Abstract
Background
Colorectal cancer is common among obese individuals. The purpose of the current study was to determine changes in DNA methylation status and mRNA expression of thyroid hormone receptor beta (THRB), as a tumor suppressor, and thyroid hormone inactivating enzyme, type 3 deiodinase (DIO3) genes, in human epithelial colon tissues of healthy obese individuals.
Methods
Colon biopsies were analyzed by methylation sensitive-high resolution melting (MS-HRM) to investigate promoter methylation of DIO3 and THRB, and by quantitative real-time polymerase chain reaction to assay expression of DIO3 and THRB mRNA on eighteen obese and twenty-one normal-weight healthy men.
Results
There was no significant difference in mean methylation levels at the THRB promoter region between the two groups. Nevertheless, obesity decreased THRB expression levels, significantly (P < 0.05; fold change: 0.19). Furthermore, obesity attenuated DNA methylation (P < 0.001) and enhanced mRNA expression of DIO3 (P < 0.05; fold change: 3).
Conclusions
Our findings suggest that obesity may alter expression of THRB and DIO3 genes through epigenetic mechanism. Alterations of THRB and DIO3 expressions may predispose colon epithelium of obese patients to neoplastic transformation.
Similar content being viewed by others
Background
Obesity is associated with an increased risk of some types of cancer including, colorectal, breast, endometrial, esophageal, pancreatic, and kidney [1]. Despite a recognized role of obesity in increasing oncogenesis, the mechanisms by which obesity regulates tumor initiation and growth are not yet clear. Among the most important proposed mechanisms linking obesity and cancer are obesity-induced low-grade inflammation and obesity-associated dysfunction of insulin/IGF1 signaling [2]. According to a study conducted by Homayounfar et al. (2015), p53 tumor suppressor, as a main guardian of cells against neoplastic transformation, is upregulated in intestine and some other tissues of obese rats [3]. Therefore, predisposition of obese patients to neoplastic alterations might result from the loss of other tumor preventive signaling through genetic or epigenetic inactivation.
The link between thyroid hormones (THs) and cancer was proposed more than a decade ago. Most epidemiological evidence shows that hypothyroidism prevents tumor initiation and growth, however some other studies provide opposite results [4]. THs exert their effects through genomic and non-genomic pathways. Genomic actions of THs take place through thyroid hormone receptors (TRs) including TRα and TRβ and their various isoforms [5]. Non-genomic actions of THs are exerted via signaling originated from membrane receptors, integrin avβ3, or through interaction with cytoplasmic kinases, such as phosphoinositide 3-kinase (PI3K). Plasma membrane integrin avβ3 is upregulated in cancer cells and promotes its intracellular signaling through PI3K and mitogen-activated protein kinase (MAPK)/ERK1/2 pathways [6,7,8].
According to several lines of evidence, pro-cancerous effects of THs are exerted via either TRα or integrin avβ3 by promoting angiogenesis, proliferation, apoptosis suppression, evasion from immune surveillance, and metabolic reprogramming [9]. Convincing evidence suggests that TRβ is a tumor suppressor in several types of cancer, including thyroid, breast, colorectal and other solid tumors [10]. Downregulation of TRβ is the characteristic of many cancers [10]. TRβ1 is able to suppress proliferation and migration in human colorectal cancer cells, and has been proposed to be a target for treating CRC [11].
Expression of type 3 deiodinase, as an inactivating thyroid hormone enzyme, is negligible in adult tissues; however, its re-expression was shown in some pathological conditions such as tissue regeneration after injury, inflammation and cancers such as breast, endometrium carcinoma, basal cell carcinoma, and colon [12]. In most colon tumors, β-catenin/T-cell factor (TCF) complex is activated. Increased DIO3 levels, as a direct downstream target of the Wnt/β-catenin pathway, reduce intracellular T3 and E-cadherin, promote cellular proliferation, and prevent differentiation [13].
Our present research studied the alterations of THRB and DIO3 genes at epigenetic and mRNA expression levels in colonic epithelium specimens of obese individuals. This can provide evidence for obesity-related changes in THs metabolism that predispose colon tissues to cancer development.
