Abstract
Background
Ethno-veterinary practices could be used as a sustainable developmental tool by integrating traditional phytotherapy and husbandry. Phytotherapeutics are available and used worldwide. However, evidence of their antiparasitic efficacy is currently very limited. Parasitic diseases have a considerable effect on pig production, causing economic losses due to high morbidity and mortality. In this respect, especially smallholders and organic producers face severe challenges. Parasites, as disease causing agents, often outcompete other pathogens in such extensive production systems. A total of 720 faecal samples were collected in two farms from three age categories, i.e. weaners, fatteners, and sows. Flotation (Willis and McMaster method), modified Ziehl–Neelsen stained faecal smear, centrifugal sedimentation, modified Blagg technique, and faecal cultures were used to identify parasites and quantify the parasitic load.
Results
The examination confirmed the presence of infections with Eimeria spp., Cryptosporidium spp., Balantioides coli (syn. Balantidium coli), Ascaris suum, Oesophagostomum spp., Strongyloides ransomi, and Trichuris suis, distributed based on age category. A dose of 180 mg/kg bw/day of Allium sativum L. and 90 mg/kg bw/day of Artemisia absinthium L. powders, administered for 10 consecutive days, revealed a strong, taxonomy-based antiprotozoal and anthelmintic activity.
Conclusions
The results highlighted the therapeutic potential of both A. sativum and A. absinthium against gastrointestinal parasites in pigs. Their therapeutic effectiveness may be attributed to the content in polyphenols, tocopherols, flavonoids, sterols, sesquiterpene lactones, and sulfoxide. Further research is required to establish the minimal effective dose of both plants against digestive parasites in pigs.
Similar content being viewed by others
Background
In pig farming, management and preventive measures against parasitic diseases improve overall feed conversion and reproductive performance, while decreasing morbidity and mortality [1, 2]. Gastrointestinal parasites pose a significant economic burden on pig farms, leading to various detrimental effects. These include inefficient feed consumption, poor growth rate, reduced weight gain, decreased litter size, fertility disorders, compromised post-vaccination immunity against infectious diseases, lower meat quality, and diminished animal welfare [1, 3, 4].
The diversity and intensity of gastrointestinal parasitism depends on the type of swine production system [3, 5]. Low-input farming faces several constraints, among which parasitic diseases are of significant importance [6]. The prevalence of digestive parasites in pigs is broadly reported upon worldwide. The most common helminths are Ascaris suum, Trichuris suis, Strongyloides ransomi, Hyostrongylus rubidus, Trichostrongylus axei, and Oesophagostomum spp. Furthermore, pigs can also harbour intestinal protozoan parasites, such as Eimeria spp., Cystoisospora suis, Cryptosporidium spp., Balantioides coli, and Giardia lamblia [1, 7, 8].
Controlling parasite infections in livestock farming is increasingly crucial globally. Antiparasitic medications, like avermectins, triazine, and benzimidazole, are commonly used to combat these parasitic infections in swine [5, 9]. Their primary drawback is the emergence of antiparasitic resistance across most compounds, coupled with the presence of residues in animal products. The residues of such chemicals in the environment can disrupt ecosystems, posing significant threats to human health and welfare [10].
There is renewed international interest in using herbal products as safer alternatives to control parasite infections and lower the risk of developing resistance to antiparasitic drugs [11]. Ethno-medicine holds an integral position within traditional medical practices in numerous developing countries. This type of empirical medicine is mainly used in rural areas, where standard treatment protocols, especially for livestock, have prohibitive costs [12]. Ethno-veterinary practices have the potential to serve as sustainable development tools by leveraging local veterinary and husbandry knowledge. Thus, herbal medicine may very well become a frontrunner in turning local into global knowledge through the recognition of local expertise as an essential source of wide-reaching sustainable development for both people and animals [4, 13]. Over the last decade, there has been a notable increase in the utilization of phytotherapeutic remedies, which can be attributed to their enhanced bioavailability, reduced toxicity, and environmentally friendly characteristics [14].
Allium sativum L., commonly known as garlic, belongs to the Amaryllidaceaa family [15, 16]. The plant forms a bulb that is commonly used either as food or medicine. Garlic contains several enzymes, 17 amino acids, along with minerals and more than 33 sulfur compounds, a content higher than in any other Allium species. The latter are responsible for both the garlic’s pungent odour as well as many of its medicinal effects [16,17,18]. The bioactive compounds of garlic are allicin, alliin, ajoene, diallyl sulfide, dithiin, vinyldithiins, and allylcysteine [11, 18, 19]. Dried, powdered garlic contains approximately 1% alliin (S-allyl cysteine sulfoxide). The main anthelmintic compound, allicin (diallyl thiosulfinate or diallyl disulfide) only becomes available in garlic once the bulb is crushed, through activation of the enzyme alliinase, which then metabolizes alliin into allicin [16,17,18]. Garlic has a wide range of properties, such as antibacterial, antiviral, antifungal, antiprotozoal, and anthelmintic. It can also act as an immune stimulating agent, while reducing the risk of cardiovascular disease [16, 20].
Artemisia absinthium L., commonly known as wormwood, is a species belonging to the Artemisia genus, distributed mainly in the temperate zone [21]. It contains various compounds, such as: sesquiterpene lactones, essential oils (thujones, trans-sabinyl acetate, cis-chrysanthenyl-acetate, and cis-epoxyimene), along with phenolic acids, flavonoids, carotenoids, coumarins, thiophene, tannins, and lignans [21, 22]. A. absinthium and its extracts have several therapeutic properties such as: antioxidant, anticarcinogenic, immuno-modulatory, anti-inflammatory, antipyretic, cardio-protective, gastro-protective, hepato-protective, hypoglycaemic, neuroprotective, and antidepressant [23,24,25]. In traditional medicine, A. absinthium has been used in both humans and animals as an anthelmintic and antiprotozoal drug and is still regarded as an effective natural remedy against parasites [21, 22, 24]. Sesquiterpene lactones, such as artemisinin, dihydroartemisinin, ridentin, hanphyllin, dehydroleucodine, and santonin, constitute the most important molecules responsible for the antiparasitic activity of plants from the Artemisia genus [26,27,28]. In the poultry industry, the bulb of A. sativum and whole plant of A. absinthium have been used as phytogenic growth promoters, by stimulating the secretion of digestive enzymes, leading to enhanced digestion and absorption [29, 30].
Due to continuously increasing drug resistence in parasites and prohibited use of antiparasitic medications in organic pig farming practices, phytotherapy could represent a valid, biologically available and cost effective alternative for parasite control. Since numerous plants are cited for their antiparasitic effects in different animals species and many of them are commonly locally available, this study focused on exploring the antiparasitic potential of garlic (Allium sativum) and wormwood (Artemisia absinthium) plants native to Romanian’s flora and renown for their manifold beneficial properties against naturally occurring gastrointestinal parasites of pigs on two low-input (free-range) farms from NW Romania. The primary objective of this research was to identify a plant-based formula that exhibits effectiveness in combating pig parasitoses without interfering with their welfare and health.
Results
Chemical analysis of plant extracts
Following chemical analysis of the alcoholic plant extracts, the main biologically active compounds identified were: polyphenols (caffeic acid, ferulic acid, sinapic acid), tocopherols (α-tocopherol), sulfoxide (aliin) for A. sativum and polyphenols (chlorogenic acid, p-coumaric acid, ferulic acid, vitexin, isoquercitrin, rutoside, quercitrin, quercetol, luteolin, kaempferol, apigenin, syringic acid, protocatechuic acid, and vanillic acid), tocopherols (α-tocopherol, γ-tocopherol, Δ-tocopherol), sterols (ergosterol, stigmasterol, β-sitosterol, campesterol), methoxylated flavones (hispidulin, eupatorin, casticin), sesquiterpene lactones (α-santonin, vulgarin) for A. absinthium.
