Abstract
Background
In order to explore the pathophysiology underlying type 2 diabetes we examined the impact of gene variants associated with type 2 diabetes on circulating levels of glucagon during an oral glucose tolerance test (OGTT). Furthermore, we performed a genome-wide association study (GWAS) aiming to identify novel genomic loci affecting plasma glucagon levels.
Methods
Plasma levels of glucagon were examined in samples obtained at three time points during an OGTT; 0, 30 and 120 min, in two separate cohorts with a total of up to 1899 individuals. Cross-sectional analyses were performed separately in the two cohorts and the results were combined in a meta-analysis.
Results
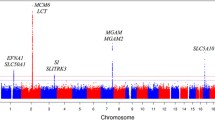
A known type 2 diabetes variant in EYA2 was significantly associated with higher plasma glucagon level at 30 min during the OGTT (Beta 0.145, SE 0.038, P = 1.2 × 10–4) corresponding to a 7.4% increase in plasma glucagon level per effect allele. In the GWAS, we identified a marker in the MARCH1 locus, which was genome-wide significantly associated with reduced suppression of glucagon during the first 30 min of the OGTT (Beta − 0.210, SE 0.037, P = 1.9 × 10–8), equivalent to 8.2% less suppression per effect allele. Nine additional independent markers, not previously associated with type 2 diabetes, showed suggestive associations with reduced glucagon suppression during the first 30 min of the OGTT (P < 1.0 × 10–5).
Conclusions
A type 2 diabetes risk variant in the EYA2 locus was associated with higher plasma glucagon levels at 30 min. Ten additional variants were suggestively associated with reduced glucagon suppression without conferring increased type 2 diabetes risk.
Similar content being viewed by others
Background
Glucagon is a key regulator of hepatic glucose production and is therefore intimately linked to type 2 diabetes pathophysiology. Key regulators of glucagon secretion are insulin and glucose [1]. Patients with type 2 diabetes have disproportionately elevated fasting glucagon levels and also exhibit reduced early glucose-induced suppression of glucagon secretion in response to an oral glucose tolerance test (OGTT) [2]. These alterations in glucagon secretion have been proposed to represent a primary defect but may be secondary to other metabolic defects in type 2 diabetes [2, 3].
Genetic influence on glucagon secretion in response to a glucose challenge has not been widely studied in large populations, mostly due to the strict sample handling protocol necessary to preserve the labile hormone and obtain valid results along with the use of validated assays. The most comprehensive genome-wide association study (GWAS) for glucagon so far was reported by Almgren et al. [4] in 2017. They performed a meta-analysis on circulating levels of glucagon measured at two time points, 0 and 120 min, during an OGTT including 3344 Swedish individuals from Malmö Diet and Cancer study (MDC) and 4905 Finnish individuals from the Prevalence, Prediction and Prevention of diabetes Botnia study (PPP-Botnia). Six suggestively associated loci to glucagon levels were reported, four for fasting levels of glucagon and two for 2 h glucagon levels [4].
Here we investigated the impact of genetic variants on circulating plasma levels of glucagon at three time points, 0, 30 and 120 min, during an OGTT. In order to explore the pathophysiology underlying type 2 diabetes, we examined the impact of known gene variants demonstrated to associate with type 2 diabetes. In addition, we tested three suggestive loci for fasting and 2 h plasma glucagon levels reported previously. Furthermore, we performed a GWAS aiming to identify novel genomic loci affecting glucagon plasma levels.
Methods
Study population
The ADDITION-PRO study (2009–2011) [5] is a continuation of the Danish arm of the ADDITION study (2001–2006) [6], in which individuals with normal glucose tolerance (NGT), impaired fasting glycaemia (IFG), impaired glucose tolerance (IGT) or type 2 diabetes (free from medical treatment) are followed. In total, 2082 participants have undergone an extensive examination, including detailed characterization of glycemic status based on a 3-point OGTT at baseline. Information regarding both glucagon levels and GWAS was available in 1346 individuals. Absolute glucagon levels and the percentage of glucagon suppression during the OGTT in individuals with different glycemic status have been reported previously [7]. Ethical approval was obtained from the Scientific Ethics Committee of the Central Denmark Region (journal no. 20080229), and all participants provided oral and written informed consent before participating in the study.
