Abstract
Background
Cerebral ischaemia‒reperfusion (I/R) frequently causes late-onset neuronal damage. Breviscapine promotes autophagy in microvascular endothelial cells in I/R and can inhibit oxidative damage and apoptosis. However, the mediation mechanism of breviscapine on neuronal cell death is unclear.
Methods
First, transcriptome sequencing was performed on three groups of mice: the neuronal normal group (Control group), the oxygen-glucose deprivation/ reoxygenation group (OGD/R group) and the breviscapine administration group (Therapy group). Differentially expressed genes (DEGs) between the OGD/R and control groups and between the Therapy and OGD/R groups were obtained by the limma package. N6-methyladenosine (m6A) methylation-related DEGs were selected by Pearson correlation analysis. Then, prediction and confirmation of drug targets were performed by Swiss Target Prediction and UniProt Knowledgebase (UniProtKB) database, and key genes were obtained by Pearson correlation analysis between m6A-related DEGs and drug target genes. Next, gene set enrichment analysis (GSEA) and Ingenuity pathway analysis (IPA) were used to obtain the pathways of key genes. Finally, a circRNA-miRNA‒mRNA network was constructed based on the mRNAs, circRNAs and miRNAs.
Results
A total of 2250 DEGs between the OGD/R and control groups and 757 DEGs between the Therapy and OGD/R groups were selected by differential analysis. A total of 7 m6A-related DEGs, including Arl4d, Gm10653, Gm1113, Kcns3, Olfml2a, Stk26 and Tfcp2l1, were obtained by Pearson correlation analysis. Four key genes (Tfcp2l1, Kcns3, Olfml2a and Arl4d) were acquired, and GSEA showed that these key genes significantly participated in DNA repair, e2f targets and the g2m checkpoint. IPA revealed that Tfcp2l1 played a significant role in human embryonic stem cell pluripotency. The circRNA-miRNA‒mRNA network showed that mmu_circ_0001258 regulated Tfcp2l1 by mmu-miR-301b-3p.
Conclusions
In conclusion, four key genes, Tfcp2l1, Kcns3, Olfml2a and Arl4d, significantly associated with the treatment of OGD/R by breviscapine were identified, which provides a theoretical basis for clinical trials.
Similar content being viewed by others
Background
Stroke is the leading cause of death in humans [1, 2]. Moreover, ischaemic stroke accounts for 60-80% of all brain strokes, with high morbidity, mortality and long-term severe disability [3]. Cerebral ischaemia‒reperfusion (I/R) injury is the major pathophysiological process that causes delayed neuronal injury [4]. The clinical treatment strategies are far from expected, with the secondary injury mechanism being complex [5] and still a huge health problem worldwide [6]. An increasing number of studies have shown that I/R injury activates a variety of cell death programs, such as acute necrosis, apoptosis, and autophagy [7], which lead to cognitive and memory dysfunction [8]. Novel and effective therapeutic targets require further exploration. However, N6-methyladenosine (m6A) is regarded as the most common and key regulator of mRNA modification [9] and affects the wide expression of genes in different pathophysiological processes by “writer” methyltransferases, “eraser” demethylases and “reader” proteins in eukaryotes [10]. Recent studies have demonstrated that m6A is involved in the occurrence and development of stroke, epilepsy and schizophrenia [11], but the relevant regulatory mechanism has not been fully elucidated.
Breviscapine is a traditional Chinese herbal medicine containing ≥ 90% scutellarin and ≤ 10% apigenin-7-O-glucronide that has been widely used to treat cerebrovascular disease in the past three decades [12] due to its functions of dilating cerebral vessels, reducing cerebral vascular resistance, increasing cerebral blood flow, improving microcirculation, decreasing blood viscosity and inhibiting platelet conglomeration [13,14,15]. In addition, breviscapine administration can promote autophagy of microvascular endothelial cells, exert antioxidative damage effects and attenuate neuronal cell apoptosis after I/R injury, which improves neurobehavioural functions [16]. However, the specific mechanism of breviscapine in I/R injury still needs to be further explored.
In this article, we aim to investigate the key genes modulated by breviscapine against cerebral I/R injury from the perspective of m6A methylation. According to the self-sequencing data from mice, four key m6A methylation-related genes (Tfcp2l1, Kcns3, Olfml2a and Arl4d) were identified, and these key genes have been considered to be significantly associated with the treatment of I/R injury by breviscapine, which provides a theoretical basis for clinical trials and a novel breakthrough point in the treatment of I/R.
