-transporter and requires dimerization to be functional, unlike ABCA1 which is a full-transporter that functions independently [42]. Whereas ABCA1 contributes to formation of nascent HDL particles, ABCG1 is thought to export lipids to larger, more mature HDL subclasses [43]. It has been suggested from cell culture as well as murine knockout models that ABCA1 and ABCG1 may synergise together [42, 44].
ABCG1 has also mostly been studied in the context of atherosclerosis, however there are currently no reported genetic ABCG1 mutations reported that are associated with an increased risk for cardiovascular disease in humans. Interestingly, ABCG1 deficiency in human alveolar macrophages has been associated with pulmonary alveolar proteinosis (PAP), a rare condition characterised by accumulation of surfactant proteins and lipids in the alveolar space [18]. This will be discussed in detail below.
Similarly to ABCA1, ABCG1 utilises ATP to actively export its substrates (see Table 1 and Fig. 1) to the exoplasmic leaflet of the cell membrane [42]. However, direct binding of ABCG1 with an acceptor (e.g. HDL) is not required for transport activity, and there is currently some debate in the literature whether the transporter is exclusively located intracellularly or travels and acts on the cell surface [45].
ABCG1 and its role in lipid homeostasis and pulmonary inflammation
There is strong evidence derived from Abcg1-knockout murine models to suggest that this transporter also plays an essential role in maintaining pulmonary lipid homeostasis. ABCG1 is widely expressed in various lung cell types, including alveolar macrophages, epithelial cells, ATII pneumocytes, ASM cells, T-lymphocytes and dendritic cells [43, 46, 47]. Several studies in Abcg1-knockout mice have demonstrated disrupted lipid homeostasis in murine lungs. Compared to heterozygote Abcg1 +/− mice, homozygote knockouts revealed massive accumulation of lipids in the alveolar macrophages. The subpleural region of Abcg1 −/− lungs also revealed build-up of lymphocytes, multinucleated giant cells, and macrophage foam cells containing an abundance of cholesterol clefts that indicated impaired lipid processing. These observations were progressive and age-dependent, and were exacerbated upon administration of a high-fat high-cholesterol diet [16, 43]. Furthermore, human ABCG1-transgenic mice overexpressing the ABCG1 transporter were protected from this diet-induced pulmonary lipidosis [43].
Absence of ABCG1 also results in progressive and chronic pulmonary inflammation [19]. Immunofluorescence studies and bronchoalveolar lavages obtained from chow-fed Abcg1 −/− mice revealed multiple markers of inflammation, including elevated levels of foamy macrophages, lymphocytes and pro-inflammatory cytokines. None of these features were observed in lungs of wild-type littermates [19]. Coupled with the observations that administration of a high-fat high-cholesterol diet significantly accelerated lipid deposition and induced macrophage cytokine expression in wild-type and (to an even greater extent in) Abcg1 −/− lungs, these findings imply that ABCG1 indirectly controls inflammation by modifying intracellular cholesterol levels [19]. However, the timeline of events was less clear in a separate study, which also reported inflammatory cytokine, neutrophil and lymphocyte infiltration alongside lipidosis in the lungs of Abcg1 −/− mice [48]. Wojcik and coauthors proposed that one of two mechanisms could be occurring: i) lipid accumulation causes inflammation or ii) absence of Abcg1 triggers inflammation that subsequently leads to the observed lipidosis [48]; which one of these events predominates is still unclear. Nevertheless, pulmonary ABCG1 expression also appears to protect against exogenous inflammation-inducing factors. Abcg1 −/− mice challenged with LPS or gram-negative bacteria displayed an exaggerated pulmonary response, characterised by elevated neutrophil, leukocyte, cytokine and chemokine levels in the airspace and tissue remodelling as compared to wild-type mice [49]. These effects were attributed specifically to disrupted Abcg1 −/− activity in alveolar macrophages. Despite the enhanced pulmonary bacterial clearance in these knockout mice, the excessive inflammation meant they also had a much higher mortality rate [49]. In a murine model of allergic asthma, ovalbumin-challenged Abcg1 −/− mice displayed increased airway neutrophils and IL-17, which are implicated in severe forms of asthma [46]. These findings suggest that ABCG1 plays a critical role in regulating the host defence response in the lung. Overall, results from murine models provide strong evidence for the critical role of ABCG1 in maintaining cellular lipid homeostasis and controlling pulmonary inflammation.