Methods
Participants
The participants in this case–control study were consecutively selected from Modarres hospital and Masoud clinic (18 obese and 21 normal-weight men), between December 2018 and April 2019 in Tehran, Iran. They were enrolled from the ambulatory endoscopy clinic of the hospital and colonoscopy unit of the clinic. Inclusion criteria were: individuals between the ages of 25 and 60, BMI ≥ 30 kg/m2 for obese men as case and BMI between 18 and 25 kg/m2 for normal-weight men as control, with no acute or chronic illness and taking no medication. Individuals with a personal history of colonic neoplasia, colitis, polyposis, previous colon resection, and CRC or different types of cancer were excluded. DNA methylation and mRNA expression analyses were conducted on all participants.
All men were on a low-fiber diet for 3 days before the colonoscopy. On the day of colonoscopy, they were fasted. Standard optical colonoscopy was carried out under the direct supervision of attending gastroenterologists. All colonic biopsies were taken from mid-rectum by using jumbo pinch forceps. Finally, the biopsies were rapidly snap-frozen in liquid nitrogen, transported to laboratory, and stored at − 80 °C.
Weight and height of participants were measured with standard protocols, and body mass index (BMI) was calculated as weight in kilograms divided by height in square meter. Waist: hip ratio (WHR), waist circumference (WC), and hip circumference were also measured using a non-stretchable tape. General information was also recorded from the participants. This study was conducted according to the criteria set by the declaration of Helsinki [14], and written informed consent forms were obtained from all participants before recruitment.
DNA extraction and sodium bisulfite treatment
DNA was extracted from the colon tissue samples using the animal DNA Mini-Preps Kit (Bio Basic Inc.), according to the manufacturer’s protocol. Extracted DNA samples were treated by sodium bisulfite conversion, using an EZ DNA Methylation Gold kit (Zymo Research). The treated DNA samples were rapidly stored at − 80 °C.
Methylation sensitive-high resolution melting
MS-HRM was used to assess the methylation status of DIO3 and THRB genes. Primers for MS-HRM assay were designed according to HRM primer design principles. PCR was carried out in a 20 μL total volume containing 1 µL bisulfite modified template, 4 µL of 5 × Hot FIREPOL Eva Green HRM Mix-Rox Kit (Solis BioDyne), 14 µL double-distilled water, and 6 pmol/µL of forward and reverse primers (1 µL). DNA melting with high resolution was a three-stage process. The first stage included initial denaturation at 95 °C for 15 min. The second stage consisted of three steps in 40 cycles: 95 °C for 15 s, appropriate annealing temperature for each primer set (Table 1) for 20 s, 72 °C for 30 s (extension). The third stage (melting curve continuous stage) was performed as follows: 95 °C for 15 s, 60 °C for 1 min, followed by HRM step ramping from 60 to 95 °C, rising at 0.3 °C per second (StepOnePlus, Applied Biosystems, Paisley, UK).
Human methylated and unmethylated DNA sets from Zymo Research were used as 100% methylated and 0% unmethylated controls. Series of standard dilutions of methylated DNA were prepared. For THRB gene, methylation percentages of 0, 1, 5, 10, 25, 50, and 100% were used to draw the standard curve, while standards of 0, 50, 60, 70, 90, and 100% were used for DIO3. Melting curves were normalized relative to two normalization regions before and after a major decrease in fluorescence. This indicated the melting region of the PCR product using the HRM version 2.2 software (ThermoFisher Scientific).
RNA extraction, cDNA synthesis, and quantitative real-time polymerase chain reaction
Total RNA was isolated from colon tissues using RNX-Plus solution according to the manufacturer's protocol (Cinaclone, Iran). Total RNA (1 μg) was used for cDNA synthesis (Cinaclone, Iran), according to the manufacturer’s instructions. The polymerase chain reaction (PCR) for THRB, DIO3, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (as an internal control) was performed in duplicate by 10 μL BIOFACT™ 2X real-time PCR master mix (for SYBR Green I; High Rox, BIOFACT, South Korea), 7 μL double-distilled water, 0.5 μL forward primer (10 pmol/µL), 0.5 μL reverse primer (10 pmol/µL), and 2 μL cDNA in a final volume of 20 μL. After an initial denaturation step of 15 min at 95 °C, 50 cycles of amplification were carried out. Each two step cycle included a denaturation step, 15 s at 95 °C and an annealing step, 25 s at 55 °C. The melt curve was between 60 and 95 °C (StepOnePlus; Real-Time PCR, Applied Biosciences, Paisley, UK). The gene expression values were calculated as the fold change defined by 2−ΔΔct. The applied primers are listed in Table 2.