Evaluation of plants’ antiparasitic activity
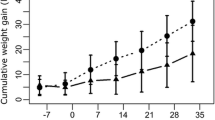
In this experiment, the animals readily consumed the feed without any hesitation and did not exhibit any noticeable adverse effects. Although it was not the primary objective of the study, clinical observations indicated that animals in both experimental groups across all age categories showed enhanced feed intake and a higher growth rate compared to those in the control groups. This effect was particularly evident among the weaners and fatteners.
The coproparasitological examination revealed co-infections with seven species of gastrointestinal parasites, including Eimeria spp., Balantioides coli, Cryptosporidium spp, Ascaris suum, Trichuris suis, Oesophagostomum spp., and Strongyloides ransomi, in combinations depending on the category of pigs. Oocysts culture examination successfully attributed the parasites to the Eimeria genus. Faecal cultures, containing strongylid eggs, showed that all L3 larvae belonged to the Oesophagostomum genus. Neither the centrifugal sedimentation nor Blagg methods revealed positive results. The flotation, oocysts/egg culture, and McMaster methods showed that the prevalence and the average intensity of infections varied according to farm, age category, and tested plant.
S. ransomi was only found on farm 1 (F1), but it was present in all age categories. In weaners from farm 2 (F2), only Eimeria spp., B. coli and Cryptosporidium spp. were diagnosed, while on F1, Oesophagostomum spp. was additionally found. In fatteners, Eimeria spp., B. coli, T. suis, and A. suum were identified on both farms. In sows from both farms, Eimeria spp., B. coli, A. suum, Oesophagostomum spp., and Cryptosporidium spp. were observed (Figs. 1, 2, 3, 4).
A. sativum demonstrated a strong antiparasitic (antiprotozoal, anthelmintic) activity against all diagnosed parasites and across all pig categories, except for Cryptosporidium, in the case of which both plants showed limited antiparasitic activity. While A. absinthium demonstrated a good antiparasitic efficacy against Eimeria, B. coli, A. suum, Oesophagostomum, and T. suis, it had no noticeable effect against S. ransomi. In both plants, the strongest antiparasitic activity against all parasites was demonstrated on day 14, with a decrease of the therapeutic effect following day 28 (Figs. 1, 2, 3, 4; Table 1, 2, 3).
Both control and experimental groups were homogeneous at the beginning of the experimental period, before the treatment (Table 1 and 2). For all of Eimeria spp., B. coli, A. suum, T. suis, Oesophagostomum spp., and S. ransomi, the differences between experimental and control groups (EG 14 / CG 14, EG 28 / CG 28) on one hand, and between experimental groups (EG 0 / EG 14 / EG 28) on the other hand, were statistically significant for each farm, plant, and age category. The A. sativum group had statistically significant values (SSVs) for Eimeria spp. in sows from F1 and in all age groups from F2, for B. coli in sows from F1 and in all age groups from F2, for A. suum in fatteners and sows from both farms, for S. ransomi in weaners from F1, and for Oesophagostomum spp. in sows from both farms (Table 1 and 2). The A. absinthium group showed SSVs for Eimeria spp. and B. coli in all age groups from both farms, and for A. suum in sows from F2 and in fatteners from both farms (Table 1 and 2).
The therapeutic efficacy (reduction %) of A. sativum (AS) and A. absinthium (AA) against diagnosed parasites, in all age groups ranged as follows: AS = 20–100%, AA = 33.1–100% for Eimeria spp., AS = 47.9–82.3%, AA = 31.6–88.4% for B. coli, AS = 62.8–87.6%, AA = 30.2–80.5% for A. suum, AS = 54.1–76.6%, AA = 39.5–79.2%, for T. suis, AS = 45.8–100%, AA = 25.1–66.7% for Oesophagostomum spp., and AS = 57.3–100%, AA = 31.3–69.1% for S. ransomi (Table 3). Due to a lack of quantitative sensitivity of usual coproparasitological methods for Cryptosporidium in terms of the oocysts numbers (intensity), the infection prevalence was the only indicator provided.
Discussion
In this experiment, the evaluation of the antiparasitic efficacy of two plants from the Romanian flora (A. sativum and A. absinthium) was carried out successfully on two low-input farms. Despite the lack of scientific data on the effect of the abovementioned plants on swine parasites, we extrapolated our results by comparing them to studies on humans [19], and domestic species [13]. Overall, all three age groups (weaners, fatteners and sows) had similar co-infections with protozoa and nematodes. The efficacy of plants was estimated by calculating the difference between the parasitic intensity before and after the garlic and wormwood therapy. In most of the cases, the intensity was highly influenced along with the prevalence, while in others, the prevalence remained unchanged in spite of decreasing the intensity of the infection.
Previous reports mention a daily A. sativum dose ranging from a minimum of 30 mg to a maximum of 1052 mg/kg bw [31]. In the present study, the selected dose of 180 mg garlic powder/kg bw/day was administered to infected swine, for ten consecutive days. This dose was deemed safe, avoiding any potential adverse effects, i.e. lack of appetite, weight loss, liver injury, pulmonary oedema, inhibition of spermatogenesis, partial paralysis, allergy, anaemia and death, previously attributed to compounds like diallyl disulfide, allylpropyl sulfide, and allicin [20, 32]. For A. absinthium, a dosage of 90 mg/kg bw/day for 10 days was used under the same considerations as for garlic. There was no available information on the therapeutic dose of wormwood used in pigs, therefore the choice of tested doses was based on studies on other species. These included rats (300 mg/kg/day), goats (2000 mg/kg, single dose) and humans (2000–3000 mg/individual/day) [33,34,35]. Long-term administration of A. absinthium can cause neurotoxicity due to the presence of thujone and trans-sabinyl acetate. Additionally, adverse side effects, at least in humans, may include gastrointestinal disorders, brain injury, vertigo, insomnia, restlessness, urine retention, seizures, tremors, and even death [14, 22]. In our study, no adverse reactions were observed, if the therapeutic doses were respected.
The chemical composition of the two Romanian medicinal plants used in the present study resembles those from previous reports, only differing in concentration. Biological compounds, such as polyphenols, tocopherols, flavonoids, sesquiterpene lactones, and sulfoxide, have demonstrated strong antiprotozoal and anthelmintic properties, both in vitro and in vivo. Therefore, they could be used as substitutes for classic antiparasitics [4, 11, 12, 15, 22, 36].