Inflammatory profile of ABCG1-knockout mice
Amongst the myriad of inflammatory cytokines and chemokines released in asthma and COPD, several have also been identified in Abcg1-knockout mice. Levels of the inflammatory cytokines tumour necrosis factor (TNF)-α and IL-1β were markedly elevated in lungs of Abcg1-deficient chow-fed mice [19]. TNF-α is expressed in multiple lung cell types, including macrophages, lymphocytes, mast cells and ASM cells, and promotes oxidative damage that subsequently activates NF-κB and AP-1. Results of this activation include recruitment of adhesion molecules, lymphocytes, and other inflammatory cytokines, and increased airway hyperresponsiveness [50]. IL-1β has been shown to cause inflammation, airway fibrosis, bronchiolar thickening and mucus metaplasia – features that are prominent in COPD and asthma. Furthermore, IL-1β enhanced production of matrix metalloproteinases (MMP)-9 and −12 in neutrophils and macrophages of murine airways respectively [51]. MMPs that degrade extracellular matrix are overexpressed in patients with COPD and asthma, and have been associated with airway inflammation and remodelling [52]. Elevated levels of MMP-8 and MMP-12 have also been reported in lungs of Abcg1 −/− mice [19]. Alveolar macrophages also play a pivotal role in orchestrating the inflammatory processes in COPD and asthma by secreting numerous pro-inflammatory cytokines and chemokines [3]. However, they also secrete inhibitory mediators such as IL-10 that dampen inflammation, but this secretion is thought to be impaired in patients with asthma [53]. These activities were reflected in the alveolar macrophages of Abcg1 −/− mice, with Wojtik and coauthors reporting significantly elevated pro-inflammatory IL-1, IL-6 and IL-12 levels, and decreased expression of the anti-inflammatory cytokine IL-10 [48]. Another study indicated that following an asthma allergen challenge in Abcg1 −/− mice, airway neutrophil and IL-17 levels were elevated compared to wild-type [46]. Although it was not specified which IL-17 subtype was elevated, a separate study showed that IL-17A contributes to glucocorticoid-insensitivity by decreasing the activity of histone deacetylase-2, an enzyme that normally mediates the anti-inflammatory effects of glucocorticoids [54]. Taken together, evidence from Abcg1 −/− mouse models suggest that disruption to ABCG1 activity results in a phenotype reflective of inflammatory lung disease.
ABCG1 and pulmonary surfactant homeostasis
ABCG1 deficiency has been identified in patients with pulmonary alveolar proteinosis (PAP), a condition characterised by pulmonary surfactant accumulation [18]. Pulmonary surfactant is produced in lamellar bodies of ATII pneumocytes, and degraded by alveolar macrophages. Studies in chow-fed Abcg1 −/− mice showed accumulation of ATII pneumocytes that were enlarged and engorged with surfactant-rich lamellar bodies, suggesting abnormal surfactant clearance. Esterified cholesterol levels were also elevated in alveolar macrophages, which occurred despite increased Abca1 expression, which was thought to be due to compensatory upregulation. The authors hypothesised that ABCG1 disruption in both these cell types resulted in defective lipid/surfactant secretion, thereby resulting in compensatory cell hypertrophy and severe pulmonary lipidosis [16]. In 90% of PAP cases, autoantibodies that neutralise granulocyte macrophage-colony stimulating factor (GM-CSF) impair alveolar macrophage maturation, consequently hindering their ability to clear surfactant [55]. Thomassen and colleagues recognised that both PAP patients and GM-CSF-knockout mice demonstrate surfactant accumulation in alveolar macrophages, and ABCG1 deficiency, despite increased ABCA1 activity [18]. Induction of ABCG1 expression via LXRα was able to reverse intracellular lipid accumulation and restore lung compliance in GM-CSF-knockout mice [56, 57]. These results demonstrate that ABCG1 is critical for normal surfactant metabolism.
Altogether, the importance of ABCG1 in regulating lipid levels and inflammation in the lung is clear from murine studies, as well as the phenotype of patients with PAP. However, the underlying mechanisms and pathways by which ABCG1 mediates its protective effects are currently unclear.
