Abstract
Rationale
The demographics of patients with idiopathic pulmonary arterial hypertension (IPAH) are changing and this diagnosis is increasingly being made in older patients. However, diagnostic misclassifications are common as it may be difficult to differentiate between IPAH and pulmonary hypertension due to heart failure with preserved ejection fraction (PH-HFpEF). We investigated the hypothesis that the capillary pCO2 (pcCO2) may help distinguishing between idiopathic pulmonary arterial hypertension (IPAH) and pulmonary hypertension due to heart failure with preserved ejection fraction (PH-HFpEF).
Methods
In a cross-sectional study, we retrospectively assessed pcCO2 levels (obtained from arterialized capillary blood at the time of diagnosis) from patients with IPAH or PH-HFpEF, respectively. Receiver operated characteristics (ROC) were used to determine the pcCO2 level providing the best discrimination between these two conditions. PcCO2 values were considered helpful if they were associated with a negative predictive value >0.9 to excluded either IPAH or PH-HFpEF.
Results
The study enrolled 185 patients, 99 with IPAH (74% female; age 47 ± 17 years; body mass index 26 ± 5 kg/m2, PAPm 53 ± 12 mmHg, PAWP 8 ± 3 mmHg), and 86 with PH-HFpEF (64% female; age 69 ± 10 years; body mass index 30 ± 6 kg/m2, PAPm 47 ± 10 mmHg, PAWP 21 ± 5 mmHg). PcCO2 at time of diagnosis was 33 ± 4 mmHg in the IPAH group and 40 ± 5 mmHg in the PH-HFpEF group (p < 0.001), respectively. According to ROC analysis, a pcCO2 of 36 mmHg was the best discriminator between both entities with an area under curve of 0.87 (p < 0.001). The likelihood of PH-HFpEF was <10% in patients with a PcCO2 < 34 mmHg, whereas the likelihood of IPAH was <10% in patients with a PcCO2 > 41 mmHg.
Conclusions
PcCO2 levels were significantly lower in IPAH compared to PH-HFpEF and may provide useful information in differentiating between both conditions.
Similar content being viewed by others

Introduction
According to the current classification, pulmonary hypertension (PH) is divided into 5 distinct groups: (i) pulmonary arterial hypertension (PAH), (ii) PH due to left heart disease, (iii) PH due to lung disease and/or hypoxia, (iv) chronic thromboembolic pulmonary hypertension (CTEPH), and (v) PH with unclear multifactorial mechanisms [1]. For most patients with PH, the diagnostic classification is straightforward but in occasional patients, the distinction between some of these conditions may be difficult.
An increasing diagnostic challenge in the work-up of patients with PH is the discrimination between idiopathic PAH (IPAH) and PH due to heart failure with preserved ejection fraction (PH-HFpEF). The current criteria for the distinction between IPAH and PH-HFpEF have limitations [2,3]. By definition, patients with IPAH have pre-capillary PH, i.e. a pulmonary artery wedge pressure (PAWP) or a left ventricular end-diastolic pressure (LVEDP) ≤15 mmHg, whereas patients with PH-HFpEF are characterized by post-capillary PH as defined by a PAWP/LVEDP >15 mmHg [2]. However, the invasive measurements of the left ventricular filling pressures can be misleading, both for technical as well as for physiological reasons [4]. Hence, PAWP/LVEDP measurements may yield values >15 mmHg in patients with PAH and - arguably more common - values ≤15 mmHg in patients with HFpEF, especially if left heart disease is optimally treated [5-7].
Thus, a single PAWP/LVEDP cut-off value is not always sufficient to allow an accurate diagnosis of pre- or post-capillary PH in each individual patient. This distinction, however, is of fundamental practical importance as the treatment of IPAH differs substantially from the treatment of patients with PH-HFpEF [8].