Eimeria spp. was diagnosed in weaners, fatteners and sows, in both farms, with high prevalence. A. sativum and A. absinthium powders demonstrated a strong anticoccidial activity (reducing oocyst excretion) in all age groups, in both farms, with wormwood (33.1%-100%) displaying superior efficacy to that of the garlic (20%-100%) (Table 3). Although eimeriosis is of low pathogenicity in swine compared to other species (birds, rabbits, ruminants) in which the disease is more clinically relevant, the efficacy provided by garlic and wormwood powders in our survey is comparable with the results obtained in respective studies. The propylene glycol extract from A. sativum and Thymus serpyllum reduced duodenal lesions, increasing the feed conversion ratio and oocysts output, as well as reducing the weight gain in broilers compared to control groups, treated with amprolium [37]. Abu-Akkada et al. [38] showed that oral administration of crude garlic in a daily dosage of 500 mg/kg bw for five consecutive days limited the adverse impacts of hepatic coccidiosis in rabbits, resulting in improved body weight gain and lowered numbers of oocysts. Alcoholic garlic extract was proved to possess a remarkable anticoccidial effect against rabbit intestinal coccidiosis, both in vitro and in vivo, and could be used as a natural feed additive for both prophylaxis and treatment of coccidiosis, thus minimizing the economic losses caused by the parasite [39]. Sidiropoulou et al. [40] showed that supplementation of oregano and garlic essential oils had a significant anticoccidial effect in vitro, along with promoting growth in broilers, reared in the absence of anticoccidial drugs. Artemisinin is a sesquiterpene lactone isolated from Artemisia absinthium, with an unclear action mechanism, still used in controlling poultry coccidiosis [27]. Several studies have demonstrated that A. absinthium (essential oil, leaf powder), used as an additive in chickens, exhibited anticoccidial activity by lowering the severity of diarrhoea, as well as reducing oocyst excretion, improving feed conversion efficiency, and strengthening the immune system [30, 41, 42]. Therapy with A. absinthium extracts, using a dose of 1–3 mg/kg, was able to reduce the severity of Eimeria infection, thus decreasing the number of oocysts per gram of faeces in chickens infected with Eimeria tenella [43]. Popović et al. [44] also noticed that wormwood supplementation (100 g/kg) in the diet of rabbits had an anticoccidial effect, along with a positive influence on growth performance, as well as on antioxidative systems. The positive results obtained in this experiment are highly valuable for the prevention and control of porcine coccidiosis, especially in organic and low-input farming systems, particularly because in vivo studies regarding the efficacy of wormwood and garlic on Eimeria species in pigs, are lacking.
Cryptosporidiosis is an emerging zoonotic protozoan parasite that poses a global challenge in both veterinary and human medicine [45]. The findings revealed its existence, while the usual coproparasitological tehniques lacked the ability to detect the average intensity, due to the absence of quantitative sensitivity in oocyst counting. In the present study, Cryptosporidium spp. was only identified in weaners and sows, which were considered to serve as a permanent source of infection. Both plants exhibited limited efficacy against Cryptosporidium, causing a decrease in prevalence (Fig. 1 and Fig. 3). In Cryptosporidium infected mice, treatment with raw garlic juice (50 mg/kg, for seven days) also reduced shedding of Cryptosporidium oocysts [45]. In humans, therapy with allicin against Cryptosporidium parvum and Cryptosporidium hominis was successful, indicating a lethal effect on Cryptosporidium, while improving patient immune function [46, 47]. Gaafar et al. [48] performed an in vivo experiment, administering aqueous garlic extract on immunosuppressed mice, and observed a destruction of Cryptosporidium oocysts. The use of artemisin against C. parvum infection showed limited effectiveness, causing a minor decrease in average oocyst numbers, when compared to macrolides [49], similar to the results obtained in the present study in pigs. Artemisia ethanolic extracts were used as a remedy in mouse cryptosporidiosis and the results revealed significantly reduced oocyst shedding, while the symptoms disappeared in the treated groups [50]. A. absinthium essential oil (2 mg/ml) was able to destroy Cryptosporidium baileyi and Cryptosporidium galli oocysts, in vitro [51]. Considering that the selected age groups were only carriers of the parasite, the practical importance of the effect of garlic and wormwood on cryptosporidiosis might be limited. Cryptosporidium clinically affects only piglets during neonatal period. Therefore, the positive results obtained by both plants, might not have a significant impact in this frame.
Balantioides coli (former Balantidium coli) was diagnosed in all age groups, in both farms. A. sativum (47.9%-82.3%) and A. absinthium (31.6%-88.4%) were both effective against balantidiasis. However, garlic had a more pronounced antiparasitic activity (Table 3). No studies were found on the antiparasitic activity of garlic and wormwood against balantidiasis, although these plants have been proven efficient against numerous protozoa, such as: Toxoplasma spp., Plasmodium spp., Entamoeba histolytica, Leishmania spp., Trypanosoma cruzi, Trichomonas vaginalis, Giardia lamblia [16, 22, 23, 46, 47, 52,53,54,55,56,57]. An in vivo study revealed that aqueous garlic extracts (200 mg/kg bw) had the highest antitrichomonal effect, thus shortening the duration of the treatment of pigeons from seven to five days [58]. Therefore, it may be concluded that the present report is at the forefront of evaluating the antiparasitic activity of these plants against B. coli.
Ascaris suum, another important parasite, was identified in fatteners and sows. Both A. sativum (62.8%-87.6%) and A. absinthium (30.2%-80.5%) demonstrated a strong anthelmintic activity against A. suum and may represent an alternative therapy to classic antiparasitic drugs (Table 3). The antiparasitic efficacy of both plants used in the present experiment has been confirmed by previous literature reports. Akoh et al. [19] indicated that the ethanolic garlic extract was used against Ascaris lumbricoides in humans with egg counts decreasing by 80.73% (200 mg/kg bw of garlic extract), 84.26% (400 mg/kg bw) and by 91.78% (800 mg/kg bw), respectively. According to the studies conducted by Raza et al. [13], A. sativum also had an anthelmintic activity against A. galli in chickens, attributed to the presence of allicin. However, this effects was not as pronounced as that of flubendazole. In an in vitro study, ethanolic and aqueous extracts of A. sativum inhibited hatching, by killing 100% of the larvae of Ancilostoma caninum and Toxocara canis, at concentrations between 1.25 and 10 mg/ml [32]. A. absinthium extract administrated orally to cats at a dose between 300–600 mg/kg bw showed a good anthelmintic activity [59]. Wormwood methanolic extracts demonstrated a strong in vitro inhibitory effect on the embryonation rate of A. galli eggs [60]. Several reports highlighted the anthelmintic activity of A. absinthium against A. suum and A. galli [61,62,63]. Since A. suum infection has a negative impact on the health and welfare of pigs, the excellent results obtained in this study are highly beneficial, offering a promising alternative for the treatment of this parasitosis mainly on organic and low-input farms.
Oesophagostomum spp. was the only strongyle diagnosed. This parasite was identified in both farms, in weaners and sows. Our results showed that A. sativum (45.8%-100%) was very effective against Oesophagostomum, while A. absinthium (25.1%-66.7%) expressed a weak antiparasitic activity (Table 3). In an in vivo study, the effect of aqueous and alcoholic extracts of A. sativum on gastrointestinal endoparasites of sheep (Haemonchus, Cooperia, Trichostrongylus) was evaluated, inducing a reduction in EPG in treated groups [13, 64]. A. sativum showed also a good efficacy against nematodes of cattle using a dose of 100 mg/kg bw for 28 days [65]. A good efficacy in reduction of strongyle eggs in goats treated with raw garlic juice with different concentrations (between 20–80%) was obtained by Masamha et al. [66], the effect increasing with higher concentrations. Oral administration of garlic formulations had no effect on the egg shedding of intestinal strongyles in horses [67]. In the present study in swine, A. absinthium exhibited only poor effects on nematodes compared to previous findings revealing the anthelmintic effectiveness of A. absinthium against small ruminants nematodes (Haemonchus contortus, Teladorsagia circumcincta, and Chabertia ovina), both in vivo and in vitro [62, 68,69,70].