ABCA3
Unlike ABCA1 and ABCG1 that are expressed in multiple lung cell types, ABCA3 is exclusively expressed in the lamellar bodies of ATII pneumocytes [58] and crucial for the proper formation of lamellar bodies in these cells [59]. Due to its intracellular expression, ABCA3 does not require an extracellular lipid acceptor. Substrates of ABCA3 include phosphatidylcholine, phosphatidylglycerol and also cholesterol (see Table 1 and Fig. 1), which are transported into the lamellar bodies [60, 61]. Within these organelles, phospholipids are combined with surfactant proteins to produce pulmonary surfactant, which is subsequently exocytosed into the alveolar airspace to reduce surface tension and prevent alveolar collapse [15, 62]. The exact mechanism of ABCA3-mediated lipid transport remains elusive [63].
ABCA3 and its role in lipid homeostasis and pulmonary inflammation
Dysfunctional ABCA3 disrupts lamellar body phospholipid export, causing respiratory distress syndrome (RDS), and pediatric and adult interstitial lung disease [14, 60], which is a family of related conditions characterised by inflammation and fibrosis of the pulmonary interstitium [60]. RDS typically affects neonates, and is caused by surfactant deficiency that results in alveolar collapse, hypoxaemia and pulmonary oedema [14], hence this condition is often lethal. Inflammation accompanying RDS is fuelled by neutrophils and cytokines that are likely controlled via the NF-κB pro-inflammatory signalling pathway [64]. Corticosteroids can be used for their ability to stimulate surfactant phospholipid synthesis in surviving infants, but can have significant side effects. These compounds have also been found to upregulate ABCA3 expression and modulate transcription factors including NF-κB in chronic inflammatory lung diseases [58, 65]; these may be additional mechanisms by which they facilitate foetal lung maturation.
Several murine knockout studies have also confirmed the critical role for ABCA3 in surfactant production. Absence of mature lamellar bodies and surfactant phospholipids in the alveolar space of Abca3 −/− mice resulted in surfactant deficiency and fatal respiratory failure [15, 61]. One proposed mechanism for the disrupted surfactant production seen in RDS suggests that ABCA3 mutations elevate endoplasmic reticulum stress and trigger apoptosis of ATII cells [62]. Elaborating on this finding, a more recent study showed that ABCA3 expression protects ATII cells from free cholesterol-induced cytotoxicity by exporting free cholesterol. Cells transfected with a non-functional mutant of ABCA3, ABCA3-Q215K, displayed excessive accumulation of cholesteryl esters and total phospholipids. The study showed that the excess lipids in these cells were rerouted away from lamellar bodies towards the ER, where the subsequent lipid accumulation resulted in endoplasmic reticulum stress and thus ATII apoptosis [60]. There is also increasing evidence that ATII cell dysfunction and surfactant abnormalities are implicated in the pathogenesis of COPD. Surfactant dysfunction is thought to contribute to airway resistance, oxidative stress, inflammation and increased protease activity that promotes tissue remodelling [66]. A mouse model expressing a common ABCA3 mutation observed in patients with diffuse parenchymal lung disease, the ABCA3E292V mutation, reported spontaneous lung remodelling in these mice with an emphysema-like phenotype [67]. However, no clinically significant ABCA3 mutations have been identified in COPD patients as yet [68].
Evidence is currently limited with respect to a potential role for ABCA3 in inflammation, despite suggestions that it may be involved in transport of cholesterol. Bronchoalveolar lavage cells from lungs of Abca3 −/− mice showed no statistically significant increase in expression of inflammatory cytokines, although measurements were only made for five cytokines [69]. In patients with ABCA3 mutations, levels of the representative cytokine IL-8 were significantly elevated in ATII cells as compared to normal cells [17]. It is unknown whether the increase in IL-8 was due to lipid accumulation within the ATII cells.
An alternate mechanism by which ABCA3-deficiency may lead to inflammation may be due to the associated reduction in the secretion of surfactant proteins. The hydrophilic collectins SP-A and SP-D have immunomodulatory functions, and have been suggested to reduce inflammation in the lungs by enhancing phagocytosis of apoptotic cells and pathogens, and inhibiting T-cell function. A deficiency in their secretion has been associated with the pathogenesis of COPD and asthma, although previous attempts at administering surfactants to asthma patients had mixed results [70–72]. Chiba and colleagues also demonstrated that both SP-A and phosphatidylglycerol in surfactant have anti-inflammatory effects [72]. Since it is clear that ABCA3 is critical for lamellar body formation, it is possible that ABCA3 indirectly modulates lung inflammation by helping to form the organelles that are necessary to produce these anti-inflammatory surfactant components.