Methods
Animals and cell culture
The experimental animals were SPF grade C57BL/6 mouse aged 1–3 days old, both male and female, provided by the Animal Experimental Center of Kunming Medical University (SYXK(Dian)K2020-0006), the number of qulitative qualification is SCK(Dian)K2020-0004. Neonatal mouse were placed in an anaesthetic induction box containing 3–4% isoflurane, which was inhaled for 3 min. All mouse were eventually sacrificed, and after separating the meninges, bilateral cerebral cortex was obtained. All experimental procedures were approved by the Animal Experimentation Ethics Review Committee of Kunming Medical University(approve number kmmu20221507). Primary cortical neuron culture was established by digesting cerebral cortex with 0.25% trypsin [17] and was divided into a control group, an OGD/R group, and a breviscapine administration group. The neurons were incubated with glucose-free RPMI 1640 medium and placed in an anaerobic chamber under an atmosphere of 95% N2 and 5% CO2 at 37 °C for 3 h. Then, the normal medium was replaced, and the cells were returned to normoxic conditions for reperfusion at 37 °C for 24 h, indicating that the OGD/R model was successfully established [18]. The breviscapine administration group was pretreated with 50µM breviscapine [19] before OGD and during reperfusion. Breviscapine were provided by the Kunming Longjin Pharmaceutical Co., Ltd ,Yunnan, China. With the content of 50 mg and the batch number is Z53020666 .
RNA extraction and library construction sequencing
Total RNA was isolated and purified by TRIzol reagent following the instruction manual. The RNA amount and purity were quantified by a NanoDrop ND-1000. Agarose gel electrophoresis was used to verify the integrity of RNA (concentrations > 50 ng/µL, RIN values > 7.0, OD260/280 > 1.8, and total RNA > 1 µg satisfy downstream experiments). Oligo (dT) magnetic beads were used for specific capture of mRNA with polyA (polyadenylation) in them, and the captured mRNA was fragmented under high temperature conditions. Then, the fragmented RNA was reverse transcribed into cDNA. E. coli DNA polymerase I with RNase H was used for two-strand synthesis. These compound doublets of DNA and RNA were converted into DNA doublets, dUTP solution was incorporated into the doublets at the same time, and the ends of the double-stranded DNA were complemented to flat ends. An additional base was added to each end to enable ligation with a linker with a T base at the end, and its fragment size was screened and purified using magnetic beads. The second strand was digested with UDG enzyme, and then PCR was performed to form a library with a fragment size of 300 bp ± 50 bp. Finally, we used Illumina NovaSeq™ 6000 to perform double-end sequencing according to standard practice.
Data sources
In this study, transcriptome sequencing was performed on three groups of mice: the neuronal normal group (Control group), the cerebral ischaemia and reperfusion group (OGD/R group) and the breviscapine administration group (Therapy group), with 7 mice in each group. Seven pairs of mice were sequenced for mRNA transcriptome analysis, and 3 pairs were sequenced for whole transcriptome analysis. Twenty-one m6A regulatory factors were obtained from published literature [20].
The acquisition of differentially expressed genes (DEGs)
The mRNA transcriptome data and whole transcriptome sequencing data were used for differential analysis. The limma package (version 3.48.3) [21] was used to obtain the differentially expressed genes (DEGs) between the OGD/R and control groups and between the Therapy and OGD/R groups. Then, the upregulated DEGs between the OGD/R group and control group and the downregulated DEGs between the Therapy group and OGD/R group were intersected to obtain DEGs. The ggplot2 package (version 3.3.5) [22] and the pheatmap package (version 1.0.12) were used to plot the volcano plots and heatmaps, respectively.
Construction of a protein‒protein interaction (PPI) network
According to the expression of 21 m6A regulatory factors, the Wilcoxon test was used to compare the m6A between the OGD/R and control groups and between the Therapy and OGD/R groups. The box plot was drawn using the ggpubr package for visualization. Then, in the OGD/R and control groups and the Therapy and OGD/R groups, the correlation between differentially expressed m6A regulatory factors and the DEGs was calculated by Pearson correlation coefficient (|cor| > 0.5 and p.value < 0.01). Then, the m6A-related DEGs between the OGD/R and control groups crossed with the m6A-related DEGs between the Therapy and OGD/R groups. Finally, the PPI network of m6A regulatory factors and m6A-related DEGs was constructed.
Functional enrichment analysis and drug target prediction
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) functional enrichment analyses of m6A-related DEGs were performed by clusterprofiler (version 4.0.2) [23] (p.adjust < 0.05 and count ≥ 1). The Swiss Target Prediction database (http://www.swisstargetprediction.ch/) and UniProt Knowledgebase (UniProtKB) database (http://www.uniprot.org) were used to predict the target compounds and target genes of breviscapine. Then, the target compound and target gene regulatory network was constructed by Cytoscape.
Identification and gene set enrichment analysis (GSEA) of key genes
The correlation between the m6A-related DEGs and drug target genes was performed by Pearson correlation coefficient, and the significant drug target genes were selected as key genes (|cor| > 0.7, p value < 0.01). Then, the PPI network was constructed by the drug target genes and key genes. The ClusterProfiler (version 4.0.2) package [23] and org.Hs.eg.db package (version 3.13.0) were used to explore the related pathways and molecular mechanisms of key genes.
