Abstract
Streptococcus suis (S. suis) is an important gram-positive pathogen and an emerging zoonotic pathogen that causes meningitis in swine and humans. Although several virulence factors have been characterized in S. suis, the underlying mechanisms of pathogenesis are not fully understood. In this study, we identified Zinc metalloproteinase C (ZmpC) probably as a critical virulence factor widely distributed in S. suis strains. ZmpC was identified as a critical facilitator in the development of bacterial meningitis, as evidenced by the detection of increased expression of TNF-α, IL-8, and matrix metalloprotease 9 (MMP-9). Subcellular localization analysis further revealed that ZmpC was localized to the cell wall surface and gelatin zymography analysis showed that ZmpC could cleave human MMP-9. Mice challenge demonstrated that ZmpC provided protection against S. suis CZ130302 (serotype Chz) and ZY05719 (serotype 2) infection. In conclusion, these results reveal that ZmpC plays an important role in promoting CZ130302 to cause mouse meningitis and may be a potential candidate for a S. suis CZ130302 vaccine.
Similar content being viewed by others
Introduction
Streptococcus suis (S. suis) is a major pathogen causing swine streptococcus infection and is an emerging zoonotic pathogen [1]. S. suis causes a wide range of diseases, including septicemia, meningitis, pneumonia, and arthritis [2]. S. suis isolates are classified into 33 serotypes based on capsular polysaccharides and to date, 28 novel cps types (NCL1- NCL27 and Chz) S. suis strains have been identified [3,4,5,6,7]. Although several virulence factors, such as extracellular protein factor (EPF), muramidase-related protein (MRP), and suilysin (SLY), have been identified in S. suis, the Chz type strain CZ130302, which causes acute meningitis, lacks these virulence markers [8, 9]. The pathogenic molecular mechanism of CZ130302 requires further exploration.
Zinc metalloproteases (Zmps) are a widely distributed and diverse family of proteolytic enzymes with a conserved HEXXH…E motif [10]. An increasing body of evidence demonstrates that Zmp plays important roles in the pathogenic processes of bacteria. The lethal toxin, the major virulence factor of Bacillus anthracis, contains the effector moiety lethal factor, which acts as a Zmp specific target protein kinase, thereby contributing to the anthrax [11]. In Listeria monocytogenes, Mp1 is a Zmp that activates the virulence factor phospholipase C [12]. It is well known that IgA, as an immunoglobulin, plays an important role in the elimination of pathogens from the host. To colonize the host, bacteria have evolved mechanisms to resist the host’s innate immunity by expressing IgA proteases [13]. The IgA protease is a Zmp that cleaves human IgA and has been characterized in various bacterial species [14,15,16]. There are a large number of Zmps on the cell surface of Streptococcus pneumoniae, and these Zmps are classified into four different groups: ZmpA (IgA protease), ZmpB, ZmpC, and ZmpD [17]. The N-terminal region of these Zmps contains the LPXTG motif, which is important for the localization of Zmps. The C-terminal part of Zmps comprises a proteolytic domain and contains motifs characteristic of zinc metalloproteinases. Many Zmps have been identified as critical for the virulence of S. pneumoniae [17]. Phylogenetic analysis has confirmed that the Zmp of S. suis is a homologue of S. pneumoniae ZmpC [17]. These studies propose that Zmp in S. suis might play an important role in the pathogenic process and deserves further exploration.
It has been demonstrated that S. pneumoniae ZmpC can activate human matrix metalloproteinase 9 (MMP-9) [18]. MMP-9 is a zinc-dependent matrix-degrading enzyme that can disrupt the blood-brain barrier (BBB) [19]. High levels of MMPs are present in the cerebrospinal fluid of patients with bacterial meningitis [20]. In a rat model of meningitis, the transcriptional level of MMP-9 in brain tissue was significantly increased [21]. These studies suggest that the activation of MMP-9 plays a critical role in the development of meningitis. Can Zmp in S. suis serotype Chz also activate MMP-9? What is the role of Zmp in the meningitis-caused strain CZ130302 in the progression of meningitis? The exploration of these questions will help to understand the molecular mechanism of S. suis meningitis.