In the past, this problem was less evident as IPAH was originally considered predominantly a disease of younger women, and these patients are usually not at risk for developing HFpEF. More recently, however, IPAH is increasingly diagnosed in older patients, many of whom presenting with risk factors for developing left heart disease [9-11]. In a recently published report United Kingdom Pulmonary Hypertension registry, 13.5% of the patients were diagnosed with IPAH at an age >70 years, and in the European-based COMPERA registry, this proportion was even 50% [9,11]. It is possible that some of these patients were misclassified. Several conditions may mimic PAH and among those, HFpEF is the most common [2]. However all of the older patients in the abovementioned registries had a pulmonary arterial wedge pressure (PAWP) ≤15 mmHg, which – in a strict sense – would exclude a diagnosis of PH-HFpEF [9,11].
Hence measuring PAWP/LVEDP alone is not always sufficient to delineate IPAH from PH-HFpEF, and a comprehensive diagnostic assessment is required in order to ensure an accurate distinction between these two conditions. Risk factors for HFpEF include an older age, obesity, hypertension, diabetes and coronary heart disease [2,3]. The presence of echocardiographic signs of left ventricular diastolic dysfunction including an enlarged left atrium as well as the presence of permanent atrial fibrillation increase the likelihood of HFpEF, but none of these features excludes a diagnosis of IPAH.
It would be useful to have additional non-invasive variables that help distinguishing IPAH from PH-HFpEF. One potential candidate could be capillary pCO2 (pcCO2). PcCO2 can be obtained from arterialized earlobe sampling and accurately reflects arterial pCO2 [12-15]. Hyperventilation at rest and during exercise is a known feature of heart failure and PAH [16-19]. The mechanisms causing hyperventilation in these patients are incompletely understood, but increased physiologic dead space and, probably more importantly, increased chemosensitivity seem to play an important role [17,20]. Capillary pcCO2 tends to be more profoundly reduced in patients with IPAH [16,21] than in patients with PH-HFpEF [20,22]. Hence, we hypothesized that pcCO2 measurements may be helpful to discriminate between both conditions.
Methods
Since April 2012, the Pulmonary Hypertension Clinic at Hannover Medical School has implemented an electronic database capturing all patients treated for PH. We used this database for a cross-sectional analysis of patients with well characterized IPAH or PH-HFpEF, respectively, based on the diagnostic criteria listed below. All variables analyzed and presented in this manuscript were obtained at the time of diagnosis, i.e. the time of the first diagnostic right heart catheterization.
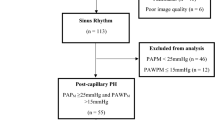
General inclusion criteria for both groups were a body mass index <40 kg/m2, normal or near normal pulmonary function test results including a total lung capacity >80% predicted, a forced expiratory capacity in 1 s >60% predicted, a diffusion capacity for carbon monoxide >40% predicted, the absence or more than mild parenchymal abnormalities on chest computed tomography, and the need for non-invasive ventilation support for sleep-related breathing disorders. CTEPH was ruled out by ventilation-perfusion scintigraphy, and pulmonary angiography if needed, in all patients. All patients underwent right heart catheterization because of suspected severe PH or PAH, respectively, at the time of diagnosis with determination of right atrial pressure, mean pulmonary artery pressure (PAPm), PAWP and mixed venous oxygen saturation (SvO2). The pressure transducer was set at mid-thoracic level for all procedures. Cardiac output was measured by thermodilution; pulmonary vascular resistance (PVR), cardiac index (CI), transpulmonary gradient (TPG) and diastolic pulmonary gradient (DPG) were calculated by standard formula.
Patients with IPAH were included if they fulfilled the following criteria: PAPm ≥25 mmHg, PAWP ≤15 mmHg, PVR >240 dyn · s · cm−5, sinus rhythm at time of diagnosis, left ventricular ejection fraction >60% and normal size of the left atrium on echocardiography. A diagnosis of PH-HFpEF was based on the following criteria: PAPm ≥25 mmHg, PAWP >15 mmHg, left ventricular ejection fraction >50%, normal end-systolic and end-diastolic left ventricular diameters, and signs of diastolic dysfunction including the presence of an enlarged left atrium on echocardiography.