The Rigshospitalet cohort (RigCoh) [8] is a cohort of 597 adult offspring to mothers with either gestational diabetes, type 1 diabetes or no diabetes during pregnancy. All participants were born at the Department of Obstetrics, Rigshospitalet, Denmark, from 1978 to 1985. Only singletons above the age of 18 years were included, and if more than one sibling from the study period met criteria for inclusion, only the oldest was invited. After an overnight fast, participants without diagnosed diabetes had a standard 75 g OGTT, with venous plasma samples collected at 0, 30 and 120 min. Measurements of plasma glucagon were available from 567 participants of the study [9]. In total, 553 individuals with available data on glucagon levels and GWAS data were included in the analysis. The protocol was in accordance with the Declaration of Helsinki and approved by the local ethical committee (journal no. KF01-061/03). All participants gave a written consent before taking part in the study. Clinical characteristics of the study participants are provided in Table 1.
Measurements
Height and weight were measured as previously described [5] and BMI calculated as weight in kilograms divided by height in meters squared. Blood samples were drawn at 0, 30 and 120 min during a standard 75 g OGTT after an overnight fast of ≥ 8 h for assessment of serum insulin and plasma glucose and glucagon concentrations. Blood samples for measurement of glucagon were drawn into EDTA tubes and were put on ice immediately and frozen. Radioimmunological determination of total plasma glucagon concentration was performed as previously described [10] using an antibody against the C-terminus of glucagon and therefore devoid of cross-reactivity with gut derived proglucagon moieties (glicentin and oxyntomodulin). The analytical detection limit was 1 pmol/l, sensitivity around 1 pmol/l and intra- and inter-assay coefficients of variation were < 6% and < 15%, respectively. The applied methodology was recently evaluated [11]. Glycated haemoglobin A1c (HbA1c) was measured by high-performance liquid chromatography (HPLC) as described previously [5].
The decremental areas under the curves (dAUCs) of plasma glucagon concentrations were calculated using the trapezoid rule, from 0 to 30 min (dAUC 0–30 min) and 0 to 120 min (dAUC 0–120 min) during the OGTT. In a secondary analysis, we calculated the fractional change in plasma glucagon levels from 0 to 30 and from 0 to 120 min by dividing the value at 30 min with that at fasting.
Genotyping
A total of 1899 individuals from the two cohorts were genotyped by the Illumina Infinium HumanCoreExome Beadchip platform (Illumina, San Diego, CA). Genotypes were called using the Genotyping module (version 1.9.4) of GenomeStudio software (version 2011.1, Illumina). Closely related individuals, individuals with an extreme inbreeding coefficients, individuals with mislabeled sex, individuals with a call rate < 95%, duplicates and individuals identified as ethnic outliers, as well as genetic markers with a call rate < 98%, a minor allele frequency < 0.01 and a Hardy–Weinberg equilibrium P < 1 × 10–5, were removed during quality control. Imputation was performed on the Michigan imputation server [12] using haplotypes from the Haplotype Reference Consortium (HRC version r1.1) panel [13].