In the present study, we analyzed the distribution of Zmp in S. suis and identified the role of ZmpC in the virulence of serotype Chz strain CZ130302. Further research has found that ZmpC plays a significant role in the meningitis induced by CZ130302. ZmpC, located on the bacterial surface, induces the host to produce a high antibody titer against lethal infection caused by S. suis CZ130302. Understanding the role of Zmp will provide new insights for the study of the pathogenic mechanism of S. suis CZ130302.
Materials and methods
Ethics statement
Five-week-old female specific pathogen free (SPF) BALB/c mice were purchased from the Comparative Medicine Center of Yangzhou University. All animal experiments were performed in strict accordance with the animal welfare standards of the Guidelines of the Jiangsu Provincial Animal Research Committee (License Number: SYXK (SU) 2017–0007), and were approved by the Animal Ethics Committee of Nanjing Agricultural University.
Bacterial strains, plasmids, and culture conditions
Chz type strain CZ130302 was isolated from the brain tissue of piglets with acute meningitis [4]. The strains and plasmids used in this study are shown in Table 1. All S. suis strains were cultivated in Todd-Hewitt broth (THB, BD, USA) containing 3% fetal bovine serum, or on solid medium containing 5% sheep blood (v/v), and were cultured at 37 °C in an incubator with 5% CO2. To screen for mutants, 100 µg/mL spectinomycin (Spc, Sigma-Aldrich, USA) or 10% sucrose (w/v) was added to the medium as needed. Escherichia coli strains were grown in Luria-Bertani (LB, BD, USA) medium at 37 °C. 50 µg/mL kanamycin (Kan, Sigma-Aldrich, USA) was added to LB medium to construct recombinant plasmids when necessary.
Bioinformatics
The Zmp of S. suis in the NCBI database (https://www.ncbi.nlm.nih.gov/) was searched and analyzed according to the conserved HEXXH…E motif. The data was visualized by GraphPad Prism 8. IgA1 protease, ZmpC, ZmpD, and ZmpB in S. pneumoniae were used as references, and a phylogenetic tree of S. suis Zmp was constructed by Neighbor-Joining Tree using MEGA-X software. The three-dimensional structures of ZmpC in S. suis CZ130302 and S. pneumoniae TIGR4 were predicted by the online prediction website SWISS-MODEL (https://swissmodel.expasy.org).
Construction of the Zmp gene deletion mutants
Zmp mutants were constructed via natural DNA transformation with some modifications [22]. The sequences of all primers used to construct deletion strains are listed in Supplementary material 1. The upstream and downstream fragments of Zmp were amplified by polymerase chain reaction (PCR) with primer pairs from the genomic DNA of CZ130302. The upstream and downstream fragments were fused with the sacB-spc cassette through overlap PCR. The fusion fragment and synthetic peptide were added to the 100 µl bacterial suspension (OD600 ≈ 0.042). Samples were incubated at 37 °C for 2 h under static conditions and then plated on THB containing Spc. The positive mutations carrying sacB-spc were then detected by PCR with primers. The sacB gene is sensitive to sucrose and can be used as a negative control. The fusion fragment without the cassette was transformed into the primary mutant to obtain the positive clone that did not carry the resistance gene.
Growth curve and CFU determination
The CZ130302, ΔzmpC, ΔzmpE, ΔzmpN and ΔzmpB strains in log phase were diluted 1:100 in fresh THB broth. The strains were cultured in a shaker at 37 °C, and the OD600 value was measured and recorded every 1 h. In addition, samples were diluted with sterile PBS every 2 h. Serial 10-fold dilutions of the bacterial suspensions were plated on THB agar and then incubated for 24 h at 37 °C, after which the colony forming units (CFU) were counted. The experiment was repeated independently three times. The growth curves and CFU assay were drawn using GraphPad Prism 8 software.
Mouse infection tests
To assess the virulence of the Δzmp strains, BALB/c mice were randomly divided into 5 groups of 10 mice each and challenged with the strains at a dose of 5 × 107 colony forming units (CFU)/mouse by intraperitoneal injection. Another group of 10 mice was challenged with PBS as a control group. The clinical symptoms (neurological symptoms: lethargy, coma, circling, and convulsions) and survival of the mice were continuously monitored for 7 days. In addition, bacterial load analysis was used to assess the proliferation capacity of ΔzmpC in mice. Two groups of 10 mice each were challenged with the indicated S. suis CZ130302 or ΔzmpC at a dose of 1 × 108 CFU/mouse through intraperitoneal injection, respectively. Subsequently, the brain, liver, and spleen were harvested, weighed, and homogenized in phosphate buffer solution (PBS) at 12 h post-infection. The infected mice were anaesthetised with 3% isoflurane inhalation and euthanised with CO2 at 12 h after infection according to the reference [23]. Bacteria were isolated by plating serial 10-fold dilutions on a THB agar (THA) medium to enumerate CFU.