All patients provided written informed consent and the study was approved by the local ethics committee.
Right heart catheterization
Right heart catheterizations were performed via a jugular approach following a standardized protocol. The pressure transducer was zeroed at the mid-thoracic level and all pressure readings were done at end-expiration [23]. Cardiac output was measured by thermodilution technique with the reported value being the average of at least three recordings with less than 10% variation.
Blood gas analyses
Experienced technicians obtained arterialized capillary blood gases from earlobes after a resting period ≥10 min while patients were breathing room air. The blood samples were analyzed without delay using a standard device (Radiometer, Copenhagen).
Statistical analysis
Data are shown as mean ± SD, unless indicated otherwise. For comparison of the two patient populations, Fisher’s exact test, Chi-square test and two-sided paired T-test were used as appropriate. Potential associations between pcCO2 and clinical variables were assessed with Pearson’s correlation analysis and two-sided testing for significance. In order to identify the pcCO2 level with the highest power to discriminate between IPAH and PH-HFpEF, receiver operated characteristics (ROC) curves were drawn and the area under the curve (AUC) was calculated. The cut-off value that resulted in the highest product of sensitivity and specificity was considered the best diagnostic pcCO2 value. Sensitivity, specificity, positive predictive values and negative predictive values were calculated assuming an equal pre-test probability of both conditions. PcCO2 values were arbitrarily considered useful for diagnostic purposes if they were associated with a negative predictive value >0.9 to excluded either IPAH or PH-HFpEF.
Results
The study enrolled 185 patients; 99 with IPAH and 86 with PH-HFpEF. The patient characteristics are shown in Table 1. Compared to patients with PH-HFpEF, patients with IPAH were younger, had a lower body mass index and a lower likelihood of diabetes, while exercise capacity was less compromised. On right heart catheterization, patients with IPAH had higher values of PAPm and PVR whereas cardiac output, cardiac index, and right atrial pressures were lower. The arterial oxygen tension (paO2) was mildly reduced in both groups.
PcCO2 at time of diagnosis was 33 ± 4 mmHg in the IPAH group and 40 ± 5 mmHg in the PH-HFpEF group (p < 0.001), respectively (Figure 1). According to ROC analysis, a pcCO2 of 36 mmHg was the best discriminator between both entities with an area under curve of 0.868 (95% confidence interval, 0.816 – 0.920; p < 0.001; Figure 2). The lower pcCO2, the higher was the likelihood of IPAH and vice versa (Figure 3). PcCO2 values between 34 and 41 mmHg had limited discriminatory power, but PcCO2 values outside these margins provide valuable information. Assuming equal pre-test probability for each diagnosis, any PcCO2 < 34 mmHg excluded the presence of PH-HFpEF with a likelihood of >90%, whereas the likelihood of IPAH was <10% in patients with any PcCO2 > 41 mmHg (Figure 3). PcCO2 values >41 mmHg were found in 14% and PcCO2 levels <34 mmHg in 35% of the patients in this study, respectively; thus PcCO2 measurements provided relevant diagnostic information in 49% of the patients in this series.
Correlations between pcCO2 and clinical variables
In patients in with IPAH, pcCO2 correlated with BMI and inversely with PVR. In the PH-HFpEF group, pcCO2 correlated with age at diagnosis and cardiac output. Even if statistically significant, all these correlations were weak (Table 2).
Discussion
The present data confirm clinical observations that pcCO2 values tend to be lower in patients with IPAH compared to patients with PH-HFpEF. The average pcCO2 in patients with PH-HFpEF was 40 mmHg, i.e. in the normal range. In contrast, the average pcCO2 in patients with IPAH was 33 mmHg, i.e. markedly reduced compared to normal values. A pcCO2 of 36 mmHg was the best cut-off for discriminating between IPAH and PH-HFpEF. According to ROC analysis, the AUC was 0.868 for this value, suggesting that pcCO2 may be helpful in distinguishing between both conditions. The lower the pcCO2, the lower the likelihood of PH-HFpEF and vice versa.