Statistical analyses
Statistical tests were performed using SPSS software (version 20), and a two-sided P -value < 0.05 was considered significant. Independent-samples t-test was used to check differences in the distribution of continuous variables between two groups. Chi-square (χ2) test and Fisher's exact or Fisher–Freeman–Halton test were applied for categorical variables. Regression analyses were performed to assess the relationship between variables and to control confounders. As suggested elsewhere [15] confounders with univariate regression P value < 0.2 were included in an adjusted model. Thus, among demographic and lifestyle factors of the study subjects, only those which met the above criteria were considered as confounders.
Accordingly, relationships of DIO3 methylation levels, DIO3 delta Ct, and THRB delta Ct with BMI, weight and central obesity of participants were adjusted for omega 3 fatty acid intake, red meat consumption and smoking, NSAID use, consumption of vitamin D and colon cancer family history, respectively.
Results
Characteristics of cases and controls
The demographic and lifestyle characteristics of participants are demonstrated in Table 3. The mean (SD) age of participants was 42.9 ± 9.5 and 38.4 ± 10.1 years in cases and controls, respectively (P = 0.168). Weight, BMI, WC, hip circumference, and WHR were significantly different between the two groups (P < 0·001). However, there was no significant difference in other characteristics.
Promoter methylation status of THRB
MS-HRM aligned melt curves analysis for THRB controls is shown in Fig. 1a. Figure 1b and c illustrate THRB promoter methylation of normal-weight group compared to obese participants, in colon sample tissues. Results indicated no significant difference in mean methylation levels at THRB promoter region between obese cases (1.9 ± 1.67) and normal weight controls (1.35 ± 1.20) (Fig. 1c).
Graphs and diagram related to MS-HRM analysis of THRB gene promoter methylation. Aligned melt curves for THRB controls (a) and normal-weight compared to obese samples (b). No significant difference in mean ± SD methylation levels at THRB promoter region between normal-weight compared to obese samples was observed (c)
There was no significant relation between methylation levels of THRB and demographic/lifestyle factors of the participants. There were also no significant relations between THRB methylation levels and BMI, weight or central obesity, in both crude and adjusted models.
Promoter methylation status of DIO3
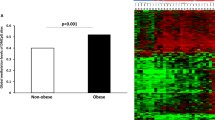
MS-HRM aligned melt curves analysis for DIO3 controls and normal-weight compared to obese samples are presented in Fig. 2a and b. MS-HRM analysis of DIO3 showed that there was a significant difference in mean methylation levels at the DIO3 promoter region between obese cases (70.32 ± 6.16) and normal-weight controls (85.26 ± 14.34) (P < 0.001) (Fig. 2c).
Graphs and diagram related to MS-HRM analysis of DIO3 gene promoter methylation. Aligned melt curves for DIO3 controls (a) and normal-weight compared to obese samples (b). There was a significant difference in mean ± SD methylation levels at DIO3 promoter region between obese and normal-weight samples. c *P < 0.001 between normal-weight and obese subjects
As shown in Table 4, simple linear regression was used to examine the relation between DIO3 methylation levels and demographic/lifestyle factors of the study participants. Among demographic/lifestyle factors only omega-3 fatty acid intake showed a significant direct association with DIO3 methylation levels (P < 0.05).
The relations between DIO3 methylation levels and BMI, weight, and central obesity of cases are demonstrated in Table 5. A significant inverse relationship was observed between BMI and methylation levels of DIO3 in crude (P < 0.001) and adjusted (P < 0.001) analyses. A significant inverse relation was also seen between crude and adjusted analyses of central obesity and methylation levels (P < 0.05). As for weight, the crude model showed a significant inverse relation (P < 0.05) while, the adjusted model showed an approximately significant inverse relation with DIO3 methylation levels (P = 0.057).
Analyses of THRB and DIO3 gene expressions in case and control groups
After analysis of real-time PCR raw data and analysis of expression fold change, our results indicated that the level of THRB gene expression was significantly lower in obese group compared to controls (P < 0.05; fold change: 0.19). The level of DIO3 gene expression in obese cases was significantly higher than in controls (P < 0.05; fold change: 3) (Fig. 3).