Statistical analysis
Associations between genetic variants and plasma levels of glucagon were studied using a linear mixed model (EMMAX) implemented in the EPACTS software package [14] by the use of inverse-normalized residuals of trait values adjusted for age, sex and BMI in each cohort separately. Individuals with missing information were excluded from the analysis. The results were subsequently combined in a meta-analysis using METAL (http://csg.sph.umich.edu/abecasis/Metal). P values below 5.0 × 10–8 were considered genome-wide significant, while P values < 1 × 10–5 were considered to be suggestively associated. Since the ADDITION-PRO cohort contains individuals with different glucose tolerance status, we performed the analyses with and without adjustment for glucose tolerance status as well as in individuals with normal glucose tolerance separately. The results of the analyses were similar and adjustment for glucose tolerance did not affect the results. We here present the results with adjustments for age, sex and BMI in both cohorts. We selected 403 markers that have previously been associated with type 2 diabetes [15]. We used a threshold for minor allele frequency (MAF) of < 0.01, which resulted in 367 markers left for analysis. Bonferroni adjustment was used to correct for multiple testing, while analyzing the selected variants, thus P < 1.4 × 10–4 was considered to be significant in those analyses. For the replication analyses of genetic variants previously reported to be suggestively associated with plasma glucagon levels, P values < 0.05 was considered to be nominally significant. The presented P values are uncorrected.
Results
Known type 2 diabetes associated variant associates with increased 30 min glucagon levels
When analyzing the variants previously reported to associate with type 2 diabetes in the paper by Mahajan et al. [15], the variant in the EYA2 locus was associated with higher 30 min plasma glucagon levels during the OGTT, after correction for multiple testing (rs6063048 Beta 0.145, SE 0.038, P = 1.2 × 10–4, see Additional file 1: Table S1). None of the selected variants were significantly associated with either fasting or 120 min glucagon levels.
Nominal association of previously reported glucagon associated variant
We identified a nominal association for the variant rs28929474 on chromosome 14 in SERPINA1, reported by Almgren et al. [4] to be associated with increased fasting glucagon levels. In our meta-analysis, we could replicate the reported association of the T-allele of rs28929474 with increased fasting glucagon levels (Beta 0.345, SE 0.114, P = 0.0024, see Additional file 1: Table S2). Three of the remaining suggestive variants for fasting and 120 min glucagon levels were excluded in our data set due to low allele frequency. Results for the remaining three markers can be found in Additional file 1: Table S2.
Novel gene locus associated with reduced suppression of early glucagon secretion
The strongest association was found for early glucagon suppression (dAUC 0–30 min). The C-allele of rs4691991 in the MARCH1 locus was genome-wide significantly associated with reduced suppression of glucagon during the first 30 min of the OGTT (Beta − 0.210, SE 0.037, P = 1.9 × 10–8, see Table 2) corresponding to 8.2% reduction per effect allele. We also discovered a suggestive locus for dAUC 0–30 min of glucagon on chromosome 7, between PRKAG2 and GALNTL5 (Beta -0.334, SE 0.067, P = 6.9 × 10–7). Other suggestive loci for dAUC 0–30 min of glucagon can be found in Table 2. The suggestive associations for fasting, 30 and 120 min glucagon as well as dAUC 0–120 min for glucagon are shown in Additional file 1: Table S3–6.
Discussion
Here we performed a GWAS of plasma levels of glucagon during an OGTT in 1899 individuals and identified a previously described type 2 diabetes variant in EYA2, we replicated a previously reported suggestive association with fasting glucagon level and identified a novel genome-wide significant variant, rs4691991 in the MARCH1 locus.
Our results indicate that genetic influence of fasting glucagon levels and suppression of glucagon secretion during an OGTT have a limited impact on type 2 diabetes development. We only identified one of the current known type 2 diabetes risk alleles to associate with glucagon levels during OGTT. The variant in EYA2 was associated with increased glucagon levels at 30 min during the OGTT. EYA2 is involved in DNA repair and has, besides type 2 diabetes, also been reported to associate with triglyceride levels [16] and waist-hip ratio [17].
We plotted all the selected markers in a QQ-plot, to investigate if we had more significant associations of the type 2 diabetes risk increasing alleles to plasma glucagon levels during the OGTT than expected by chance (Additional file 2: Figure S1) and could conclude that it is most probably not the same genetic markers that is associated with type 2 diabetes that also affect glucagon levels.