In summary, our understanding of a role for ABCA3 in regulating lung lipid levels and inflammation is not as expansive as that for ABCA1 and ABCG1. Its role in the lung has been well defined in the context of surfactant production, however how it modulates pulmonary inflammation warrants further investigation.
Statins as alternate therapy for inflammatory lung disease
Recently, attention has been given to statins to explore their effect on lung inflammation in asthma and COPD. In addition to their lipid-lowering effects, statins have pleiotropic anti-inflammatory, antioxidant and anti-proliferative properties. In vivo studies in rodents have been promising, with simvastatin attenuating tobacco-induced infiltration of macrophages, neutrophils and leukocytes into the airways [9], and reducing the expression of macrophages, neutrophils, eosinophils, MMPs and various inflammatory cytokines in bronchoalveolar lavage fluid in ovalbumin-induced allergic asthma [73]. However, the benefits of statins in humans with inflammatory lung conditions have so far been inconsistent. Observational studies in humans have shown that statins can reduce exacerbation and mortality rates, and improve symptom control in patients with asthma and COPD [8, 74, 75]. Furthermore, a randomised placebo-controlled study in 12 COPD patients reported significantly reduced levels of inflammatory cells and mediators in the lung following atorvastatin treatment, although this did not translate into improved lung function [76]. On the other hand, one randomised double-blind crossover trial found no anti-inflammatory activity in asthma patients given simvastatin [77], while a large-scale randomised placebo-controlled trial in 884 patients concluded that simvastatin was ineffective in reducing exacerbations in moderate-to-severe COPD [78]. Two systematic reviews have reported statistically insignificant improvements in symptom control or lung function in asthma patients, however data did support statin-associated reductions in airway inflammation [79, 80]. A possible explanation for the inconsistent effects of statins may be their negative effects on ABC transporter expression. It has been well established that statin treatment in cells negatively affect ABCA1 and ABCG1 expression via an LXR dependent mechanism [81, 82]. This may have offset some of the benefits associated with their cholesterol-lowering and pleiotropic anti-inflammatory effects.
Conclusions
Although much of our understanding of the function of ABC lipid transporters has been derived from studies in the context of cardiovascular disease, there has been increasing interest in their activities in the lung. Studies utilising murine knockout models have reported congruent results that support the crucial roles of ABCA1 and ABCG1 in maintaining lipid homeostasis in alveolar macrophages, ASM cells, and ATI and ATII pneumocytes. Furthermore, many studies have affirmed a role of ABCG1 in protecting against lung inflammation, whereas positive, albeit less evidence is available for ABCA1, and thus this is an area requiring further investigation. Human pathologies such as RDS demonstrate a clear link between ABCA3 dysfunction and lipid disruption in ATII cells. Although a direct relationship between ABCA3 and inflammation has yet to be established, currently evidence suggests it plays an indirect role via the secretion of surfactant proteins. Statins have also shown mixed clinical benefits in improving outcomes in asthma and COPD, and any positive effects have been attributed to their pleiotropic anti-inflammatory activity. Other potential therapies such as inhaled apoA-I mimetic peptides have been suggested but so far not tested. Overall, ABC transporters are a promising area to further explore in the search for more effective therapies for inflammatory lung diseases.
Abbreviations
- ABC:
-
ATP-binding cassette
- AP-1:
-
Activator protein-1
- apo:
-
Apolipoprotein
- ASM:
-
Airway smooth muscle
- ATI:
-
Alveolar type I
- ATII:
-
Alveolar type II
- ATP:
-
Adenosine triphosphate
- COPD:
-
Chronic obstructive pulmonary disease
- GM-CSF:
-
Granulocyte macrophage-colony stimulating factor
- HDL:
-
High-density lipoprotein
- HMG-CoA:
-
3-hydroxymethyl-3-glutaryl coenzyme A
- IL:
-
Interleukin
- LPS:
-
Lipopolysaccharide
- LXR:
-
Liver X receptor
- MMP:
-
Matrix metalloproteinase
- NF-κB:
-
Nuclear factor κB
- PAP:
-
Pulmonary alveolar proteinosis
- RCT:
-
Reverse cholesterol transport
- RDS:
-
Respiratory distress syndrome
- SP:
-
Surfactant protein
- TNF-α:
-
Tumour necrosis factor-α
References
Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, Nair H, Gasevic D, Sridhar D, Campbell H, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. 2015;5(2):020415.