Strongyloides ransomi mainly infects young pigs, causing only sporadically a clinical disease [71]. In this study, it was only diagnosed in farm 1, in all age groups. The presented results demonstrated that garlic (57.3%-100%) was strongly effective against S. ransomi, while wormwood (31.3%-69.1%) had only a mild effect (Table 3). Fawzi and Elsohaby [72] investigated the role of allicin in treatment of gastrointestinal nematodes (Trichostrongylus, Cooperia, and Strongyloides) in cattle and demonstrated that allicin had an anthelmintic action comparable to that of albendazol. Suttun et al. [16] showed that A. sativum is effective against Strongyloides infection in donkeys. Consistently, Ahmed et al. [73] evaluated in vivo effects of ethanolic A. sativum extract (100 mg/kg bw) and demonstrated a good anthelmintic effect against sheep gastrointestinal nematodes, including Strongyloides. To our best knowledge, no other reports on the antiparasitic activity of A. absinthium against strongyloidosis were published, therefore, our results could provide a valuable resource for future studies in farm species.
Lastly, Trichuris suis was diagnosed. The whipworm was identified in both farms, affecting only fatteners, thus supporting the life cycle of this parasite. Our study revealed that both plants, A. sativum (54.1%-76.6%) and A. absinthium (39.5%-79.2%), were effective against T. suis (Table 3). Consistently, aqueous garlic extracts showed a good activity against Trichuris muris [74]. A. sativum, extracted with various solvents and used in different experimental models, showed a good anthelmintic activity against other nematodes, such as: Heterakis gallinarum, Angiostrongylus cantonensis, and Trichinella spiralis [53, 75, 76]. Mirza et al. [28] demonstrated the antiparasitic efficacy of terpenes, chemical compounds found in A. absinthium, against T. muris and Ancylostoma ceylanicum. Similarly, wormwood had a potent anthelmintic activity against T. spiralis and H. gallinarum [33, 77].
In summary, the aforementioned studies corroborated with our current study demonstrated that A. sativum and A. absinthium could be prospective sources for the development of new and potent antiparasitic herbal remedies, for both humans and animals. Plant powders, along with essential oils, aqueous and alcoholic extracts, have been found to exhibit strong antiprotozoal and anthelminthic effects. Allicin (garlic) and artemisinin (wormwood) are two of the most important bioactive molecules responsible for the antiparasitic activity. This study identified a co-infection parasitic status, including protozoa and helminths on all studied farms. On one of the farms (F1) a supplementary parasite, S. ransomi, was present. Nevertheless, the effects of both plants were similar on both farms.
The results obtained from our research have practical implications for organic livestock systems and the welfare and health of pigs. Moreover, considering the zoonotic potential of several of the identified parasites (A. suum, Cryptosporidium, and B. coli), the dissemination of such studies is of the utmost importance.
Conclusion
The current study demonstrated that administering powdered A. sativum bulbs and A. absinthium aerial parts at doses of 180 mg/kg/day and 90 mg/kg/day, respectively, for ten consecutive days, may be be effective against digestive parasites in swine.
The findings of the present study revealed that A. sativum and A. absinthium have the potential of treating gastrointestinal parasitoses in swine. The curative efficacy may be attributed to the presence of polyphenols, sterols, tocopherols, flavonoids, sesquiterpene lactones, and sulfoxide, which exhibit antiparasitic activity. Therefore, A. sativum and A. absinthium have satisfactory antiparasitic (antiprotozoal and anthelmintic) potential and may still be of value as part of an integrated approach, specifically designed to achieve sustainable parasite control in low-input swine production systems.
The absence of toxicity observed for garlic and wormwood, coupled with our study results, leads us to propose that these medicinal plants could serve as a foundation for developing a novel line of antiparasitic herbal medication.
Further studies on the standardization of dosage and identification of precise action mechanisms of these plants against the gastrointestinal parasites are thereby demanded. The findings from the present study contribute to the field of plant antiparasitic therapies allowing sustainable, effective and safe alternative development, along with preventive care through additives based on these compounds.
Methods
Ethics statement and ontologies
Before and during the experiment, the behaviour and clinical condition of the pigs was monitored. The described experiment complied with national (Law No. 43 of 2014) and European law (EU Directive No. 63 of 2010) with respect to animal experimentation and care of animals under study. The ontologies related to medicinal plants, chemical compounds, diseases, and pathogens utilised in the experimental protocol were detailed in Additional file 1.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania (Permit no. 231/02.11.2020). The current experiment was conducted on two farms, and informed consent was obtained from the owners of both farms.
Chemical analysis of A. sativum and A. absinthium
The aerial parts of A. absinthium (wormwood) and A. sativum (garlic) bulbs were processed into powder and were used for the chemical analysis. High performance liquid chromatography coupled with mass spectrometry (HPLC–MS) was used for the analysis of biologically active compounds present in the alcoholic plant extracts (Additional file 2). All the procedures were performed at the”Iuliu Haţieganu “ University of Medicine and Pharmacy Cluj-Napoca (Romania). The techniques, equipment and methods used for analysis of A. sativum L. and A. absinthium L. alcoholic extracts have been described in detail in a previous article [78].
Swine husbandry
The faecal samples were obtained from two low-input farms (referred to as F1 and F2), both of which raised native Bazna and Mangalitza pig breeds. These breeds are renowned for their superior organoleptic characteristics of meat, resistance to diseases, minimal requirement for complex feed regimens, and suitability for free-range (low-input) farming practices. At the beginning of the experiment, in April 2021, F1 had a pig herd of 350 animals, while F2 had 320 animals. The farms were located in Northwestern Romania, in a hilly area characterized by pastures and forests with a specific temperate-continental climate [8, 79]. From an infrastructure standpoint, both farms were similar: the interior of the barns was divided into compartments, the flooring consisted of concrete with drainage holes incorporated, there were automatic waterers, and feed was supplied in troughs. Drinking water for the animals was provided from a local potable water source, which meets all necessary hygiene and quality standards for human use. The shelters underwent regular hygiene maintenance twice daily throughout the year. Additionally, prior to starting the experimental protocol, a rigorous mechanical cleaning and disinfection of barns and paddocks was carried out. The outdoor environment, comprising an earth paddock, was bordered by an electric fence for protection against predators and also wild boars. The pasture and enrichments were accessible to the animals at all times [8, 79].
Experimental design
To ensure precise results, a pilot study was conducted on a limited number of animals before initiating the experimental protocol. It involved assessing various reports detailing doses of A. sativum and A. absinthium. Hence, the feeding behaviour of the pigs, potential toxic effects, and antiparasitic efficacy of the tested plants were carefully observed and monitored.
The plants were sourced from Romania through an authorized company (Plafar SA, Romania), which brings over 50 years of expertise in producing, processing, storing, and marketing medicinal and aromatic plants for human use throughout Romania. The identification of the plant material occurred in the laboratory of Plafar SA. The control samples of garlic and wormwood have been meticulously preserved and stored at both the Parasitology and Parasitic Diseases Department at the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca and the Department of Pharmaceutical Botany within the”Iuliu Haţieganu “ University of Medicine and Pharmacy of Cluj-Napoca.