To determine the protective potential of recombinant protein in BALB/c mice, mice immunized with recombinant protein ZmpC-M26 or adjuvant ISA201 (control) (Seppic, France) were challenged with CZ130302 (Chz type) and ZY07519 (serotype 2), respectively. Mice were randomly divided into 4 groups, and 8 mice in each group were immunized 24 days and then challenged with CZ130302 at a dose of 2 × 107 CFU/mouse or ZY05719 at a dose of 2 × 108 CFU/mouse. Finally, the clinical symptoms (neurological symptoms: lethargy, coma, circling, and convulsions) and survival of the mice were continuously observed and recorded.
RNA isolation, RT-PCR, and qRT-PCR
Total RNA from bacteria in log phase and the infected host brain tissue cells was extracted using TRIzol (Vazyme, Nanjing, China) according to the manufacturer’s instructions. After removing contaminating DNA with DNase I (Vazyme, Nanjing, China), RNA was used as a template to synthesize cDNA using the PrimeScript RT reagent kit (Vazyme, Nanjing, China). The QuantStudio 6 Flex RT-PCR System and ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) were used to determine the transcription of selected genes. Transcript of β-Actin acts as the control to normalize the relative amount of host cell target gene mRNA [24]. The relative amount of S. suis target gene mRNA was normalized to the gene gapdh [25].
Purification of protein and preparation of polyclonal antibody
The sequence containing the M26 domain was amplified from the CZ130302 genomic DNA. The sequences were digested and ligated into pET-28a plasmid and the recombinant plasmids were transferred into BL21 (DE3). The recombinant proteins were purified by Ni-NTA Spin Columns (QIAGEN, Germany) from BL21 (DE3) carrying the recombinant pET-28a plasmid after isopropyl-β-d-thiogalactopyranoside (IPTG) (Thermo Fisher Scientific, USA) induction (0.1 mM) for 16 h at 16° C. Protein concentrations were determined by BCA (Thermo Fisher Scientific, USA) protein assays. BALB/C mice in each group were immunized via multipoint intradermal injection for three times at 14 days intervals with 100 µg recombinant proteins. The polyclonal antiserum was collected from immunized mice after the third immunization, and titers were determined by enzyme-linked immunosorbent assay (ELISA).
Gelatin zymographic analysis
Gelatin zymography assay was performed according to the literature with some modifications [26]. Briefly, the purified ZmpC-M26 (900 ng to 1300 ng) and ZmpE-M26 (900 ng to 1300 ng) were incubated with human MMP-9 (Shanghai You Ning Wei Biotechnology Co. Ltd, Shanghai, China) in a buffer containing zinc ions for 1 h at 37 °C, respectively. The samples were separated on SDS-PAGE (1% gelatin, Invitrogen, USA) at 4 °C. The protein gel was then washed four times with the eluent (2.5% Triton-X, 50 mmol/L Tris-HCl, 5 mmol/L CaCl2, 1µmol/L ZnCl2, pH 7.6) at 4 °C for 1 h each time. The protein gel was washed twice with rinse solution (50 mmol/L Tris-HCl, 5 mmol/L CaCl2, 1µmol/L ZnCl2, pH 7.6) at 4 °C for 40 min each. The protein gel was then placed in the incubation solution (50 mmol/L Tris-HCL, 5 mmol/ CaCl2, 1 µmol/L ZnCl2, 0.02% Brij-35) and incubated at 37 °C for 48–72 h. Finally, the protein gel was stained overnight in staining solution (0.05% Coomassie brilliant blue R-250, 30% methanol, 10% acetic acid). Images were collected after the protein gel was destained.