George L, Brightling CE. Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther Adv Chronic Dis. 2016;7(1):34–51.
Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–92.
Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol. 2014;205(5):621–31.
Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2016;138(1):16–27.
Gowdy KM, Fessler MB. Emerging roles for cholesterol and lipoproteins in lung disease. Pulm Pharmacol Ther. 2013;26(4):430–7.
Howard ML, Vincent AH. Statin effects on exacerbation rates, mortality, and inflammatory markers in patients with chronic obstructive pulmonary disease: a review of prospective studies. Pharmacotherapy. 2016;36(5):536–47.
Huang CC, Chan WL, Chen YC, Chen TJ, Chou KT, Lin SJ, Chen JW, Leu HB. Statin use in patients with asthma – a nationwide population-based study. Eur J Clin Invest. 2011;41(5):507–12.
Davis BB, Zeki AA, Bratt JM, Wang L, Filosto S, Walby WF, Kenyon NJ, Goldkorn T, Schelegle ES, Pinkerton KE. Simvastatin inhibits smoke-induced airway epithelial injury: implications for COPD therapy. Eur Respir J. 2013;42(2):350–61.
Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289(6):L980–9.
Yin K, Liao DF, Tang CK. ATP-binding membrane cassette transporter A1 (ABCA1): a possible link between inflammation and reverse cholesterol transport. Mol Med. 2010;16(9–10):438–49.
Tarling EJ, de Aguiar Vallim TQ, Edwards PA. Role of ABC transporters in lipid transport and human disease. Trends Endocrinol Metab. 2013;24(7):342–50.
Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol. 2010;204(3):233–40.
Shulenin S, Nogee LM, Annilo T, Wert SE, Whitsett JA, Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. New Eng J Med. 2004;350(13):1296–303.
Fitzgerald ML, Xavier R, Haley KJ, Welti R, Goss JL, Brown CE, Zhuang DZ, Bell SA, Lu N, McKee M, et al. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res. 2007;48(3):621–32.
Baldan A, Tarr P, Vales CS, Frank J, Shimotake TK, Hawgood S, Edwards PA. Deletion of the transmembrane transporter ABCG1 results in progressive pulmonary lipidosis. J Biol Chem. 2006;281(39):29401–10.
Flamein F, Riffault L, Muselet-Charlier C, Pernelle J, Feldmann D, Jonard L, Durand-Schneider AM, Coulomb A, Maurice M, Nogee LM, et al. Molecular and cellular characteristics of ABCA3 mutations associated with diffuse parenchymal lung diseases in children. Hum Mol Genet. 2012;21(4):765–75.
Thomassen MJ, Barna BP, Malur AG, Bonfield TL, Farver CF, Malur A, Dalrymple H, Kavuru MS, Febbraio M. ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J Lipid Res. 2007;48(12):2762–8.
Baldan A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol. 2008;180(5):3560–8.
Wellington CL, Walker EK, Suarez A, Kwok A, Bissada N, Singaraja R, Yang YZ, Zhang LH, James E, Wilson JE, et al. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab Invest. 2002;82(3):273–83.
Jessup W, Gelissen I, Gaus K, Kritharides L. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr Opin Lipidol. 2006;17:247–57.
Duong PT, Collins HL, Nickel M, Lund-Katz S, Rothblat GH, Phillips MC. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-I. J Lipid Res. 2006;47(4):832–43.
Vedhachalam C, Ghering AB, Davidson WS, Lund-Katz S, Rothblat GH, Phillips MC. ABCA1-induced cell surface binding sites for ApoA-I. Arterioscler Thomb Vasc Biol. 2007;27(7):1603–9.
Bochem AE, van der Valk FM, Tolani S, Stroes ES, Westerterp M, Tall AR. Increased systemic and plaque inflammation in ABCA1 mutation carriers with attenuation by statins. Arterioscler Thomb Vasc Biol. 2015;35(7):1663–9.
Puntoni M, Sbrana F, Bigazzi F, Sampietro T. Tangier disease: epidemiology, pathophysiology, and management. Am J Cardiovasc Drugs. 2012;12(5):303–11.