We were able to replicate one of the suggestive associations with fasting glucagon level reported by Almgren et al. [4]. Importantly, in both this and the present study, the same glucagon assay has been used. One of the variants, rs142179968 in MACF1, for which Almgren et al. [4] found a suggestive association with fasting glucagon, has a MAF of < 0.0005 according to the UCSC Genome Browser (GRCh37/hg19) [18, 19], although it was slightly higher in the meta-analysis of the MDC and PPP-Botnia cohorts described in the paper (MAF = 0.002) [4]. Since we restricted our analyses to include only markers with a MAF > 0.01, this variant was not analyzed in our primary data set. Another variant associated with fasting glucagon in the aforementioned paper, rs140436257, located in TDC, has a reported allele frequency < 0.003, but is ten times more common in the meta-analysis by Almgren et al. (MAF = 0.026). However, this variant was not included in the analysis reported here and neither was the variant rs111485612 with a MAF below 1%.
We identified a genome-wide significant association with 0–30 min glucagon suppression. The closest gene to the signal is MARCH1, which mediates ubiquitination of proteins and promotes their subsequent endocytosis and sorting to lysosomes. Variants in MARCH1 have previously been shown to be nominally associated with BMI and increased risk of type 2 diabetes in the UK Biobank [20]. However, whether this gene or another gene in the locus is responsible for the effect on plasma levels of glucagon remains to be discerned.
We did not find any strong evidence of an impact of previously published type 2 diabetes genetic variants on glucagon levels during OGTT, except for EYA2. Neither did we find a large number of novel genetic variants affecting glucagon levels during OGTT in the GWAS performed here. We have not been able to identify any previous studies investigating the heritability of glucagon levels in the fasting state and during glucose stimulation in other cohorts. The sample size in this study is modest and might be a contributing factor to the lack of strong genetic signals. Larger studies are required to get a more comprehensive picture of the genetic influence on glucagon levels during OGTT. We are also strongly encouraging heritability studies of glucagon levels in family or twin studies in the future, since there is an obvious lack of these kinds of studies published.
A larger sample size would allow for studies of polygenic risk scores (PRS), which might be clinically useful in the future. The PRS could be used to identify individuals genetically prone to deviating glucagon levels and thereby monitor them before any adverse effect manifests.
The accuracy of measurements of plasma glucagon has been debated for many years. Apart from the usual reliability concerns, specificity and sensitivity have been the main issue. Glucagon assays may react with the glucagon-containing products from the gut, glicentin and oxyntomodulin, and with proglucagon 1–61 from the pancreas as well as the gut. Regarding glucagon secretion, inhibition of secretion, e.g. by glucose, is as important as stimulation with e.g. arginine, which puts great demands on the sensitivity of assay systems. In addition, interfering matrix effects have been a problem. The assay used in this study is a radioimmunoassay directed against the C-terminus based on a C-terminal-wrapping antibody, 4305, which grants the assay complete specificity towards molecular forms with a free glucagon C-terminal which characterizes pancreatic glucagon as opposed to the gut derived peptides, as well as a sufficient sensitivity (< 1 pmol/l) to pick up all relevant changes. The assay has been validated in several recent studies against mass spectrometry [21] as well as against sandwich ELISA [22, 23]. However, in patients with major surgical operations on the human GI tract, such as gastrectomy and bypass, the sandwich ELISA has in some cases shown aberrant behavior. The radioimmunoassay (4305) also measures proglucagon 1–61 because of the identical C-terminus, but this component plays a very minor role under normal circumstances and has glucagon-like bioactivity, if any [24]. We are therefore convinced that we have used the ideal assay for measurement of glucagon in this study [11].
A strength of our study is the measurement of the physiological important 30 min glucagon levels during an OGTT. In fact, the strongest genetic influence in our data set, was seen for plasma levels of glucagon at 30 min of the OGTT. However, it is a limitation to the study that we have not been able to validate our suggestive associations at this time point in additional cohorts, since plasma glucagon at 30 min is a rare measurement.