Ingenuity pathway analysis (IPA) and construction of a regulatory network
IPA (www.ingenuity.com) was performed to acquire the signalling pathways of key genes. Then, we used IPA to analyse the upstream regulatory factors and downstream target genes of key genes, and we found the regulatory relationship of key genes. Transcription factors (TFs) in mice were downloaded from the AnimalTFDB database (http://bioinfo.life.hust.edu.cn/AnimalTFDB#!/), and the expression levels of TFs were obtained from the self-sequencing dataset. The Psych package was used to calculate the Pearson correlation coefficient between TFs and key genes. The TFs were selected to construct the TF-mRNA network (|cor| ≥ 0.8 and P ≤ 0.01).
Construction of a ceRNA network
First, differentially expressed lncRNAs (DELs) between the OGD/R and control groups and between the Therapy and OGD/R groups were obtained by the limma package (version 3.48.3) (|logFC| ≥ 0.5, p value < 0.05) in 7 pairs of mRNA transcriptomes and 3 pairs of whole transcriptome samples. Then, the downregulated DELs between the OGD/R and control groups and upregulated DELs between the Therapy and OGD/R groups were intersected to obtain DELs. The limma package (version 3.48.3) was used to obtain the differentially expressed circRNAs (DECs) between the OGD/R and control groups and between the Therapy and OGD/R groups in the 3 pairs of whole transcriptome samples. Then, DECs were obtained with the intersection of downregulated DECs in the OGD/R and control groups and upregulated DECs in the Therapy and I/R groups. Heatmaps and volcano plots were plotted by the pheatmap (version 1.0.12) and ggplot2 (version 3.3.5) [22] packages, respectively. Next, the target binding miRNAs of key genes and the miRNAs of intersecting DECs were predicted based on the ENCORI database (https://starbase.sysu.edu.cn/). The circRNA-miRNA‒mRNA regulatory network was constructed by the common miRNA of circRNA and mRNA, and Cytoscape was used to visualize the network. In addition, the target genes of codifferentially expressed lncRNAs were predicted by the coexpression relationship, and the correlation between codifferentially expressed lncRNAs and key genes was calculated using Pearson correlation analysis. Finally, coexpressed lncRNAs and mRNAs were selected to construct the lncRNA‒mRNA regulatory network (|cor| ≥ 0.7 and p value ≤ 0.01), and the key gene regulation-pathway network was created based on the predicted ceRNA networks and lncRNAs of key genes.
Results
Analysis of differential expression
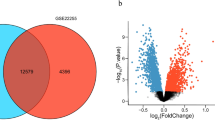
A total of 2250 DEGs between the OGD/R and control groups were selected, including 1089 upregulated and 1461 downregulated DEGs (Fig. 1A, B). There were 757 DEGs between the Therapy and OGD/R groups (314 upregulated and 443 downregulated) (Fig. 1C, D). Fifty-seven upregulated DEGs and 34 downregulated DEGs were obtained between the OGD/R and control groups (Fig. 1E, F).
Differential expression analysis. (A, B) Differentially expressed genes (DEGs) between the OGD/R and control groups in a volcano plot (A) and heatmap (B). (C, D) DEGs between the Therapy and OGD/R groups in the volcano plot (C) and heatmap (D). (E-F) Venn diagram identifying overlapping DEGs between different groups
Screening of m6A regulatory factor-related genes
Nine m6A regulatory factors had significant differences (ELAVL1, HNRNPA2B1, HNRNPC, IGF2BP1, RBM15, RBM15B, WTAP, YTHDC2, and YTHDF2) between the OGD/R and control groups. One m6A regulatory factor (ALKBH5) was remarkably different between the Therapy and OGD/R groups (Fig. 2A). Forty-nine m6A-related DEGs between the OGD/R and control groups and 9 m6A-related DEGs between the Therapy and OGD/R groups were obtained by Pearson analysis. Seven m6A-related DEGs were obtained after intersection, including Arl4d, Gm10653, Gm1113, Kcns3, Olfml2a, Stk26, and Tfcp2l1 (Fig. 2B). There were 7 m6A-related DEGs connected with 8 m6A regulatory factors (Fig. 2C).
Identification of m6A regulatory factor-related genes. (A) Expression patterns of m6A regulatory factors in the OGD/R and control groups (top) and the Therapy and OGD/R groups (bottom). (B) Venn diagram identifying overlapping m6A-related DEGs. (C) Protein‒protein interaction (PPI) diagram of m6A- and m6A-related differentially expressed genes
Functional enrichment of m6A-related DEGs and breviscapine drug target prediction
There were 9 biological processes (BPs) of GO enriched for 7 m6A-related DEGs, such as cytoplasm organization, epithelial cell maturation and microvillus assembly (Fig. 3A). Eighty-six target compounds and 176 target genes of breviscapine were obtained by the Swiss Target Prediction and UniProtKB databases, and CHEMBL1075275 of the target compounds regulated 8 genes, including Aurkb, Aik2, Aim1, Airk2, Ark2, Stk1, Stk12 and Stk5 (Fig. 3B).