ΔCT mean ± SD values of DIO3 and THRB gene expressions between normal-weight and obese participants. There was a significant difference in ΔCT mean values of THRB between obese (9.69 ± 2.85) and normal-weight samples (7.28 ± 3.66). A significant difference in ΔCT mean values of DIO3 between obese (17.12 ± 1.89) and normal-weight samples (18.7 ± 2.35) was observed. Gene expression results were expressed as the fold change defined by 2– ΔΔct. The higher ΔCT values indicate lower gene expression. *P < 0.05 between normal-weight and obese subjects
There was no significant association between delta Ct of THRB and demographic/lifestyle factors. In addition, no significant relation was observed between delta Ct of DIO3 and demographic/lifestyle factors.
The relations of THRB and DIO3 delta Ct with BMI, weight, and central obesity of the study subjects are demonstrated in Table 6. There was a direct significant relation between THRB delta Ct and BMI in the crude analysis (P < 0.05). A significant direct relation was observed between THRB delta Ct and weight in the crude analysis (P < 0.05). Nevertheless, for the adjusted analysis, the relation of THRB delta Ct with BMI and its relation to weight were marginally significant, (P = 0.054 and P = 0.056, respectively). Moreover, THRB delta Ct relation with central obesity was not significant.
A statistically significant inverse relation was observed between DIO3 delta Ct and BMI in the crude model (P < 0.05. A perceivable statistically significant inverse association was seen in the adjusted model (P = 0.05). A significant inverse relation was also observed between DIO3 delta Ct and weight in the crude analysis (P < 0.05). The adjusted model approached near significance (P = 0.061). Moreover, the inverse relation of DIO3 delta Ct with central obesity approached statistical significance in the crude analysis (P = 0.063), and was not significant in the adjusted model.
Discussion
The present study showed that THRB is downregulated in epithelial colon tissues of obese individuals. Moreover, obesity induces hypomethylation of DIO3 promoter and its upregulation, consistently. Obesity has a possible influence on epigenetic landscape that is related to CRC [16]. Moreover, the results of other studies also showed that DNA methylation changes in obesity-related CRC, can be considered as a marker for predicting CRC [17, 18]. A study showed that alterations of DNA methylation and gene expression induced by obesity in the colonic epithelium of a mouse model, promoted pro-proliferative signaling pathways, but, weight loss reversed these changes [19].
A little increase in promoter methylation and low expression of THRB, in obese individuals, may arise from the unequal link between gene expression and methylation. [20]. Based on a study, liver THRB expression negatively correlated with a severe nonalcoholic steatohepatitis in obese individuals who had undergone bariatric surgery [21]. In another study, THRB gene expression was significantly lower in adipose tissues of obese patients compared to normal-weight counterparts [22]. Furthermore, Zhu et al. (2016) revealed that THRB1 gene expression was reduced in CRC tumors compared to normal colorectal mucosal tissues [11]. Even though obesity predisposes individuals to oncogenic events, some studies have reported that p53 and even p16 tumor suppressors became activated in some tissues of obese individuals [23,24,25]. Although the role of THs in cancer is uncertain, several pioneering studies have identified THRB1 as a tumor suppressor gene in different cancers, particularly in CRC [11]. A possible suggested mechanism is that THRB1 in colon cells can stimulate degradation of β-catenin, thus, inactivating Wnt signaling pathway [26]. THRB1 expression can also suppress progression and migration by preventing PI3K/Akt signaling in CRC tissues and cells [11]. An innovative study revealed that TRβ1 was highly expressed in differentiated cells of the surface and upper colon crypt, compared to cells with high stemness located in lower crypt. However, THRA expression showed an opposite manner [27].