Conclusions
The present meta-analysis identified a genome-wide significant association to suppression of early glucagon secretion during OGTT for a locus containing MARCH1. Type 2 diabetes risk variant in the EYA2 locus associated with plasma glucagon levels at 30 min of the OGTT, while other variants influence glucagon levels without conferring increased type 2 diabetes risk. More studies of the genetics underlying fasting glucagon levels and levels of glucagon during an OGTT are needed in order to understand the genetic regulation of α-cell function and to develop genetic tools for mendelian randomization to examine the causal effect of glucagon in various metabolic pathways.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to data protection regulations in Denmark, but are available from the corresponding author on reasonable request.
Abbreviations
- OGTT:
-
Oral glucose tolerance test
- GWAS:
-
Genome-wide association study
- EYA2 :
-
EYA transcriptional coactivator and phosphatase 2
- MARCH1 :
-
Membrane associated ring-CH-type finger 1
- MDC:
-
Malmö Diet and Cancer study
- PPP-Botnia:
-
Prevalence, Prediction and Prevention of diabetes Botnia study
- NGT:
-
Normal glucose tolerance
- IFG:
-
Impaired fasting glycaemia
- IGT:
-
Impaired glucose tolerance
- RigCoh:
-
Rigshospitalet cohort
- HbA1c:
-
Glycated haemoglobin A1c
- HPLC:
-
High-performance liquid chromatography
- dAUC:
-
Decremental area under the curve
- MAF:
-
Minor allele frequency
- EA:
-
Effect allele
- Chr:
-
Chromosome
- NEA:
-
Non effect allele
- EAF:
-
Effect allele frequency
References
Unger RH. Glucagon physiology and pathophysiology in the light of new advances. Diabetologia. 1985;28(8):574–8.
Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sorensen M, Suppli MP, Janah L, et al. The liver-alpha-cell axis and type 2 diabetes. Endocr Rev. 2019;40(5):1353–66.
Holst JJ, Wewer Albrechtsen NJ, Pedersen J, Knop FK. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-alpha-cell axis. Diabetes. 2017;66(2):235–40.
Almgren P, Lindqvist A, Krus U, Hakaste L, Ottosson-Laakso E, Asplund O, et al. Genetic determinants of circulating GIP and GLP-1 concentrations. JCI Insight. 2017;2(21):e93306.
Johansen NB, Hansen AL, Mygind Jensen T, Philipsen A, Rasmussen SS, Jorgensen ME, et al. Protocol for ADDITION-PRO: a longitudinal cohort study of the cardiovascular experience of individuals at high risk for diabetes recruited from Danish primary care. BMC Public Health. 2012;12(1):1078.
Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G, et al. The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with Type 2 diabetes detected by screening. Int J Obes Relat Metab Disord. 2000;24(Suppl 3):S6-11.
Faerch K, Vistisen D, Pacini G, Torekov SS, Johansen NB, Witte DR, et al. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes. 2016;65(11):3473–81.
Clausen TD, Mathiesen ER, Hansen T, Pedersen O, Jensen DM, Lauenborg J, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008;31(2):340–6.
Kelstrup L, Clausen TD, Mathiesen ER, Hansen T, Holst JJ, Damm P. Incretin and glucagon levels in adult offspring exposed to maternal diabetes in pregnancy. J Clin Endocrinol Metab. 2015;100(5):1967–75.
Holst J. Molecular heterogeneity of glucagon in normal subjects and in patients with glucagon-producing tumours. Diabetologia. 1983;24(5):359–65.
Holst JJ, Wewer Albrechtsen NJ. Methods and guidelines for measurement of glucagon in plasma. Int J Mol Sci. 2019;20(21):5416.
Das S, Forer L, Schonherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284–7.
McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet. 2016;48(10):1279–83.
Kang HM, Sul JH, Service SK, Zaitlen NA, Kong SY, Freimer NB, et al. Variance component model to account for sample structure in genome-wide association studies. Nat Genet. 2010;42(4):348–54.