Acquisition of key genes and gene set enrichment analysis (GSEA)
Four key genes, Tfcp2l1, Kcns3, Olfml2a and Arl4d, were obtained by correlation analysis (Supplementary Table 1). It is worth noting that 15 drug target genes (Pik3cg, Pygl, Akt1, Cdk1, Tyms, Casp1, Aurkb, Adora2a, Dhfr, Dnmt1, Drd2, Eif4e, Gart, Hdac1 and Mme) were connected with 4 key genes in the PPI network, and CHEMBL2687 regulated Olfml2a by Rnasel in the key gene‒target gene-compound network (Fig. 4A). The expression of the 4 key genes in the OGD/R groups was the lowest among the three groups (Fig. 4B). In the GO functional enrichment, Tfcp2l1 and Olfml2a were mainly enriched in ribosome biogenesis, rRNA processing and ribosomal subunit; Kcns3 participated in cellular response to biotic stimulus, DNA replication and ribosomal subunit; and Arl4d was involved in cytoplasmic translation, ribosome biogenesis and ribosomal subunit. Hallmark enrichment showed that the 4 key genes including Tfcp2l1, Kcns3, Olfml2a and Arl4d, mainly participated in DNA repair, e2f targets and the g2m checkpoint (Fig. 4C F).
IPA enrichment and construction of the TF-mRNA regulatory network
Tfcp2l1 was significantly enriched in human embryonic stem cell pluripotency (Fig. 5A). In Tfcp2l1 regulatory networks, Tfcp2l1 was regulated by POU5F1, and ESRRB and TFCP2 were directed to regulate Tfcp2l1. Arl4d was regulated by PPARG in the Arl4d regulatory network (Fig. 5B). A total of 1482 TFs were obtained from the self-sequencing dataset. A total of 109 TFs were selected by correlation analysis, and a TF-mRNA regulatory network was constructed by the TFs (Fig. 5C, Supplementary Table 2).
Construction of circRNA-miRNA‒mRNA and lncRNA‒mRNA networks
There were 667 DELs between the OGD/R and control groups, including 299 upregulated and 368 downregulated lncRNAs (Fig. 6A). A total of 295 DELs were obtained between the Therapy and OGD/R groups (142 upregulated and 153 downregulated) (Fig. 6B). Sixteen DELs were obtained by the intersection of downregulated DELs between the OGD/R and control groups and upregulated DELs between the Therapy and I/R groups (Fig. 6C, Supplementary Table 3). There were 238 DECs obtained between the OGD/R and control groups, of which 154 DECs were upregulated and 84 DECs were downregulated (Fig. 6D). In the Therapy and OGD/R groups, 189 DECs were obtained, including 145 upregulated and 44 downregulated DECs (Fig. 6E). Eighteen DECs were obtained by taking the intersection of downregulated DECs between the OGD/R and control groups and upregulated DECs between the Therapy and OGD/R groups (Fig. 6F, Supplementary Table 4). The regulatory network of circRNA-miRNA‒mRNA was constructed based on 2 circRNAs, 15 miRNAs and 1 mRNA. The mmu_circ_0001258 circRNA regulated Tfcp2l1 by 11 miRNAs, including mmu-miR-493-3p, mmu-miR-301b-3p, mmu-miR-301a-3p, mmu-miR-130c, mmu-miR-6389, mmu-miR-291b-3p, mmu-miR-350-5p, mmu-miR-130a-3p, mmu-miR-721, mmu-miR-130b-3p and mmu-miR-6341 (Fig. 6G). Four key genes, Tfcp2l1, Kcns3, Olfml2a and Arl4d, were regulated by 5 lncRNAs (Gm33408, Gm52552, Gm13110, Gm32335, and Gm31113) (Fig. 6H). Only Tfcp2l1 was co-regulated by miRNA and lncRNA (Fig. 6I).