Attie AD. ABCA1: at the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem Sci. 2007;32(4):172–9.
Kielar D, Dietmaier W, Langmann T, Aslanidis C, Probst M, Naruszewicz M, Schmitz G. Rapid quantification of human ABCA1 mRNA in various cell types and tissues by real-time reverse transcription-PCR. Clin Chem. 2001;47(12):2089–97.
Bates SR, Tao J-Q, Yu KJ, Borok Z, Crandall ED, Collins HL, Rothblat GH. Expression and biological activity of ABCA1 in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2008;38(3):283–92.
Birrell MA, Catley MC, Hardaker E, Wong S, Willson TM, McCluskie K, Leonard T, Farrow SN, Collins JL, Haj-Yahia S, et al. Novel role for the liver X nuclear receptor in the suppression of lung inflammatory responses. J Biol Chem. 2007;282(44):31882–90.
Delvecchio CJ, Bilan P, Nair P, Capone JP. LXR-induced reverse cholesterol transport in human airway smooth muscle is mediated exclusively by ABCA1. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L949–957.
McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, et al. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci U S A. 2000;97(8):4245–50.
Wang W, Xu H, Shi Y, Nandedkar S, Zhang H, Gao H, Feroah T, Weihrauch D, Schulte ML, Jones DW, et al. Genetic deletion of apolipoprotein A-I increases airway hyperresponsiveness, inflammation, and collagen deposition in the lung. J Lipid Res. 2010;51(9):2560–70.
Nagao K, Takahashi K, Azuma Y, Takada M, Kimura Y, Matsuo M, Kioka N, Ueda K. ATP hydrolysis-dependent conformational changes in the extracellular domain of ABCA1 are associated with apoA-I binding. J Lipid Res. 2012;53(1):126–36.
Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282(34):25123–30.
Nicholas BL, Skipp P, Barton S, Singh D, Bagmane D, Mould R, Angco G, Ward J, Guha-Niyogi B, Wilson S, et al. Identification of lipocalin and apolipoprotein A1 as biomarkers of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(10):1049–60.
Gordon EM, Figueroa DM, Barochia AV, Yao X, Levine SJ. High-density Lipoproteins and Apolipoprotein A-I: Potential New Players in the Prevention and Treatment of Lung Disease. Front Pharmacol. 2016;7:323.
Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280(23):21763–72.
Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9(2):213–9.
Dai C, Yao X, Vaisman B, Brenner T, Meyer KS, Gao M, Keeran KJ, Nugent GZ, Qu X, Yu ZX, et al. ATP-binding cassette transporter 1 attenuates ovalbumin-induced neutrophilic airway inflammation. Am J Respir Cell Mol Biol. 2014;51(5):626–36.
Park SW, Lee EH, Lee EJ, Kim HJ, Bae DJ, Han S, Kim D, Jang AS, Uh ST, Kim YH, et al. Apolipoprotein A1 potentiates lipoxin A4 synthesis and recovery of allergen-induced disrupted tight junctions in the airway epithelium. Clin Exp Allergy British (British Society for Allergy and Clinical Immunology). 2013;43(8):914–27.
Kerr ID, Haider AJ, Gelissen IC. The ABCG family of membrane-associated transporters: you don’t have to be big to be mighty. Br J Pharmacol. 2011;164(7):1767–79.
Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thomb Vasc Biol. 2006;26(3):534–40.
Kennedy MA, Barrera GC, Nakamura K, Baldán Á, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1(2):121–31.
Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, et al. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thomb Vasc Biol. 2008;28(2):258–64.
Tarling EJ, Edwards PA. Intracellular Localization of Endogenous Mouse ABCG1 Is Mimicked by Both ABCG1-L550 and ABCG1-P550-Brief Report. Arterioscler Thomb Vasc Biol. 2016;36(7):1323–7.
Draper DW, Gowdy KM, Madenspacher JH, Wilson RH, Whitehead GS, Nakano H, Pandiri AR, Foley JF, Remaley AT, Cook DN, et al. ATP binding cassette transporter G1 deletion induces IL-17-dependent dysregulation of pulmonary adaptive immunity. J Immunol. 2012;188(11):5327–36.
Baldan A, Gonen A, Choung C, Que X, Marquart TJ, Hernandez I, Bjorkhem I, Ford DA, Witztum JL, Tarling EJ. ABCG1 is required for pulmonary B-1 B cell and natural antibody homeostasis. J Immunol. 2014;193(11):5637–48.
Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180(6):4273–82.
Draper DW, Madenspacher JH, Dixon D, King DH, Remaley AT, Fessler MB. ATP-binding cassette transporter G1 deficiency dysregulates host defense in the lung. Am J Respir Crit Care Med. 2010;182(3):404–12.
Mukhopadhyay S, Hoidal JR, Mukherjee TK. Role of TNFα in pulmonary pathophysiology. Respir Res. 2006;7(1):1–9.
Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32(4):311–8.
Mattos W, Lim S, Russell R, Jatakanon A, Chung KF, Barnes PJ. Matrix metalloproteinase-9 expression in asthma: effect of asthma severity, allergen challenge, and inhaled corticosteroids. Chest. 2002;122(5):1543–52.
Bloemen K, Verstraelen S, Van Den Heuvel R, Witters H, Nelissen I, Schoeters G. The allergic cascade: Review of the most important molecules in the asthmatic lung. Immunol Let. 2007;113(1):6–18.
Zijlstra GJ, Ten Hacken NH, Hoffmann RF, van Oosterhout AJ, Heijink IH. Interleukin-17A induces glucocorticoid insensitivity in human bronchial epithelial cells. Eur Respir J. 2012;39(2):439–45.
Ben-Dov I, Segel MJ. Autoimmune pulmonary alveolar proteinosis: Clinical course and diagnostic criteria. Autoimmun Rev. 2014;13(4–5):513–7.
Malur A, Huizar I, Wells G, Barna BP, Malur AG, Thomassen MJ. Lentivirus-ABCG1 instillation reduces lipid accumulation and improves lung compliance in GM-CSF knock-out mice. Biochem Biophys Res Commun. 2011;415(2):288–93.
Baker AD, Malur A, Barna BP, Kavuru MS, Malur AG, Thomassen MJ. PPARgamma regulates the expression of cholesterol metabolism genes in alveolar macrophages. Biochem Biophys Res Commun. 2010;393(4):682–7.
Yoshida I, Ban N, Inagaki N. Expression of ABCA3, a causative gene for fatal surfactant deficiency, is up-regulated by glucocorticoids in lung alveolar type II cells. Biochem Biophys Res Commun. 2004;323(2):547–55.
Cheong N, Zhang H, Madesh M, Zhao M, Yu K, Dodia C, Fisher AB, Savani RC, Shuman H. ABCA3 is critical for lamellar body biogenesis in vivo. J Biol Chem. 2007;282(33):23811–7.
Zarbock R, Kaltenborn E, Frixel S, Wittmann T, Liebisch G, Schmitz G, Griese M. ABCA3 protects alveolar epithelial cells against free cholesterol induced cell death. Biochim Biophys Acta. 2015;1851(7):987–95.
Ban N, Matsumura Y, Sakai H, Takanezawa Y, Sasaki M, Arai H, Inagaki N. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem. 2007;282(13):9628–34.
Weichert N, Kaltenborn E, Hector A, Woischnik M, Schams A, Holzinger A, Kern S, Griese M. Some ABCA3 mutations elevate ER stress and initiate apoptosis of lung epithelial cells. Respir Res. 2011;12(1):1–12.
Paolini A, Baldassarre A, Del Gaudio I, Masotti A. Structural Features of the ATP-Binding Cassette (ABC) Transporter ABCA3. Int J Mol Sci. 2015;16(8):19631–44.
Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27(4):337–49.
Bolt RJ, van Weissenbruch MM, Lafeber HN, Delemarre van de Waal HA. Glucocorticoids and lung development in the fetus and preterm infant. Pediatr Pulmonol. 2001;32(1):76–91.
C-z Z, X-c F, Wang D, Tang F-d, Wang X-d. Involvement of type II pneumocytes in the pathogenesis of chronic obstructive pulmonary disease. Respir Med. 2010;104(10):1391–5.
Beers MF, Knudsen L, Tomer Y, Maronn J, Zhao M, Ochs M, Mulugeta S. Aberrant lung remodeling in a mouse model of surfactant dysregulation induced by modulation of the ABCA3 gene. Ann Anat. 2016. doi:10.1016/j.aanat.2016.11.015 (ePub ahead of print).