Mahajan A, Taliun D, Thurner M, Robertson NR, Torres JM, Rayner NW, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018;50(11):1505–13.
Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45(11):1274–83.
Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Magi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–96.
UCSC Genome Browser database [Human GRCh37/hg19]. http://genome.ucsc.edu/. Accessed 1 July 2020.
Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006.
Canela-Xandri O, Rawlik K, Tenesa A. An atlas of genetic associations in UK Biobank. Nat Genet. 2018;50(11):1593–9.
Lund A, Bagger JI, Wewer Albrechtsen NJ, Christensen M, Grondahl M, Hartmann B, et al. Evidence of extrapancreatic glucagon secretion in man. Diabetes. 2016;65(3):585–97.
Wewer Albrechtsen NJ, Hartmann B, Veedfald S, Windelov JA, Plamboeck A, Bojsen-Moller KN, et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia. 2014;57(9):1919–26.
Wewer Albrechtsen NJ, Veedfald S, Plamboeck A, Deacon CF, Hartmann B, Knop FK, et al. Inability of some commercial assays to measure suppression of glucagon secretion. J Diabetes Res. 2016;2016:8352957.
Wewer Albrechtsen NJ, Kuhre RE, Hornburg D, Jensen CZ, Hornum M, Dirksen C, et al. Circulating Glucagon 1–61 regulates blood glucose by increasing insulin secretion and hepatic glucose production. Cell Rep. 2017;21(6):1452–60.
55th EASD Annual Meeting of the European Association for the Study of Diabetes. Diabetologia. 2019;62(1):1–600.
Acknowledgements
We acknowledge the work and essential contribution by the study centers, the staff, and the participants. Preliminary results have been presented at the 55th EASD Annual Meeting of the European Association for the Study of Diabetes [25].
Funding
The ADDITION-Denmark study was supported by the National Health Services in the counties of Copenhagen, Aarhus, Ringkøbing, Ribe, and Southern Jutland in Denmark; the Danish Council for Strategic Research; the Danish Research Foundation for General Practice; Novo Nordisk Foundation; the Danish Centre for Evaluation and Health Technology Assessment; the Diabetes Fund of the National Board of Health; the Danish Medical Research Council; and the Aarhus University Research Foundation. The ADDITION-PRO study was funded by an unrestricted grant from the European Foundation for the Study of Diabetes/Pfizer for Research into Cardiovascular Disease Risk Reduction in Patients with Diabetes (74550801), by the Danish Council for Strategic Research and by internal research and equipment funds from Steno Diabetes Center. The RigCoh-study was supported by The Lundbeck Foundation, Civilingeniør H. C. Bechgaard's Foundation, The Danish Diabetes Association, The Danish Medical Research Council, the Gangsted Foundation, A. P. Moeller og Hustru Chastine Mc-Kinney Moeller's Foundation, Aase og Ejner Danielsen's Foundation, The Augustinus Foundation, and The Research Foundation of Rigshospitalet. This work was supported by an unrestricted grant from the European Foundation for the Study of Diabetes/Pfizer for Research into Cardiovascular Disease Risk Reduction in Patients with Diabetes (74550801), the Danish Council for Strategic Research, the European Union’s Horizon 2020 research and Innovation programme (Grant Agreement No 667191) and internal research and equipment funds from Steno Diabetes Center. AJ is supported by the Danish Council for Independent Research, European Union, FP7, Marie Curie Actions, IEF, Lundbeck Foundation, Novo Nordisk Foundation and Danish Diabetes Academy (NNF17SA0031406). The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center at the University of Copenhagen partially funded by an unrestricted donation from the Novo Nordisk Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