Western blot analysis
The extraction of cell wall proteins was with reference to the literature with appropriate modifications [27]. Briefly, 30 mL of bacteria in log phase were collected and washed three times with sterile PBS. The precipitates were then resuspended in 1.2 mL of sample preparation solution (125 U/ml mutanolysin (Sigma-Aldrich, USA), 25% sucrose, 30 mM Tris-HCl (pH 7.5), 3 mM MgCl2) and incubated at 37 °C for 1 h. After incubation, the cell lysates were centrifuged at 8000 rpm for 10 min at 4 °C. 120 µL of cooled TCA (trichloroacetic acid, Sinopharm Chuan Kang Pharmaceutical Co., Ltd. China) at a final concentration of 10% was added to the supernatant, and the mixtures were incubated for 30 min in ice-water. The mixtures were then centrifuged at 8000 rpm for 10 min at 4 °C. The protein precipitate was washed twice with chilled acetone (Sinopharm Chuan Kang Pharmaceutical Co., Ltd. China) and allowed to air dry. The cell wall proteins were separated by SDS-PAGE, and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA) and blocked with 5% (w/v) skimmed milk for 2 h at 37 °C. Subsequently, the membrane was washed three times with PBST, incubated with the prepared polyclonal antibodies (anti-ZmpC-M26) at 37° C, diluted 1:1000 for 2 h. After three washes, the processed membranes were incubated with HRP-conjugated secondary antibody (1:8000) at 37 °C for 45 min. Positive bands were detected with ECL kit (Vazyme, Nanjing, China).
Indirect immunofluorescence analysis
The bacteria cultured to the log phase were washed with 1×PBS, and then 5 µl of bacteria were taken on a coverslips and fixed in 4% paraformaldehyde. Bacterial samples were blocked with 5% BSA and labeled with antiserum (anti-ZmpC-M26 or negative serum) diluted 1:200 at 37 °C for 2 h. After thrice washed, the bacteria samples were incubated with secondary antibody, Alexa Fluor 488- conjugated (Thermo Fisher, USA), diluted at 1:400. After incubation at 25 °C for 2 h, the samples were washed 3 times with 1×PBS and stained with DAPI (4′, 6-diamidino-2-phenylindole) (KeyGEN BioTECH, Nanjing, China) for 5 min. Finally, the treated samples were observed on a laser scanning confocal microscope (Leica Sp5 AOBS confocal system, Leica, Germany).
Statistical analysis
All experiments were repeated at least three times. Data were assessed for normality using the Shapiro-Wilk test. Statistical analyses were performed using GraphPad Prism version 8. The survival rate of mice was analyzed using the Log-rank (Mantel-Cox) test. Statistical significance was set at a P value of <0.05, and an unpaired two-tailed Student’s t-test was applied to analyze the data.
Results
Distribution and evolutionary analysis of Zmp in S. suis
Although Zmps are widely existent in S. pneumoniae and have been characterized [17], there are few studies on Zmp in S. suis. Therefore, we analyzed the distribution of Zmp in S. suis from the NCBI database based on the conserved HEXXH…E motif. The result showed that Zmp was distributed in different serotypes of S. suis (Fig. 1A). Furthermore, Zmps were more present in the novel cps types (Chz, NCL1, NCL3, NCL4 and NCL17) S. suis strains, suggesting a different role for Zmp in the novel cps types S. suis compared to common serotypes (e.g. serotype 2). Further phylogenetic analysis found that Zmps in S. suis were classified into five distinct groups: ZmpA, ZmpB, ZmpC, ZmpE, and ZmpN (Fig. 1B). The ZmpN cluster was distantly related to other four clusters, indicating that ZmpN is a new class of zinc metalloproteinases, which suggests that ZmpN may have different biological functions. And phylogenetic analysis also showed that ZmpC is widespread in S. suis, suggesting that ZmpC may play an important role in S. suis. Further analysis found that the Chz type strain CZ130302 contained four different Zmps: ZmpB, ZmpC, ZmpE, and ZmpN (Fig. 1B). These results reveal the biological functional importance of Zmp in CZ130302.