The physiological explanation for the low pcCO2 in IPAH is not entirely clear. A previous study on patients with IPAH also found a low pcCO2 at the time of diagnosis [16]. The median pcCO2 in that study was 32 mmHg, i.e. very similar to the average value of 33 mmHg in the present IPAH population. In the previous study, there was a significant, albeit weak, correlation between pcCO2 and cardiac output [16], which was not found in the present IPAH population.
The patients with PH-HFpEF enrolled in the present series had rather severe PH with a PAPm of 47 ± 10 mmHg. The average transpulmonary gradient was 25 ± 10 mmHg, the diastolic gradient 10 ± 8 mmHg, and the PVR 471 ± 218 dyn · s · cm−5, indicating that the majority of these patients had a combined pre- and post-capillary form of PH [2,24]. This is a population of patients that may be easily misclassified as IPAH, and it may be particularly such patients in whom pcCO2 measurements may provide valuable information.
Our study has several strength and limitations. Strengths include the relatively large sample size of well-characterized patients, all of whom had undergone a rigorous diagnostic assessment including right heart catheterization at the time of diagnosis. Limitations include the single center design, the lack of a validation cohort and the fact that our study did not further elucidate the mechanisms causing hypocarbia in patients with IPAH. In addition, our HFpEF population was unique in that most of these patients suffered from severe PH, presumably owing to a referral bias as these patients were referred to our center for evaluation of PH, and not of HFpEF.
The fact that we recorded all pressure readings at end-expiration is in line with current recommendations [23,25]. Several experts have pointed out that this approach may result in an overestimation of these pressures, most importantly the PAWP [26,27]. However, we excluded patients with lung disease so that these differences should have been marginal in our patients. The fact that the mean PAWP in our HFpEF population was 21 mmHg compared to 8 mmHg in our IPAH population, is reassuring. Finally, our results may not be applicable to patients with additional confounders, which may affect pcCO2, such as morbid obesity or underlying lung disease.
Conclusion
Our data show that pcCO2 is significantly lower in patients with IPAH compared to patients with PH-HFpEF and may help distinguishing between both conditions. Further studies are needed to determine the value of pcCO2 in the diagnostic work-up of patients with PH.
Abbreviations
- DZL:
-
German Center of Lung Research
- IPAH:
-
Idiopathic pulmonary arterial hypertension
- PH-HFpEF:
-
Pulmonary hypertension due to heart failure with preserved ejection fraction
- pcCO2 :
-
Capillary pCO2
- ROC:
-
Receiver operated characteristics
- PAPm:
-
Mean pulmonary arterial pressure
- PAWP:
-
Pulmonary arterial wedge pressure
- PH:
-
Pulmonary hypertension
- PAH:
-
Pulmonary arterial hypertension
- CTEPH:
-
Chronic thromboembolic pulmonary hypertension
- LVEDP:
-
Left ventricular enddiastolic pressure
- SvO2 :
-
Mixed venous oxygen saturation
- PVR:
-
Pulmonary vascular resistance
- CI:
-
Cardiac index
- TPG:
-
Transpulmonary gradient
- DPG:
-
Diastolic pulmonary gradient
- AUC:
-
Area under the curve
- paO2 :
-
Arterial oxygen tension
References
Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41.
Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–8.
Hoeper MM, Barbera JA, Channick RN, Hassoun PM, Lang IM, Manes A, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol. 2009;54:S85–96.
Halpern SD, Taichman DB. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest. 2009;136:37–43.
Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66.
Frost AE, Farber HW, Barst RJ, Miller DP, Elliott CG, McGoon MD. Demographics and outcomes of patients diagnosed with pulmonary hypertension with pulmonary capillary wedge pressures 16 to 18 mm Hg: insights from the REVEAL Registry. Chest. 2013;143:185–95.