AJ participated in the design of the study, carried out the analysis and drafted the manuscript. SES and KF participated in the analyses. SES, SST, JJH, TH, MEJ, DRW, KF, OP, NG, TDC, ERM, LK and PD participated in the conception and design of the study. SES, SST, TDC, LK, OP, NG, TH and JJH generated data. TH and JJH helped to draft the manuscript. All authors participated in the interpretation of the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approvals were obtained from the Scientific Ethics Committee of the Central Denmark Region for the Addition-Pro study (Sundhedssekretariatet, De Videnskabsetiske Komitéer for Region Midtjylland, Skottenborg 26, DK-8800 Viborg, Denmark, journal no. 20080229) and from the local ethical committee for the Rigshospitalet cohort (De Videnskabsetiske Komitéer for Københavns og Fredriksberg Kommuner, Københavns Kommune, Sundhetsforvaltningen, Sjællandsgade 40, 2200 København N, Denmark, journal no. KF01-061/03). All participants provided written informed consent before participating in the studies.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1
. Title of data: Impact of gene variants associated with type 2 diabetes on circulating levels of glucagon during an oral glucose tolerance test (OGTT). Description of data: Beta (SE) from linear mixed model denotes the effect of each of the effect alleles (EA, additive model) on the inverse-normalized residuals of trait adjusted for age, sex and BMI. NEA - non effect allele, CHR - chromosome, POS - position, EAF - effect allele frequency. Table S2. Title of data: Replication of previously reported markers associating with plasma glucagon levels during an oral glucose tolerance test (OGTT). Description of data: Beta (SE) from linear mixed model denotes the effect of each of the effect alleles (EA, additive model) on the inverse-normalized residuals of trait adjusted for age, sex and BMI. NEA - non effect allele, CHR - chromosome, POS - position, EAF - effect allele frequency. Table S3. Title of data: Suggestive loci for fasting plasma glucagon levels. Description of data: Beta (SE) from linear mixed model denotes the effect of each of the effect alleles (EA, additive model) on the inverse-normalized residuals of trait adjusted for age, sex and BMI. NEA - non effect allele, CHR - chromosome, POS - position, EAF - effect allele frequency. Table S4. Title of data: Suggestive loci for 30 minute plasma glucagon levels during an oral glucose tolerance test (OGTT). Description of data: Beta (SE) from linear mixed model denotes the effect of each of the effect alleles (EA, additive model) on the inverse-normalized residuals of trait adjusted for age, sex and BMI. NEA - non effect allele, CHR - chromosome, POS - position, EAF - effect allele frequency. Table S5. Title of data: Suggestive loci for 120 minutes plasma glucagon levels during an oral glucose tolerance test (OGTT). Description of data: Beta (SE) from linear mixed model denotes the effect of each of the effect alleles (EA, additive model) on the inverse-normalized residuals of trait adjusted for age, sex and BMI. NEA - non effect allele, CHR - chromosome, POS - position, EAF - effect allele frequency. Table S6. Title of data: Suggestive loci for dAUC 0-120 minute of plasma glucagon levels during an oral glucose tolerance test (OGTT). Description of data: Beta (SE) from linear mixed model denotes the effect of each of the effect alleles (EA, additive model) on the inverse-normalized residuals of trait adjusted for age, sex and BMI. NEA - non effect allele, CHR - chromosome, POS - position, EAF - effect allele frequency.
Additional file 2: Figure S1.
Title of data: QQ-plot of selected variants previously reported to associate with increased risk of type 2 diabetes. Description of data: The figure shows the observed vs. the expected P-values of the selected variants for fasting glucagon (A), 30 min glucagon (B), 120 min glucagon (C), decremental area under the curve (dAUC) 0-30 minutes of glucagon (D) and dAUC 0-120 minutes of glucagon (E) during the OGTT. There was no obvious excess of genetic variants, previously associated with type 2 diabetes, associating with circulating glucagon during OGTT.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jonsson, A., Stinson, S.E., Torekov, S.S. et al. Genome-wide association study of circulating levels of glucagon during an oral glucose tolerance test. BMC Med Genomics 14, 3 (2021). https://doi.org/10.1186/s12920-020-00841-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12920-020-00841-7