Construction of circRNA-miRNA‒mRNA and lncRNA‒mRNA networks. (A, B) Differential expression analysis of lncRNAs between the model and control samples (A) and the Therapy and OGD/R groups (B). (C) Venn diagram of differential lncRNAs. (D, E) Differential expression of circRNAs between the model and control groups (D) and the Therapy and OGD/R groups (E). (F) Venn diagram of differential circRNAs. (G) The circRNA-miRNA‒mRNA network of key genes. (H) The lncRNA‒mRNA network of key genes. (I) The regulatory-pathway network of key genes
Discussion
Although rapid restoration of blood supply is the best treatment for ischaemic stroke, secondary brain tissue damage inevitably emerges in the reperfusion territory [24, 25]. Growing evidence shows that hypoxia, oxidative stress, and the inflammatory response are common neurological events in ischaemic stroke [26, 27]. The dysregulation of m6A modification is closely associated with cancer and cerebrovascular diseases [28, 29]. However, posttranscriptional m6A modification can influence the expression of key proteins and could provide new insight into treating cerebral I/R injury [30, 31]. Breviscapine administration for ischaemic stroke is effective and reliable [12] and improves neurological function and reduces the cerebral infarction area based on neuroprotective and anti-coagulation effects [32]. Moreover, accumulating evidence has proven that breviscapine can suppress proinflammatory cytokine (IL-6, IL-1β, and TNF-α) expression, decrease reactive oxygen species (ROS) levels, and attenuate massive nerve cell death and brain tissue destruction after cerebral I/R [33]. However, the role of m6A with breviscapine in cerebral I/R injury has not been studied. In this article, we identified four m6A methylation-related genes (Tfcp2l1, Kcns3, Olfml2a and Arl4d) significantly associated with the treatment of OGD/R by breviscapine, which plays a foundation for further exploring the mechanism of breviscapine neuronal protective effects for cerebral I/R.
However, studies have shown that Tfcp2l1 is a downstream target of the canonical Wnt/β-catenin, STAT3 and FGF/MEK signalling pathways and plays a pivotal role in promoting embryonic stem cell self-renewal [34, 35]. In addition, the lower Kcns3 levels provide a new molecular mechanism of neuron dysfunction in schizophrenia, which encodes potassium channel-associated subunits [36]. Olfml2a is a key regulatory protein acting downstream of AP-1 [37]. Knockdown of Olfml2a in glioma cells could promote apoptosis and inhibit the Wnt/β-catenin signalling pathway [38]. Studies have shown that Arl4d is involved in vesicle trafficking, organelle structure, cytoskeleton organization, modulation of cell migration [39] and microtubule growth [40]. In conclusion, the molecular functions and regulatory mechanisms of four key genes (Tfcp2l1, Kcns3, Olfml2a, Arl4d) have not been reported in cerebral I/R injury, and further research is needed.
Research has also shown that DNA hypomethylation can promote learning and memory recovery in cerebral I/R rat models [41]. Our results demonstrated that Tfcp2l1, Kcns3, Olfml2a and Arl4d were significantly downregulated in cerebral OGD/R models and the control group. Additionally, upregulation with breviscapine administration most likely plays a protective role against cerebral I/R and thus provides novel treatment insights into cerebral I/R injury. However, the mechanism is poorly understood, and no reports have focused on this topic.
A study showed that ischaemia preconditioning, which resulted in an overall decrease in global DNA methylation in neuronal cultures, may improve tolerance to cell death [42]. The levels of m6A modification were significantly increased after neuronal OGD/R and rat MCAO treatment, which regulated neuronal synaptic plasticity, axonal growth, apoptosis, learning and memory, and stress responses induced by I/R [43]. In recent years, m6A-modified lncRNAs have received extensive attention. m6A modification of RNA plays an important role in pathophysiological processes in the central nervous system, in which lncRNAs are key biomarkers in cerebral ischaemic disease [44], and the m6A modification status in lncRNAs may control the biological functions of lncRNAs [45]. Noncoding RNAs are considered novel therapeutic targets for ischaemic stroke [46, 47]. However, five lncRNAs (Gm33408, Gm52552, Gm13110, Gm32335 and Gm31113) and two circRNAs (mmu_circ_0001258 and mmu_circ_0001558) were identified, and their specific functions and roles have not been reported, which may be a new entry point for cerebral I/R injury.
Owing to the limitations of current experiments, elucidating the dynamic regulatory mechanisms of individual m6A sites and their molecular functions remains challenging [48]. We analysed the sequencing results of primary neurons by bioinformatics methods and showed that four key genes were significantly decreased in the model group but significantly increased in the treatment group. We speculate that breviscapine can regulate the levels of m6A methylation during cerebral I/R, which may be a potential new mechanism for treating cerebral I/R injury. These genes were also analysed by ceRNA network, lncRNA‒mRNA coexpression network and regulation-pathway, which showed that key genes play a critical role in the regulation of m6A with cerebral ischaemia reperfusion, especially in DNA repair, e2f targets and g2m checkpoint. Of course, several limitations in our study should be noted, such as the sample size being relatively small. Further molecular experimental research and clinical applications are needed, and we will continue to pay more attention to the role of these genes.
There is no evidence of direct interaction among the four key genes, but the regulation-pathway network of the key genes suggests that the four key genes may interact with each other indirectly through the action of IncRNAs (e.g., Gm33408, Gm13110, Gm31113, and Gm52552), but the specific regulatory mechanism still needs to be further investigated. The database was also not searched for data related to the expression of the four genes after ischaemic stroke. Additionally, the expression of the four key genes was decreased after neuronal OGD/R and upregulated after the use of breviscapine, which may be involved in neuronal damage and repair after cerebral I/R injury and is also a sensitive biomarker for evaluating the efficacy of breviscapine. The results of the present study may be helpful for clinical trials and provide new perspectives for the treatment of ischaemic stroke.