Steimle AE, Stevenson LW, Chelimsky-Fallick C, Fonarow GC, Hamilton MA, Moriguchi JD, et al. Sustained hemodynamic efficacy of therapy tailored to reduce filling pressures in survivors with advanced heart failure. Circulation. 1997;96:1165–72.
Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. The task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Respir J. 2009;34:1219–63.
Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–6.
Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest. 2011;139:128–37.
Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168:871–80.
Vaquer S, Masip J, Gili G, Goma G, Oliva JC, Frechette A, et al. Earlobe arterialized capillary blood gas analysis in the intensive care unit: a pilot study. Ann Intensive Care. 2014;4:11.
Eaton T, Rudkin S, Garrett JE. The clinical utility of arterialized earlobe capillary blood in the assessment of patients for long-term oxygen therapy. Respir Med. 2001;95:655–60.
Zavorsky GS, Cao J, Mayo NE, Gabbay R, Murias JM. Arterial versus capillary blood gases: a meta-analysis. Respir Physiol Neurobiol. 2007;155:268–79.
Harrison AM, Lynch JM, Dean JM, Witte MK. Comparison of simultaneously obtained arterial and capillary blood gases in pediatric intensive care unit patients. Crit Care Med. 1997;25:1904–8.
Hoeper MM, Pletz MW, Golpon H, Welte T. Prognostic value of blood gas analyses in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2007;29:944–50.
Naeije R, van de Borne P. Clinical relevance of autonomic nervous system disturbances in pulmonary arterial hypertension. Eur Respir J. 2009;34:792–4.
Chua TP, Ponikowski P, Harrington D, Anker SD, Webb-Peploe K, Clark AL, et al. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1997;29:1585–90.
Ponikowski P, Francis DP, Piepoli MF, Davies LC, Chua TP, Davos CH, et al. Enhanced ventilatory response to exercise in patients with chronic heart failure and preserved exercise tolerance: marker of abnormal cardiorespiratory reflex control and predictor of poor prognosis. Circulation. 2001;103:967–72.
Melot C, Naeije R. Pulmonary vascular diseases. Compr Physiol. 2011;1:593–619.
Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216–23.
Wasserman K, Zhang YY, Gitt A, Belardinelli R, Koike A, Lubarsky L, et al. Lung function and exercise gas exchange in chronic heart failure. Circulation. 1997;96:2221–7.
Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–50.
Naeije R, Vachiery JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J. 2013;41:217–23.
Kovacs G, Avian A, Olschewski A, Olschewski H. Zero reference level for right heart catheterization. Eur Respir J. 2013;42:1586–94.
Kovacs G, Avian A, Pienn M, Naeije R, Olschewski H. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med. 2014;190:252–7.
LeVarge BL, Pomerantsev E, Channick RN. Reliance on end-expiratory wedge pressure leads to misclassification of pulmonary hypertension. Eur Respir J. 2014;44:425–34.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Dr. Olsson: The author has received speaker fees from Actelion, Bayer, Pfizer and GSK.
Dr. Hoeper: The author has received speaker fees and honoraria for consultations from Actelion, Bayer, GSK, and Pfizer.
The other authors report no conflict of interest associated with the content of this paper.
Authors’ contributions
KMO and MMH contributed to data acquisition, analysis and interpretation, manuscript drafting and critical review for intellectual content and final approval of the manuscript. KMO and MMH had full access to all of the data in the study and take responsibility for the integrity of the data analysis. LS contributed to data acquisition and critical review for intellectual content and final approval of the manuscript. JF contributed to data analysis and critical review for intellectual content and final approval of the manuscript. TW contributed to data acquisition and critical review for intellectual content and final approval of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Olsson, K.M., Sommer, L., Fuge, J. et al. Capillary pCO2 helps distinguishing idiopathic pulmonary arterial hypertension from pulmonary hypertension due to heart failure with preserved ejection fraction. Respir Res 16, 34 (2015). https://doi.org/10.1186/s12931-015-0194-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-015-0194-6