The aerial parts of A. absinthium and the bulbs of A. sativum were ground, resulting in a feed containing either garlic or wormwood. Each type was then mixed with cereal flour. A total of 240 pigs were included in the study, with 120 pigs allocated to each plant based experiment variant, on both farms. Three age groups were defined in each of the studied pig herds, namely weaners, aged between 10 and 12 weeks, weighing 13–15 kg, fatteners, between 5 and 6 months, weighing 55–60 kg, and sows, aged from 1 to 4 years, with a body weight of 155 to 160 kg. On each farm and for each plant, three control groups (10 weaners, 10 fatteners, and 10 sows) and 3 experimental groups (10 weaners, 10 fatteners, and 10 sows) were defined. In F1, the study was conducted on pigs belonging to the Bazna breed, while in F2, Mangalitza pigs were used [79]. Every experimental group (unit), consisting of 10 individuals, of the same breed, age, and weight, was restricted within a pen, where feeding was applied at group level while ensuring adherence to welfare conditions. After consuming the plant-based feed, the pigs were allowed to go out into the paddock. A one-month interval was inserted between the plant experiments conducted on different animals. The diet was tailored to the animals based on their respective age categories (Table 4). The average daily feed intake per animal was as follows: 3 kg for sows, 2 kg for fatteners, and 0.7 kg for weaners, respectively. Each pig received a dosage of A. sativum of 180 mg/kg bw/day, which was divided into two portions and given for 10 consecutive days. A. absinthium was administered in a dosage of 90 mg/kg bw/day, divided into two portions, for the same period of time as garlic. Before initiating the treatment (day 0), a coproparasitological examination was conducted. Following therapy, two additional assessments (on days 14 and 28) were performed across all farms, plants, and age categories. The experiment began with a 28-day test of A. sativum, followed by an equal duration testing of A. absinthium. Both the feed and medicinal plants provided to the animals were certified by the producer.
Rectal faecal samples weighing approximately 15–20 g each, were collected individually, on days 0, 14 and 28 of the experiment, placed in clean containers, and were macroscopically examined for the presence of visible parasites. The samples were then stored at 2–8ºC for 24–48 h, until further examination. Stored samples were then examined using the following methods: centrifugal sedimentation, flotation—Willis method, faecal smear stained by modified Ziehl–Neelsen technique, Blagg method, McMaster egg counting technique, and in vitro nematode larvae/protozoan oocysts cultures (Additional file 3). The individual intensity was calculated using the McMaster quantitative faecal flotation technique. In contrast, the average intensity of parasitism was established as the arithmetic means of the counted eggs, cysts, or oocysts of a particular parasite species, divided by the number of individuals of the age group (n = 10) [8, 79,80,81].
Assessment of antiparasitic efficacy
Faecal egg count reduction test (FECRT) was used to ascertain the antiparasitic efficacy of A. sativum and A. absinthium and faecal egg count reduction (FECR) was reported [82, 83]: FECR (%) = 100 x (1–[T2/T1] x [C1/C2]), where T1 and T2 are the mean pre- and post-treatment faecal egg counts (FEC) of a treated group, and C1 and C2 are the mean pre- and post-treatment FEC of control group. This formula was also utilized in a previous article [79].
Statistical analysis
Data were analyzed with Statistica program (v. 13.5, TIBCO, Tusla, OK, USA). Excel® from Microsoft Office 365 was used to visually represent the raw data. Column graphs were used to represent the prevalence of parasites by farm, age group, and plant. The presence of Cryptosporidium spp. infection was reported as absolute frequency per age group, plant (A. sativum, A. absinthium), and farm. Due to the limited sample size for each farm, group (EG-experimental groups vs. CG-control group), and plant, as well as the high variability in the data, the count of parasites were reported as the median with the 25th percentile and 75th percentile range.
To compare the EG with the CG on each farm and investigated day (0, 14, and 28), it was employed the two-sided Mann–Whitney test at a significance level (α) of 5%. The effectiveness of the investigated plants was assessed using the two-sided Friedman test, followed by the Wilcoxon test whenever statistical significance was reached. The Bonferroni adjustment was applied to reduce the change of type 1 error α in post-hoc analysis, whenever Friedman test proved statistically significance. The significance level (α*) was adjusted based on the maximum possible number of parasites (intensity) per farm and age group, which was four in our study, resulting in α* = 1.25% (Additional file 4).
Availability of data and materials
The raw data have been deposited in the repository of the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca. The data that support the study are available from the corresponding author upon reasonable request.
Abbreviations
- A. absinthium :
-
Artemisia absinthium
- A. sativum :
-
Allium sativum
- A. suum :
-
Ascaris suum
- B. coli :
-
Balantioides coli
- T. suis :
-
Trichuris suis
- S. ransomi :
-
Strongyloides ransomi
- CG:
-
Control group
- EG:
-
Experimental group
- F1:
-
Farm 1
- F2:
-
Farm 2
- T. muris :
-
Trichuris muris
- T. spiralis :
-
Trichinella spiralis
- H. gallinarum :
-
Heterakis gallinarum
- FECRT:
-
Faecal egg count reduction test
References
Opara MN, Ibekwe N, Azubuike JC, Okoli CG. Occurrence of parasitic helminths among free-range pigs in five local government areas of Owerri zone, Imo State, Nigeria. Int J Appl Nat Sci. 2006;2:72–7. https://doi.org/10.4314/ijonas.v2i1.36049.
Krishna Murthy CM, Ananda KJ, Adeppa J, Satheesha MG. Studies on gastrointestinal parasites of pigs in Shimoga region of Karnataka. J Parasit Dis. 2016;40:885–9. https://doi.org/10.1007/s12639-014-0598-0.
Knecht D, Popiołek M, Zaleśny G. Does meatiness of pigs depend on the level of gastro-intestinal parasites infection? Prev Vet Med. 2011;99:234–9. https://doi.org/10.1016/j.prevetmed.2011.01.009.
Khan AH, Bulbul KH, Shahardar RA, Wani ZA, Allaie IM. Prospects of botanical dewormers with especial reference to Kashmir. Int J Vet Sci Anim Husb. 2021;6:58–64.
Pettersson E, Sjölund M, Wallgren T, Lind EO, Höglund J, Wallgren P. Management practices related to the control of gastrointestinal parasites on Swedish pig farms. Porc Health Manag. 2021;7:1–12. https://doi.org/10.1186/s40813-021-00193-3.
Kagira JM, Kanyari PN, Githigia SM, Maingi N, Ng’ang’a JC, Gachohi JM. Risk factors associated with occurrence of nematodes in free range pigs in Busia District, Kenya. Trop Anim Health Prod. 2012;44:657–64. https://doi.org/10.1007/s11250-011-9951-9.
Whitehead B, Thamsborg SM, Denwood MJ, Nejsum P. Assessing the impact of Ascariasis and Trichuriasis on weight gain using a porcine model. PLOS Negl Trop Dis. 2022;16: e0010709. https://doi.org/10.1371/journal.pntd.0010709.
Băieş MH, Boros Z, Gherman CM, Spînu M, Mathe A, Pataky S, Cozma V. Prevalence of swine gastrointestinal parasites in two free-range farms from Nord-West region of Romania. Pathogens. 2022;11: 954. https://doi.org/10.3390/pathogens11090954.
Zajíčková M, Nguyen LT, Skálová L, Stuchlíková LR, Matoušková P. Anthelmintics in the future: current trends in the discovery and development of new drugs against gastrointestinal nematodes. Drug Discov Today. 2020;25:430–7. https://doi.org/10.1016/j.drudis.2019.12.007.
Coles GC, Jackson F, Pomroy WE, Prichard RK, von Samson-Himmelstjerna G, Silvestre A, Taylor MA, Vercruysse J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 2006;136:167–85. https://doi.org/10.1016/j.vetpar.2005.11.019.