Bækvad-Hansen M, Nordestgaard BG, Dahl M. Heterozygosity for E292V in ABCA3, lung function and COPD in 64,000 individuals. Respir Res. 2012;13(1):1–9.
Besnard V, Matsuzaki Y, Clark J, Xu Y, Wert SE, Ikegami M, Stahlman MT, Weaver TE, Hunt AN, Postle AD, et al. Conditional deletion of Abca3 in alveolar type II cells alters surfactant homeostasis in newborn and adult mice. Am J Physiol Lung Cell Mol Physiol. 2010;298(5):L646–659.
Haczku A. Protective role of the lung collectins surfactant protein A and surfactant protein D in airway inflammation. J Allergy Clin Immunol. 2008;122(5):861–79.
Wang J-Y, Reid KBM. The immunoregulatory roles of lung surfactant collectins SP-A, and SP-D, in allergen-induced airway inflammation. Immunobiology. 2007;212(4–5):417–25.
Chiba H, Piboonpocanun S, Mitsuzawa H, Kuronuma K, Murphy RC, Voelker DR. Pulmonary surfactant proteins and lipids as modulators of inflammation and innate immunity. Respirology. 2006;11(Suppl):S2–6.
Kim DY, Ryu SY, Lim JE, Lee YS, Ro JY. Anti-inflammatory mechanism of simvastatin in mouse allergic asthma model. Eur J Pharmacol. 2007;557(1):76–86.
Lawes CM, Thornley S, Young R, Hopkins R, Marshall R, Chan WC, Jackson G. Statin use in COPD patients is associated with a reduction in mortality: a national cohort study. Prim Care Respir J. 2012;21(1):35–40.
Zeki AA, Oldham J, Wilson M, Fortenko O, Goyal V, Last M, Last A, Patel A, Last JA, Kenyon NJ. Statin use and asthma control in patients with severe asthma. BMJ Open. 2013;3:e003314.
Mroz RM, Lisowski P, Tycinska A, Bierla J, Trzeciak PZ, Minarowski L, Milewski R, Lisowska A, Boros P, Sobkowicz B, et al. Anti-inflammatory effects of atorvastatin treatment in chronic obstructive pulmonary disease. A controlled pilot study. J Physiol Pharmacol. 2015;66(1):111–28.
Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J Allergy Clin Immunol. 2007;119(2):328–35.
Criner GJ, Connett JE, Aaron SD, Albert RK, Bailey WC, Casaburi R, Cooper JADJ, Curtis JL, Dransfield MT, Han MK, et al. Simvastatin for the Prevention of Exacerbations in Moderate-to-Severe COPD. N Engl J Med. 2014;370(23):2201–10.
Silva D, Couto M, Delgado L, Moreira A. A systematic review of statin efficacy in asthma. J Asthma. 2012;49(9):885–94.
Yuan C, Zhou L, Cheng J, Zhang J, Teng Y, Huang M, Adcock IM, Barnes PJ, Yao X. Statins as potential therapeutic drug for asthma? Respir Res. 2012;13:108.
Wong J, Quinn CM, Brown AJ. Statins inhibit synthesis of an oxysterol ligand for the liver x receptor in human macrophages with consequences for cholesterol flux. Arterioscler Thromb Vasc Res. 2004;24(12):2365–71.
Wong J, Quinn CM, Gelissen IC, Jessup W, Brown AJ. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis. 2008;196(1):180–9.
Quazi F, Molday RS. Lipid transport by mammalian ABC proteins. Essays Biochem. 2011;50(1):265–90.
Kobayashi A, Takanezawa Y, Hirata T, Shimizu Y, Misasa K, Kioka N, Arai H, Ueda K, Matsuo M. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J Lipid Res. 2006;47(8):1791–802.
Acknowledgements
None.
Funding
ICG and AJA received a Compact Seed funding Grant from the Faculty of Pharmacy, the University of Sydney, Australia.
Availability of data and materials
Not applicable as no data were generated or analysed.
Authors’ contributions
ABC drafted the article and the figure. AJA and ICG both edited the final article while ICG prepared the paper for submission. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chai, A.B., Ammit, A.J. & Gelissen, I.C. Examining the role of ABC lipid transporters in pulmonary lipid homeostasis and inflammation. Respir Res 18, 41 (2017). https://doi.org/10.1186/s12931-017-0526-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-017-0526-9