Our study showed that obesity attenuated DNA methylation and accordingly enhanced the expression of DIO3. Although there is a lack of research on DIO3 gene methylation and expression in obesity, one research showed that DIO3 mRNA was increased in omental and subcutaneous white adipose tissues of obese men and women patients compared to their lean counterparts [28]. The human oncofetal DIO3 is frequently re-expressed in some conditions, including chronic inflammation, critical illness, starvation and some cancers. Furthermore, signaling pathways linked to stemness such as sonic hedgehog-glioma associated oncogene 2 (Shh-Gli2), tumor growth factor-β (TGF-β), Wnt/β-catenin, and hypoxia-inducible factor-1α (HIF-1α) increase the activity of the DIO3 enzyme [29]. Furthermore, obesity as an inflammatory disease, might trigger inflammation in the colon, as well. It seems that inflammation upregulates Wnt/β-catenin through NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) [30]. DIO3 is a direct β-catenin/TCF complex target [13]. In addition, it has been indicated that local inflammation can greatly enhance DIO3 activation in inflammatory cells, particularly in invading polymorphonuclear granulocytes [31, 32]. Therefore, up and down-regulation of DIO3 and DIO2, by over-activation of the Wnt/β-catenin pathway, in colon carcinomas, are significant contributors to colorectal carcinogenesis [13, 33]. Consequently, based on our findings, high expression of DIO3 gene in obese individuals might be a factor that predisposes tissues to neoplastic transformation.
Mechanisms of resistance to TH (RTH) have been updated. Mutations in TRs are not the only condition that reduces activity and availability of TH in target tissues. Defects in the synthesis of deiodinases may also exist in RTH [34, 35]. Moreover, RTH is connected to etiology of obesity where changes occur in the expression and activity of deiodinases and TRs [36, 37]. Based on our results, altered thyroid hormone bioavailability may have happened in epithelial colon tissues of obese adults.
Among nutritional and dietary factors presented here, only omega-3 fatty acid consumption was directly related to increasing methylation levels of the DIO3 promoter gene. This finding is in agreement with studies that have reported the role of n-3 and n-6 PUFA on DNA methylation status [38]. By considering factors missed in our study, such as physical activity, stress, environmental and metabolic factors, further research is needed to confirm the exact role of omega-3 fatty acid in preventing colorectal cancer.
Conclusions
Obesity dysregulates epigenetics and expression of DIO3, and expression of THRB. It may also alter thyroid hormone bioavailability in human colon tissues. These changes induced by obesity could eventually result in CRC. Since this study provided no evidence to support CRC development in the participants, and current literature regarding clinical significance of TRβ and DIO3 at protein levels is inadequate, more evidence on obese individuals is required for further therapeutic approaches.
Availability of data and materials
The data that support the findings of this study are openly available in 4TU.ResearchData at https://doi.org/10.4121/14789856.v1, reference number [39].
Abbreviations
- CRC:
-
Colorectal cancer
- THRB:
-
Thyroid hormone receptor beta gene
- DIO3:
-
Type 3 deiodinase
- MS-HRM:
-
Methylation sensitive-high resolution melting
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- THs:
-
Thyroid hormones
- TRs:
-
Thyroid hormone receptors
- TRα:
-
Thyroid hormone receptor α protein
- TRβ:
-
Thyroid hormone receptor β protein
- PI3K:
-
Phosphoinositide 3-kinase
- MAPK:
-
Mitogen-activated protein kinase
- TCF:
-
β-Catenin/T-cell factor
- BMI:
-
Body mass index
- WHR:
-
Waist:hip ratio
- WC:
-
Waist circumference
- PCR:
-
Polymerase chain reaction
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- Shh-Gli2:
-
Sonic hedgehog-glioma associated oncogene 2
- TGF-β:
-
Tumor growth factor-β
- HIF-1α:
-
Hypoxia-inducible factor-1α
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- RTH:
-
Resistance to thyroid hormone
References
Control CfD, Prevention. Cancers associated with overweight and obesity make up 40 percent of cancers diagnosed in the United States 2017; 2017.
Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue. Ann N Y Acad Sci. 2012;1271:82–7.
Homayounfar R, Jeddi-Tehrani M, Cheraghpour M, Ghorbani A, Zand H. Relationship of p53 accumulation in peripheral tissues of high-fat diet-induced obese rats with decrease in metabolic and oncogenic signaling of insulin. Gen Comp Endocrinol. 2015;214:134–9.
Krashin E, Piekiełko-Witkowska A, Ellis M, Ashur-Fabian O. Thyroid hormones and cancer: a comprehensive review of preclinical and clinical studies. Front Endocrinol. 2019;10:59.
Tata JR. Looking for the mechanism of action of thyroid hormone. J Thyroid Res. 2011;2011:730630.
Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev Endocrinol. 2016;12(2):111–21.