Nahed A, Abd El-Hack ME, Albaqami NM, Khafaga AF, Taha AE, Swelum AA, Elbestawy AR. Phytochemical control of poultry coccidiosis: a review. Poult Sci. 2022;101:101542. https://doi.org/10.1016/j.psj.2021.101542.
Githiori JB, Höglund J, Waller PJ. Ethnoveterinary plant preparations as livestock dewormers: practices, popular beliefs, pitfalls and prospects for the future. Anim Health Res Rev. 2005;6:91–103. https://doi.org/10.1079/AHR2005099.
Raza A, Muhammad F, Bashir S, Aslam B, Anwar MI, Naseer MU. In-vitro and in-vivo anthelmintic potential of different medicinal plants against Ascaridia galli infection in poultry birds. Worlds Poult Sci J. 2016;72:115–24. https://doi.org/10.1017/S0043933915002615.
Ayrle H, Mevissen M, Kaske M, Nathues H, Gruetzner N, Melzig M, Walkenhorst M. Medicinal plants–prophylactic and therapeutic options for gastrointestinal and respiratory diseases in calves and piglets? A systematic review. BMC Vet Res. 2016;12:89. https://doi.org/10.1186/s12917-016-0714-8.
Chase MW, Reveal JL, Fay MF. A subfamilial classification for the expanded asparagalean families Amaryllidaceae, Asparagaceae and Xanthorrhoeaceae. Bot J Linn Soc. 2009;161:132–6. https://doi.org/10.1111/j.1095-8339.2009.00999.x.
Oosthuizen CB, Reid AM, Lall N. Chapter 9 - garlic (Allium sativum) and its associated molecules, as medicine. In: Lall N, editor. Medicinal plants for holistic health and well-being. Pretoria: Academic Press, University of Pretoria; 2018. p. 277–95.
Sutton GA, Haik R. Efficacy of garlic as an anthelmintic in donkeys. Isr J Vet Med. 1999;54:23–7.
Londhe VP, Gavasane AT, Nipate SS, Bandawane DD, Chaudhari PD. Role of garlic (Allium sativum) in various diseases: an overview. J Pharm Res Opin. 2011;1:129–34.
Akoh OU, Mac-Kalunta OM, Amadi OK. Phytochemical screening and in-vivo anthelmintic activity of Allium sativum leaf extract. Communication Phys Sci. 2021;7:18–23.
Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003;17:97–106. https://doi.org/10.1002/ptr.1281.
Pazhouh HK, Hamedi S, Javadi B. Gastrointestinal effects of Artemisia absinthium Linn. Based on traditional persian medicine and new studies. Tradit Med Res. 2020;5:498–506. https://doi.org/10.12032/TMR20200210160.
Beigh YA, Ganai AM. Potential of wormwood (Artemisia absinthium Linn.) Herb for use as additive in livestock feeding: a review. Pharma Innov. 2017;6:176–87.
Ahamad J. A pharmacognostic review on Artemisia absinthium. Int Res J Pharm. 2019;10:25–31. https://doi.org/10.7897/2230-8407.
Batiha GES, Olatunde A, El-Mleeh A, Hetta HF, Al-Rejaie S, Alghamdi S, Rivero-Perez N. Bioactive compounds, pharmacological actions, and pharmacokinetics of wormwood (Artemisia absinthium). Antibiotics. 2020;9: 353. https://doi.org/10.3390/antibiotics9060353.
Bisht D, Kumar D, Kumar D, Dua K, Chellappan DK. Phytochemistry and pharmacological activity of the genus Artemisia. Arch Pharm Res. 2021;44:439–74. https://doi.org/10.1007/s12272-021-01328-4.
Ivanescu B, Miron A, Corciova A. Sesquiterpene lactones from Artemisia Genus: Biological activities and methods of analysis. J Anal Methods Chem. 2015;2015(247685):1. https://doi.org/10.1155/2015/247685.
Mo P, Ma Q, Zhao X, Cheng N, Tao J, Li J. Apoptotic effects of antimalarial artemisinin on the second generation merozoites of Eimeria tenella and parasitized host cells. Vet Parasitol. 2014;206:297–303. https://doi.org/10.1016/j.vetpar.2014.09.025.
Mirza Z, Soto ER, Hu Y, Nguyen TT, Koch D, Aroian RV, Ostroff GR. Anthelmintic activity of yeast particle-encapsulated terpenes. Molecules. 2020;25: 2958. https://doi.org/10.3390/molecules25132958.
Munir MT. Effect of garlic on the health and performance of broilers. Veterinaria. 2015;3:32–9.
Kostadinović L, Lević J, Popović ST, Čabarkapa I, Puvača N, Djuragic O, Kormanjoš S. Dietary inclusion of Artemisia absinthium for management of growth performance, antioxidative status and quality of chicken meat. Eur Poul Sci. 2015;79:1612–9199. https://doi.org/10.1399/eps.2015.75.
Ayrle H, Nathues H, Mevissen M, Walkenhorst M. Allium sativum L. for prophylaxis of diarrhea in weaned piglets – how to find the right dosage? Planta Med Int Open. 2017;4:S1-202. https://doi.org/10.1055/s-0037-1608298.
Orengo KO, Maitho T, Mbaria JM, Maing N, Kitaa JM. In vitro anthelmintic activity of Allium sativum, Allium cepa and Jatropha curcas against Toxocara canis and Ancylostoma Caninum. Afr J Pharm Pharmacol. 2016;10:465–71. https://doi.org/10.5897/AJPP2016.4551.
Caner A, Döşkaya M, Değirmenci A, Can H, Baykan Ş, Üner A, Başdemir G, Zeybek U, Gürüz Y. Comparison of the effects of Artemisia vulgaris and Artemisia absinthium growing in western Anatolia against trichinellosis (Trichinella Spiralis) in rats. Exp Parasitol. 2008;119:173–9. https://doi.org/10.1016/j.exppara.2008.01.012.
Iqbal A, Tariq KA, Wazir VS, Singh R. Antiparasitic efficacy of Artemisia absinthium, toltrazuril and amprolium against intestinal coccidiosis in goats. J Parasit Dis. 2013;37:88–93. https://doi.org/10.1007/s12639-012-0137-9.
Krebs S, Omer TN, Omer B. Wormwood (Artemisia absinthium) suppresses tumour necrosis factor alpha and accelerates healing in patients with Crohn’s disease –a controlled clinical trial. Phytomedicine. 2010;17:305–9. https://doi.org/10.1016/j.phymed.2009.10.013.
El-Saber Batiha G, Magdy Beshbishy A, Wasef LG, Elewa YHA, Al-Sagan AA, Abd El-Hack ME, Tah AE, Abd-Elhakim YM, Prasad Devkota H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrients. 2020;12:872. https://doi.org/10.3390/nu12030872.
Pop LM, Varga E, Coroian M, Nedișan ME, Mircean V, Dumitrache MO, Farczádi L, Fülöp I, Croitoru MD, Fazakas M, Gyӧrke A. Efficacy of a commercial herbal formula in chicken experimental coccidiosis. Parasites Vectors. 2019;12:1–9. https://doi.org/10.1186/s13071-019-3595-4.
Abu-Akkada SS, Oda SS, Ashmawy KI. Garlic and hepatic coccidiosis: prophylaxis or treatment? Trop Anim Health Prod. 2010;42:1337–43. https://doi.org/10.1007/s11250-010-9590-6.