Conclusion
In summary, this study demonstrated that breviscapine could regulate the gene expression levels of m6A in neuronal I/R injury. This is the first study to identify these genes, which may play an important role in cerebral I/R injury. Additionally, prediction of the drug target of breviscapine might provide novel potential therapeutic targets for cerebral ischaemia stroke.
Data Availability
The datasets generated and/or analysed during the current study are available in the [ArrayExpress] repository, [Persistent web link: https://www.ebi.ac.uk/biostudies/arrayexpress/studies/E-MTAB-13017?key=0d170feb-c91b-4582-b4c8-8200f01c4b43].
Abbreviations
- I/R:
-
Ischaemia‒reperfusion
- DEGs:
-
Differentially expressed genes
- m6A:
-
N6-methyladenosine
- UniProtKB:
-
UniProt Knowledgebase
- GSEA:
-
Gene set enrichment analysis
- IPA:
-
Ingenuity pathway analysis
- OGD:
-
Oxygen-glucose deprivation
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- TF:
-
Transcription factors
- DELs:
-
Differentially expressed lncRNAs
- DECs:
-
Differentially expressed circRNAs
References
Chen X, Zhang J, Song Y, Yang P, Yang Y, Huang Z, Wang K. Deficiency of anti-inflammatory cytokine IL-4 leads to neural hyperexcitability and aggravates cerebral ischemia-reperfusion injury. Acta Pharm Sin B. 2020;10(9):1634–45.
Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, Wang L, Jiang Y, Li Y, Wang Y, Chen Z, Wu S, Zhang Y, Wang D, Wang Y, Feigin VL. NESS-China investigators. Prevalence, incidence, and mortality of stroke in China: results from a Nationwide Population-Based survey of 480 687 adults. Circulation. 2017;135(8):759–71.
Lee M, Saver JL, Liao HW, Lin CH, Ovbiagele B. Pioglitazone for secondary stroke prevention: a systematic review and meta-analysis.Stroke. 2017; 48(2): 388–93.
Han JY, Li Q, Ma ZZ, Fan JY. Effects and mechanisms of compound chinese medicine and major ingredients on microcirculatory dysfunction and organ injury induced by ischemia/reperfusion. PHARMACOL THERAPEUT. 2017;177:146–73.
Chen HD, Jiang MZ, Zhao YY, Li X, Lan H, Yang WQ, Lai Y. Effects of breviscapine on cerebral ischemia-reperfusion injury and intestinal flora imbalance by regulating the TLR4/MyD88/NF-κB signaling pathway in rats. J Ethnopharmacol. 2023;300:115691.
Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Liang S, Venketasubramanian N, Rathakrishnan R, Ahmad A, Ng KW, Loh PK, Ong JJ, Wakerley BR, Chong VF, Bathla G, Sharma VK. Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol. 2013;70(3):353–8.
Fan J, Liu Y, Yin J, Li Q, Li Y, Gu J, Cai W, Yin G. Oxygen-glucos-deprivation/ reoxygenation-induced autophagic cell death depends on JNK-mediated phosphorylation of Bcl-2. Cell Physiol Biochem. 2016;38(3):1063–74.
Sener G, Sakarcan A, Yegen BC. Role of garlic in the prevention of ischemia- reperfusion injury. Mol Nutr Food Res. 2007;51(11):1345–52.
Song H, Feng X, Zhang H, Luo Y, Huang J, Lin M, Jin J, Ding X, Wu S, Huang H, Yu T, Zhang M, Hong H, Yao S, Zhao Y, Zhang Z. METTL3 and ALKBH5 oppositely regulate m6A modification of TFEB mRNA, which dictates the fate of hypoxia/reoxygenation -treated cardiomyocytes. Autophagy. 2019;15(8):1419–37.
Yi D, Wang Q, Zhao Y, Song Y, You H, Wang J, Liu R, Shi Z, Chen X, Luo Q. Alteration of N6-Methyladenosine mRNA methylation in a rat model of cerebral ischemia-reperfusion Injury. Front Neurosci. 2021;15:605654.
Magwai T, Shangase KB, Oginga FO, Chiliza B, Mpofana T, Xulu KR. DNA methylation and Schizophrenia: current literature and future perspective. Cells. 2021;10(11):2890.
Wen L, He T, Yu A, Sun S, Li X, Wei J, Song R, Yan X, Li R, Ren X, Wang Y, Liu X, Dong Y, Fu X, She G. Breviscapine: a review on its Phytochemistry, Pharmacokinetics and Therapeutic Effects. Am J Chin Med. 2021;49(6):1369–97.
Yang X, Zheng S, Wang X, Wang J, Ali Shah SB, Wang Y, Gao R, Xu Z. Advances in pharmacology, biosynthesis, and metabolic engineering of Scutellaria-specialized metabolites. Crit Rev Biotechnol. 2022;29:1–17.