Hönes GS, Rakov H, Logan J, Liao X-H, Werbenko E, Pollard AS, et al. Noncanonical thyroid hormone signaling mediates cardiometabolic effects in vivo. Proc Natl Acad Sci. 2017;114(52):E11323–32.
Moeller LC, Broecker-Preuss M. Transcriptional regulation by nonclassical action of thyroid hormone. Thyroid Res. 2011;4(Suppl 1):S6.
Deligiorgi MV, Trafalis DT. The intriguing thyroid hormones-lung cancer association as exemplification of the thyroid hormones-cancer association: three decades of evolving research. Int J Mol Sci. 2021;23(1):436.
Davidson CD, Gillis NE, Carr FE. Thyroid hormone receptor beta as tumor suppressor: untapped potential in treatment and diagnostics in solid tumors. Cancers. 2021;13(17):4254.
Zhu L, Tian G, Yang Q, De G, Zhang Z, Wang Y, et al. Thyroid hormone receptor β1 suppresses proliferation and migration by inhibiting PI3K/Akt signaling in human colorectal cancer cells. Oncol Rep. 2016;36(3):1419–26.
Nappi A, De Stefano MA, Dentice M, Salvatore D. Deiodinases and cancer. Endocrinology. 2021;162(4):bqab016.
Dentice M, Luongo C, Ambrosio R, Sibilio A, Casillo A, Iaccarino A, et al. β-Catenin regulates deiodinase levels and thyroid hormone signaling in colon cancer cells. Gastroenterology. 2012;143(4):1037–47.
World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79(4):373.
Altman DG. Practical statistics for medical research. Boca Raton: Taylor & Francis; 1990.
Ayers D, Boughanem H, Macías-González M. Epigenetic influences in the obesity/colorectal cancer axis: a novel theragnostic avenue. J Oncol. 2019;2019:7406078.
Dong L, Ma L, Ma GH, Ren H. Genome-wide analysis reveals DNA methylation alterations in obesity associated with high risk of colorectal cancer. Sci Rep. 2019;9(1):1–11.
Crujeiras AB, Morcillo S, Diaz-Lagares A, Sandoval J, Castellano-Castillo D, Torres E, et al. Identification of an episignature of human colorectal cancer associated with obesity by genome-wide DNA methylation analysis. Int J Obes (2005). 2019;43(1):176–88.
Li R, Grimm SA, Mav D, Gu H, Djukovic D, Shah R, et al. Transcriptome and DNA methylome analysis in a mouse model of diet-induced obesity predicts increased risk of colorectal cancer. Cell Rep. 2018;22(3):624–37.
Wagner JR, Busche S, Ge B, Kwan T, Pastinen T, Blanchette M. The relationship between DNA methylation, genetic and expression inter-individual variation in untransformed human fibroblasts. Genome Biol. 2014;15(2):R37.
Krause C, Grohs M, El Gammal AT, Wolter S, Lehnert H, Mann O, et al. Reduced expression of thyroid hormone receptor β in human nonalcoholic steatohepatitis. Endocr Connect. 2018;7(12):1448–56.
Kurylowicz A, Jonas M, Lisik W, Jonas M, Wicik Z, Wierzbicki Z, et al. Obesity is associated with a decrease in expression but not with the hypermethylation of thermogenesis-related genes in adipose tissues. J Transl Med. 2015;13(1):31.
Zand H, Homayounfar R, Cheraghpour M, Jeddi-Tehrani M, Ghorbani A, Pourvali K, et al. Obesity-induced p53 activation in insulin-dependent and independent tissues is inhibited by beta-adrenergic agonist in diet-induced obese rats. Life Sci. 2016;147:103–9.
Rabhi N, Hannou SA, Gromada X, Salas E, Yao X, Oger F, et al. Cdkn2a deficiency promotes adipose tissue browning. Mol Metab. 2018;8:65–76.
Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15(9):1082–7.
Skah S, Uchuya-Castillo J, Sirakov M, Plateroti M. The thyroid hormone nuclear receptors and the Wnt/β-catenin pathway: an intriguing liaison. Dev Biol. 2017;422(2):71–82.
Hörkkö TT, Tuppurainen K, George SM, Jernvall P, Karttunen TJ, Mäkinen MJ. Thyroid hormone receptor β1 in normal colon and colorectal cancer–association with differentiation, polypoid growth type and K-ras mutations. Int J Cancer. 2006;118(7):1653–9.