Mohammed Kuraa HM, Nageib BR, El-Hendy AHM, Hassanin AAFA. Evaluation of prophylactic and anticoccidial effects of black seed and garlic extracts in rabbits. Worlds Vet J. 2021;11:124–37. https://doi.org/10.54203/scil.2021.wvj18.
Sidiropoulou E, Skoufos I, Marugan-Hernandez V, Giannenas I, Bonos E, Aguiar-Martins K, Lazari D, Blake DP, Tzora A. In vitro anticoccidial study of oregano and garlic essential oils and effects on growth performance, fecal oocyst output, and intestinal microbiota in vivo. Front Vet Sci. 2020;7: 420. https://doi.org/10.3389/fvets.2020.00420.
Ahmadov EI, ShA T, Hasanova JV, Namazova AA. Effects of herbal plants on ducks and quail infected with Eimeria species. J Entomol Zool Stud. 2016;4:1150–2.
Kostadinović LM, Popović SJ, Puvača NМ, Čabarkapa IS, Kormanjoš ŠM, Lević JD. Influence of Artemisia absinthium essential oil on antioxidative system of broilers experimentally infected with Eimeria oocysts. Vet Arh. 2016;86:253–64.
Kostadinovic L, Levic J, Galonja-Coghill T, Ruzicic L. Anticoccidian effects of the Artemisia absinthium L. extracts in broiler chickens. Arch Zootech. 2012;15:69–77.
Popović S, Kostadinović L, Puvača N, Kokić B, Čabarkapa I, Đuragić O. Potential of wormwood (Artemisia absinthium) as a feed supplement in rabbit diet: effect on controlling rabbit coccidiosis, antioxidative systems and growth performance. Vet Arh. 2017;87:769–82. https://doi.org/10.24099/vet.arhiv.160704a.
Kiros H, Bitsue FZ, Gebreyesus N, Hadush B, Afera B, Tekele Y, Werkeluel K, Gebremicael M, Gizaw F. In vivo evaluation of the therapeutic efficacy of Allium Sativum against cryptosporidiosis. Ethiop J Vet Sci Anim Prod. 2017;1:46–56.
Ma C. Treatment methods of traditional Chinese medicines against intestinal protozoan infections. Treatment of human parasitosis in traditional Chinese medicine, vol. 6. Berlin, Heidelberg: Springer; 2014. p. 11–21.
Ullah F, Ayaz M, Sadiq A, Ullah F, Hussain I, Shahid M, Yessimbekov Z, Adhikari-Devkota A, Prasad Devkota H. Potential role of plant extracts and phytochemicals against foodborne pathogens. Appl Sci. 2020;10:4597. https://doi.org/10.3390/app10134597.
Gaafar MR. Efficacy of Allium sativum (garlic) against experimental cryptosporidiosis. Alexandria J Med. 2012;48:59–66. https://doi.org/10.1016/j.ajme.2011.12.003.
Giacometti A, Cirioni O, Scalise G. In-vitro activity of macrolides alone and in combination with artemisin, atovaquone, dapsone, minocycline or pyrimethamine against Cryptosporidium parvum. J Antimicrob Chemother. 1996;38:399–408. https://doi.org/10.1093/jac/38.3.399.
Shahbazi P, Nematollahi A, Arshadi S, Farhang HH, Shahbazfar AA. The protective effect of Artemisia spicigera ethanolic extract against Cryptosporidium parvum infection in immunosuppressed mice. Iran J Parasitol. 2021;16:279–88. https://doi.org/10.18502/ijpa.v16i2.6318.
Tanghort M, Chefchaou H, Mzabi A, Moussa H, Chami N, Chami F, Remmal A. Oocysticidal effect of essential oils (EOs) and their major components on Cryptosporidium baileyi and Cryptosporidium galli. Int J Poult Sci. 2019;18:475–82. https://doi.org/10.3923/ijps.2019.475.482.
Calzada F, Yépez-Mulia L, Aguilar A. In vitro susceptibility of Entamoeba histolytica and Giardia lamblia to plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J Ethnopharmacol. 2006;108:367–70. https://doi.org/10.1016/j.jep.2006.05.025.
Mikaili P, Maadirad S, Moloudizargari M, Aghajanshakeri S, Sarahroodi S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran J Basic Med Sci. 2013;16:1031–48.
Hussain M, Raja N, Akram A, Iftikhar A, Ashfaq D, Yasmeen F, Mazhar R, Imran M, Iqbal M. A status review on the pharmacological implications of Artemisia absinthium: a critically endangered plant. Asian Pac J Trop Dis. 2017;7:185–92. https://doi.org/10.12980/apjtd.7.2017D6-385.
Foroutan-Rad M, Tappeh KH, Khademvatan S. Antileishmanial and immunomodulatory activity of Allium sativum (garlic): a review. J Evid Based Integr Med. 2017;22:141–55. https://doi.org/10.1177/21565872156231.
Luce TW. Anthelmintic potential of plant extracts on helminths in small ruminants. Unpublished thesis. San Marcos: Texas State University; 2019. https://digital.library.txstate.edu/handle/10877/9015. Accessed 2 Aug 2023.
Szopa A, Pajor J, Klin P, Rzepiela A, Elansary HO, Al-Mana FA, Mattar MA, Ekiert H. Artemisia absinthium L. - Importance in the history of medicine, the latest advances in phytochemistry and therapeutical, cosmetological and culinary uses. Plants. 2020;9: 1063. https://doi.org/10.3390/plants9091063.
Seddiek SA, El-Shorbagy MM, Khater HF, Ali AM. The antitrichomonal efficacy of garlic and metronidazole against Trichomonas Gallinae infecting domestic pigeons. Parasitol Res. 2014;113:1319–29. https://doi.org/10.1007/s00436-014-3771-6.
Yıldız K, Başalan M, Duru Ö, Gökpınar S. Antiparasitic efficiency of Artemisia absinthium on Toxocara cati in naturally infected cats. Türkiye Parazitol Derg. 2011;35:10–4.
Poulopoulou I, Horgan MJ, Siewert B, Siller M, Palmieri L, Martinidou E, Martens S, Fusani P, Temml V, Stuppner H, Gauly M. In vitro evaluation of the effects of methanolic plant extracts on the embryonation rate of Ascaridia galli eggs. Vet Res Commun. 2022;47:409–19. https://doi.org/10.1007/s11259-022-09958-9.
Lans C, Turner N, Khan T, Brauer G. Ethnoveterinary medicines used to treat endoparasites and stomach problems in pigs and pets in British Columbia, Canada. Vet Parasitol. 2007;148:325–40. https://doi.org/10.1016/j.vetpar.2007.06.014.
Urban J, Tauchen J, Langrova I, Kokoska L. In vitro motility inhibition effect of Czech medicinal plant extracts on Chabertia ovina adults. J Anim Plant Sci. 2014;21:3293–302.
Kostadinović L, Lević J. Effects of phytoadditives in poultry and pigs diseases. J Agron Technol Eng Manag. 2018;1:1–7.
Santos FCC, Carvalho NUM. Alcoholic tincture of garlic (Allium sativum) on gastrointestinal endoparasites of sheep-short communication. Ciênc Anim Bras. 2014;15:115–8. https://doi.org/10.5216/cab.v15i1.23284.
Amin MR, Mostofa M, Awal MA, Sultana MA. Effects of garlic, turmeric and betel leaf against gastrointestinal nematodes in cattle. Bangladesh J Vet Med. 2008;6:115–9.