Lin LL, Liu AJ, Liu JG, Yu XH, Qin LP, Su DF. Protective effects of scutellarin and breviscapine on brain and heart ischemia in rats. J Cardiovasc Pharmacol. 2007;50(3):327–32.
Shang YH, Tian JF, Hou M, Xu XY. Progress on the protective effect of compounds from natural medicines on cerebral ischemia. Chin J Nat Med. 2013;11(6):588–95.
Pengyue Z, Tao G, Hongyun H, Liqiang Y, Yihao D. Breviscapine confers a neuroprotective efficacy against transient focal cerebral ischemia by attenuating neuronal and astrocytic autophagy in the penumbra. Biomed Pharmacother. 2017;90:69–76.
Wang CP, Li GC, Shi YW, Zhang XC, Li JL, Wang ZW, Ding F, Liang XM. Neuroprotective effect of schizandrin A on oxygen and glucose deprivation/ reperfusion-induced cell injury in primary culture of rat cortical neurons. J Physiol Biochem. 2014;70(3):735–47.
Si W, Li Y, Ye S, Li Z, Liu Y, Kuang W, Chen D, Zhu M. Methyltransferase 3 mediated miRNA m6A methylation promotes stress granule formation in the early stage of Acute ischemic stroke. Front Mol Neurosci. 2020;13:103.
Lu L, Yang LK, Yue J, Wang XS, Qi JY, Yang F, Feng B, Liu SB. Scutellarin alleviates depression-like behaviors induced by LPS in mice partially through inhibition of astrocyte-mediated neuroinflammation. NEUROSCI LETT. 2021;11–20:765136284.
Zhang B, Wu Q, Li B, Wang D, Wang L, Zhou YL. m6A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer. 2020;19(1):53.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47.
Ito K, Murphy D. Application of ggplot2 to Pharmacometric Graphics. CPT Pharmacometrics Syst Pharmacol. 2013;2(10):e79.
Wu T, Hu E, Xu S, Chen M, Guo P, Dai Z, Feng T, Zhou L, Tang W, Zhan L, Fu X, Liu S, Bo X, Yu G. Cluster profiler 4.0: a universal enrichment tool for interpreting omics data. Innov (Camb). 2021;2(3):100141.
Han Y, Rajah GB, Hussain M, Geng X. Clinical potential of pre- reperfusion hypothermia in ischemic injury. Neurol Res. 2019;41(8):697–703.
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/ reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317.
Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54(1):34–66.
Yu L, Zhang Y, Chen Q, He Y, Zhou H, Wan H, Yang J. Formononetin protects against inflammation associated with cerebral ischemia-reperfusion injury in rats by targeting the JAK2/STAT3 signaling pathway. Biomed Pharmacother. 2022;149:112836.
Li Y, Xiao J, Bai J, Tian Y, Qu Y, Chen X, Wang Q, Li X, Zhang Y, Xu J. Molecular characterization and clinical relevance of m6A regulators across 33 cancer types. Mol Cancer. 2019;18(1):137.
Shafik AM, Allen EG, Jin P. Dynamic N6-methyladenosine RNA methylation in brain and diseases. Epigenomics. 2020;12(4):371–80.
Engel M, Eggert C, Kaplick PM, Eder M, Röh S, Tietze L, Namendorf C, Arloth J, Weber P, Rex-Haffner M, Geula S, Jakovcevski M, Hanna JH, Leshkowitz D, Uhr M, Wotjak CT, Schmidt MV, Deussing JM, Binder EB, Chen A. The role of m6A/m-RNA methylation in stress response regulation. Neuron. 2018;99(2):389–403.
Zhu R, Tian D, Zhao Y, Zhang C, Liu X. Genome-wide detection of m6A-Associated genetic polymorphisms Associated with ischemic stroke. J Mol Neurosci. 2021;71(10):2107–15.
Li F, Wang X, Zhang Z, Gao P, Zhang X. Breviscapine provides a neuroprotective effect after traumatic brain injury by modulating the Nrf2 signaling pathway. J Cell Biochem. 2019;120(9):14899–907.
Li Y, Li S, Li D. Breviscapine alleviates Cognitive Impairments Induced by transient cerebral Ischemia/Reperfusion through its anti-inflammatory and anti-oxidant Properties in a rat model. ACS Chem Neurosci. 2020;11(24):4489–98.
Kraunsoe S, Azami T, Pei Y, Martello G, Jones K, Boroviak T, Nichols J. Requirement for STAT3 and its target, TFCP2L1, in self-renewal of naïve pluripotent stem cells in vivo and in vitro. Biol Open. 2023;12(1):bio059650.