Calvo RM, Garcia L, Vesperinas G, Corripio R, Rubio MA, Obregon MJ. Deiodinases in human adipose tissue from obese patients. Differences by Gender and Anatomical Depot. JSM Thyroid Disord Manag. 2017;2(1):1009.
Ciavardelli D, Bellomo M, Crescimanno C, Vella V. Type 3 deiodinase: role in cancer growth, stemness, and metabolism. Front Endocrinol. 2014;5:215.
Sedaghat F, Cheraghpour M, Hosseini SA, Pourvali K, Teimoori-Toolabi L, Mehrtash A, et al. Hypomethylation of NANOG promoter in colonic mucosal cells of obese patients: a possible role of NF-κB. Br J Nutr. 2018;122(5):499–508.
Boelen A, Kwakkel J, Alkemade A, Renckens R, Kaptein E, Kuiper G, et al. Induction of type 3 deiodinase activity in inflammatory cells of mice with chronic local inflammation. Endocrinology. 2005;146(12):5128–34.
Gereben B, Zeöld A, Dentice M, Salvatore D, Bianco AC. Activation and inactivation of thyroid hormone by deiodinases: local action with general consequences. Cell Mol Life Sci. 2007;65(4):570.
Barca-Mayo O, Liao X-H, Alonso M, Di Cosmo C, Hernandez A, Refetoff S, et al. Thyroid hormone receptor α and regulation of type 3 deiodinase. Mol Endocrinol. 2011;25(4):575–83.
Paragliola RM, Corsello A, Concolino P, Ianni F, Papi G, Pontecorvi A, et al. Iodothyronine deiodinases and reduced sensitivity to thyroid hormones. Front Biosci (Landmark Ed). 2020;25:201–28.
Bianco AC, da Conceição RR. The deiodinase trio and thyroid hormone signalling. Thyroid hormone nuclear receptor. Springer; 2018. p. 67–83.
Fontenelle L, Feitosa M, Severo J, Freitas T, Morais J, Torres-Leal F, et al. Thyroid function in human obesity: underlying mechanisms. Horm Metab Res. 2016;48(12):787–94.
Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab. 2010;95(8):3614–7.
Milagro FI, Mansego ML, De Miguel C, Martínez JA. Dietary factors, epigenetic modifications and obesity outcomes: progresses and perspectives. Mol Aspects Med. 2013;34(4):782–812.
Shimi G, Pourvali K, Ghorbani A, Nooshin S, Zare Karizi S, Iranirad R, Zand H. Data underlying the research on methylation level changes of thyroid hormone receptors in human. 4TU.ResearchData.2021. Dataset. https://doi.org/10.4121/14789856.v1.
Acknowledgements
The data were originated from the results of an approved doctoral thesis project of National Nutrition and Food Technology Research Institute, Faculty of Nutrition Science and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We are indebted to Modarres hospital and Masoud clinic for providing us with equipment used in the study, along with gastroenterologists, especially Dr. Shahin Merat for their contributions in performing colonoscopies and taking biopsy specimens. Also, we thank all participants for their patience and collaboration.
Funding
This work was supported by the National Nutrition and Food Technology Research Institute, Tehran, Iran (Project No. 926 and Contract No. 311-3068).
Author information
Authors and Affiliations
Contributions
GS performed the main experiment and wrote the manuscript. KP, AG, S.N, SZK, and RI assisted in all the experiments. HZ designed the study and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee at the National Nutrition and Food Technology Research Institute of Shahid Beheshti University of Medical Sciences approved the study protocol (code of ethics committee: IR.SBMU.NNFTRI.REC.1398.028), and all methods were performed in accordance with the relevant guidelines and regulations. Written informed consent forms were received from all participants before recruitment.
Consent for publication
All specimens employed in this study were obtained with the written informed consent of patients or their legal guardians.
Competing interests
The authors report there are no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shimi, G., Pourvali, K., Ghorbani, A. et al. Alterations of DNA methylation and expression of genes related to thyroid hormone metabolism in colon epithelium of obese patients. BMC Med Genomics 15, 229 (2022). https://doi.org/10.1186/s12920-022-01387-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-022-01387-6