Masamha B, Gadzirayi CT, Mukutirwa I. Efficacy of Allium Sativum (garlic) in controlling nematode parasites in sheep. Int J Appl Res Vet Med. 2010;8:161–9.
Buono F, Pacifico L, Piantedosi D, Sgroi G, Neola B, Roncoroni C, Genovese A, Rufrano D, Veneziano V. Preliminary observations of the effect of garlic on egg shedding in horses naturally infected by intestinal strongyles. J Equine Vet Sci. 2019;72:79–83. https://doi.org/10.1016/j.jevs.2018.10.025.
Váradyová Z, Pisarčíková J, Babják M, Hodges A, Mravčáková D, Kišidayová S, Königová A, Vadlejch J, Várady M. Ovicidal and larvicidal activity of extracts from medicinal-plants against Haemonchus Contortus. Exp Parasitol. 2018;195:71–7. https://doi.org/10.1016/j.exppara.2018.10.009.
Karim MA, Islam MR, Lovelu MA, Nahar SF, Dutta PK, Talukder MH. In vitro evaluation of anthelmintic activity of tannin-containing plant Artemisia extracts against Haemonchus contortus from goat: anthelmintic activity of tannin-containing plants Artemisia. J Bangladesh Agric Univ. 2019;17:363–8. https://doi.org/10.3329/jbau.v17i3.43216.
Higuera-Piedrahita RI, Dolores-Hernández M, de la-Cruz-Cruz HA, Andrade-Montemayor HM, Zamilpa A, López-Arellano R, González-Garduño R, Cuéllar-Ordaz JA, Mendoza-de-Gives P, López-Arellano MA. An Artemisia cina n-hexane extract reduces the Haemonchus contortus and Teladorsagia Circumcincta fecal egg count in naturally infected periparturient goats. Trop Anim Health Prod. 2022;54:1–10. https://doi.org/10.1007/s11250-022-03103-z.
Thamsborg SM, Ketzis J, Horii Y, Matthews JB. Strongyloides spp. infections of veterinary importance. Parasitology. 2017;144:274–84. https://doi.org/10.1017/S0031182016001116.
Fawzi EMV, Elsohaby I. Prevalence of gastrointestinal nematodes and the role of allicin in treatment of cattle in Sharkia Governorate. Zagazig Vet J. 2017;45:109–17. https://doi.org/10.21608/ZVJZ.2019.28655.
Ahmed M, Laing MD, Nsahlai IV. In vivo effect of selected medicinal plants against gastrointestinal nematodes of sheep. Trop Anim Health Prod. 2014;46:411–7. https://doi.org/10.1007/s11250-013-0506-0.
Klimpel S, Abdel-Ghaffar F, Al-Rasheid KA, Aksu G, Fischer K, Strassen B, Mehlhorn H. The effects of different plant extracts on nematodes. Parasitol Res. 2011;108:1047–54. https://doi.org/10.1007/s00436-010-2168-4.
Bauri RK, Tigga MN, Kullu SS. A review on use of medicinal plants to control parasites. Indian J Nat Prod Resour. 2015;6:268–77. https://doi.org/10.56042/ijnpr.v6i4.8837.
Boros Z, Băieș MH, Gherman C, Cozma V. The effects of Artemisia absinthium (wormwood), Allium sativum (garlic), Cucurbita pepo (pumpkin), and Coriandrum sativum (coriander) on Trichinella spiralis and Trichinella britovi larvae, in vitro study. Sci Parasitol. 2021;22:70–8.
Seddiek SA, Ali MM, Khater HF, El-Shorbagy MM. Anthelmintic activity of the white wormwood, Artemisia herba-alba against Heterakis Gallinarum infecting Turkey poults. J Med Plants Res. 2011;5:3946–57.
Bǎieş MH, Gherman C, Boros Z, Olah D, Vlase AM, Cozma-Petruț A, Györke A, Miere D, Vlase L, Crişan G, Spînu M, Cozma V. The effects of Allium sativum L., Artemisia absinthium L., Cucurbita pepo L., Coriandrum sativum L., Satureja hortensis L. and Calendula officinalis L. on the embryogenesis of Ascaris suum eggs during an in vitro experimental study. Pathogens. 2022;11:1065. https://doi.org/10.3390/pathogens11091065.
Băieş MH, Cotuţiu VD, Spînu M, Mathe A, Cozma-Petruț A, Miere D, Bolboacǎ SD, Cozma V. The effects of Coriandrum sativum L. and Cucurbita pepo L. against Gastrointestinal parasites in Swine: an. Vivo Study Microorganisms. 2023;11:1230. https://doi.org/10.3390/microorganisms11051230.
Mircean V, Cozma V, Györke A. Diagnostic coproscopic în bolile parazitare la animale (Coproparasitological diagnostic in parasitic diseases in animals). Cluj-Napoca: Risoprint; 2011. p. 23–35.
Henriksen SA, Pohlenz JFL. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet Scand. 1981;22:594. https://doi.org/10.1186/BF03548684.
McKenna PB. Further comparison of faecal egg count reduction test procedures: sensitivity and specificity. N Z Vet J. 2006;54:365–6. https://doi.org/10.1080/00480169.2006.36726.
De Graef J, Sarre C, Mills BJ, Mahabir S, Casaert S, De Wilde N, Van Weyenberg M, Geldhof P, Marchiondo A, Vercruysse J, Meeus P, Claerebout E. Assessing resistance against macrocyclic lactones in gastro-intestinal nematodes in cattle using the faecal egg count reduction test and the controlled efficacy test. Vet Parasitol. 2012;189:378–82. https://doi.org/10.1016/j.vetpar.2012.04.040.
Acknowledgements
We acknowledge the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca and the ”Iuliu Haţieganu“ University of Medicine and Pharmacy of Cluj-Napoca for their support.
Funding
This research was supported by the University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca and by the project PPILOW. The project PPILOW has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement N°816172.
Author information
Authors and Affiliations
Contributions
M-HB, MS, AC, VC; Methodology: M-HB, V-DC; Software: M-HB, SDB; Validation: M-HB, MS, AC-P, SDB; Investigation: M-HB, AM; Data Curation: M-HB, V-DC; Writing—Original Draft Preparation: M-HB, V-DC; Writing—Review and Editing: M-HB, MS, AC-P, SDB, RME, AC, VC; Visualization: M-HB, MS; Formal Analysis: MS, AC-P; Resources: MS, AC, VC; Supervision: MS, RME, AC, VC; Project Administration: MS, RME, AC, VC; Funding Acquisition: MS, AC, VC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Ontologies/pathogens, diseases, medicinal plants and chemical compounds used in experiment. The ontologies utilized in this manuscript refers to a formal representation or conceptualization of parasitology and plants domains. They provide a structured method for representing the entities and the relationships that exists between them.
Additional file 2.
Aspects regarding the HPLC–MS method used for the analysis of alcoholic plant extracts.
Additional file 3.
Coproparasitological methods used for the diagnosis of parasitic infections.
Additional file 4.
Methodological aspects regarding the statistical analysis of results.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Băieş, MH., Cotuţiu, VD., Spînu, M. et al. In vivo assessment of the antiparasitic effects of Allium sativum L. and Artemisia absinthium L. against gastrointestinal parasites in swine from low-input farms. BMC Vet Res 20, 126 (2024). https://doi.org/10.1186/s12917-024-03983-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-03983-3