Qiu D, Ye S, Ruiz B, Zhou X, Liu D, Zhang Q, Ying QL. Klf2 and Tfcp2l1, two Wnt/β-Catenin targets, Act synergistically to induce and maintain naive pluripotency. Stem Cell Reports. 2015;5(3):314–22.
Georgiev D, Arion D, Enwright JF, Kikuchi M, Minabe Y, Corradi JP, Lewis DA, Hashimoto T. Lower gene expression for KCNS3 potassium channel subunit in parvalbumin-containing neurons in the prefrontal cortex in schizophrenia. Am J Psychiatry. 2014;171(1):62–71.
Zhao Q, Zhang K, Li Y, Ren Y, Shi J, Gu Y, Qiu S, Liu S, Cheng Y, Qiao Y, Liu Y. OLFML2A is necessary for anti-triple negative breast cancer effect of selective activator protein-1 inhibitor T-5224. Transl Oncol. 2021;14(8):101100.
Ma S, Duan L, Dong H, Ma X, Guo X, Liu J, Li G, Yu Y, Xu Y, Yuan G, Zhao X, Tian G, Zhai S, Pan Y, Zhang Y. OLFML2A downregulation inhibits glioma proliferation through suppression of Wnt/β-Catenin signaling. Front Oncol. 2021;11:717917.
Chen KJ, Chiang TC, Yu CJ, Lee FS. Cooperative recruitment of Arl4A and Pak1 to the plasma membrane contributes to sustained Pak1 activation for cell migration. J Cell Sci. 2020;133(3):jcs233361.
Lin SJ, Huang CF, Wu TS, Li CC, Lee FS. Arl4D-EB1 interaction promotes centrosomal recruitment of EB1 and microtubule growth. Mol Biol Cell. 2020;31(21):2348–62.
Shi G, Feng J, Jian LY, Fan XY. DNA hypomethylation promotes learning and memory recovery in a rat model of cerebral ischemia/reperfusion injury. Neural Regen Res. 2023;18(4):863–8.
Meller R, Pearson A, Simon RP. Dynamic changes in DNA methylation in ischemic tolerance. Front Neurol. 2015;6:102.
Widagdo J, Anggono V. The m6A-epitranscriptomic signature in neurobiology: from neurodevelopment to brain plasticity. J NEUROCHEM. 2018;147(2):137–52.
Xu K, Mo Y, Li D, Yu Q, Wang L, Lin F, Kong C, Balelang MF, Zhang A, Chen S, Dai Q, Wang J. N6-methyladenosine demethylases Alkbh5/Fto regulate cerebral ischemia-reperfusion injury. Ther Adv Chronic Dis. 2020;11:2040622320916024.
Lan Y, Liu B, Guo H. The role of m6A modification in the regulation of tumor-related lncRNAs. Mol Ther Nucleic Acids. 2021;24:768–79.
Ma W, Zhu K, Yin L, Yang J, Zhang J, Wu H, Liu K, Li C, Liu W, Guo J, Li L. Effects of ischemic postconditioning and long non-coding RNAs in ischemic stroke. Bioengineered. 2022;13(6):14799–814.
Sufianova G, Shumadalova A, Wenhao Y, Gareev I. Long non-coding RNAs as biomarkers and therapeutic targets for ischemic stroke. Noncoding RNA Res. 2022;7(4):226–32.
Yao W, Han X, Ge M, Chen C, Xiao X, Li H, Hei Z. N6-methyladenosine (m6A) methylation in ischemia-reperfusion injury. Cell Death Dis. 2020;11(6):478.
Acknowledgements
Not applicable.
Funding
This work was supported in part by grants from Yunnan Provincial Clinical Medical Research Center for Radiation and Treatment [Grant Number: 202102AA100067] , Yunnan Provincial Clinical Medical Center for Neurological Diseases [Grant Number: ZX2019030501] and from Yunnan Provincial Department of Science and Technology - Kunming Medical University Applied Basic Research Joint Fund [Grant Number:202301AY070001-008].
Author information
Authors and Affiliations
Contributions
ZT and WZ contributed to the study conception and design, data analysis and the manuscript text were performed by CW, DW and JH, Cell culture and model building were completed by JP, All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The experimental animals were SPF grade C57BL/6 mouse aged 1–3 days old, both male and female, provided by the Animal Experimental Center of Kunming Medical University (SYXK(Dian)K2020-0006), the number of qulitative qualification is SCK(Dian)K2020-0004. All experimental procedures were approved by the Animal Experimentation Ethics Review Committee of Kunming Medical University(approve number kmmu20221507). All methods were carried out in accordance with relevant guidelines and regulations, and all methods are reported in accordance with ARRIVE guidelines for the reporting of animal experiments.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wan, C., Pei, J., Wang, D. et al. Identification of m6A methylation-related genes in cerebral ischaemia‒reperfusion of Breviscapus therapy based on bioinformatics methods. BMC Med Genomics 16, 210 (2023). https://doi.org/10.1186/s12920-023-01651-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-023-01651-3