Distribution and phylogenetic analysis of Zmp in S. suis. (A) The NCBI database was searched for Zmp of S. suis using conserved HEXXH…E motifs. The number of Zmps in each S. suis strain was analyzed using GraphPad Prism 8. Chz type strains are highlighted in red. (B) Phylogenetic analysis was constructed by Neighbor-Joining Tree using MEGA-X software. IgA1 proteases (H020_RS0105710), ZmpC (H020_RS0100375), ZmpD (H020_RS0103275) and ZmpB (C4N11_03120) in S. pneumoniae were used as references and are highlighted with blue boxes. Zmps in the Chz type CZ130302 strain are highlighted with red boxes
ZmpC contributes to the virulence of chz type strain CZ130302
To explore the biological role of Zmp in S. suis CZ130302, we constructed a single gene deletion strain of zmp in the wild-type strain background (Fig. 2A). Next, we analyzed the effect of Zmp on the growth of CZ130302, and found that single deletion of zmpB, zmpC, zmpN, and zmpE did not affect the growth of the strain (Fig. 2B). Furthermore, CFU assay also revealed that the deletion of zmps had no effect on growth (Supplementary material 2). Since ZmpC of S. pneumoniae is required for virulence [17], we then performed a mouse infection assay to explore the role of Zmp in the virulence of CZ130302. The result showed that the mice infected with ΔzmpB, ΔzmpE, and ΔzmpN developed the same typical symptoms of meningitis (coma, drowsiness, ataxia, and walking in circles) as mice infected with CZ130302 [28], and the survival rate of mice had no significant change compared with mice infected with the wild strain (Fig. 2C). However, the survival rate of mice infected with ΔzmpC significantly increased, and the symptoms of meningitis in mice significantly reduced. To exclude the possibility of polarity effects, we assessed the transcript levels of upstream and downstream genes of zmpC. As shown in Supplementary material 3, the transcription levels of upstream and downstream genes in ΔzmpC did not change significantly compared with the wild-type strain. Furthermore, we tested the effect of ZmpC on the colonization of CZ130302 in vivo using the mouse infection model. Results indicated that the bacterial loads in the organs (brains, livers and spleens) of mice infected with CZ130302 were significantly higher than those of mice infected with ΔzmpC (Fig. 2D-F). These data suggest that ZmpC contributes to the virulence of CZ130302 and exerts certain function in the development of meningitis.
ZmpC contributes to the virulence of CZ130302. (A) Zmps in CZ130302 and Δzmp were detected by PCR using primer pairs. The original gel is presented in Supplementary material 4. (B) Effect of the deletion mutants on the growth of S. suis CZ130302. The results are indicated as the means ± SEM of the results from 3 independent experiments (P>0.05). (C) Survival curves of 5-week-old BALB/c mice infected with wild-type or mutant strains at 5 × 107 CFU/mouse. The control group received only 1×PBS. Ten mice from each group were monitored over a 7-day period. Log-rank (Mantel-Cox) test to determine differences in survival between groups: ** P < 0.01. Infected mice were euthanized 12 h after infection to determine the bacteria burden in the brain (D), liver (E), and spleen (F). Unpaired two-tailed Student’s t-test: * P < 0.05; ** P < 0.01; **** P < 0.0001
ZmpC contributes to the development of meningitis
Activation of the inflammatory response is a common feature of bacterial meningitis, and interleukin-8 (IL-8) can be used as a general marker to assess the level of inflammation. A pivotal factor in the meningeal inflammatory process is tumor necrosis factor alpha (TNF-α), which can strongly stimulate the release and activation of MMPs in brain tissue [29]. MMP-9 can lyse the subendothelial basement membrane that forms the BBB around cerebral capillaries, thereby contributing to the development of brain injury [30]. To further explore the role of ZmpC in the development of meningitis, we determined the transcript levels of TNF-α, IL-8, and MMP-9 in the brain tissue of mice infected with CZ130302 and ΔzmpC. The results indicated that the transcript levels of TNF-α, IL-8, and MMP-9 in the brain tissues of mice infected with ΔzmpC were significantly lower than those of the wild-type strain at 12 h post-infection (Fig. 3). This suggests that ZmpC plays a role in the development of meningitis in the mouse model.
ZmpC in CZ130302 contributes to the development of meningitis. (A) Transcriptional level of TNF-α (A), IL-8 (B), and MMP-9 (C) encoding gene in the brains of the mice infected with the indicated strains. Data are represented as mean ± SEM of three independent repeats. Unpaired two-tailed Student’s t-test: * P < 0.05; *** P < 0.001
ZmpC in CZ130302 can cleave human MMP-9
Previous studies have shown that ZmpC in S. pneumoniae can cleave human MMP-9 [18]. The previous phylogenetic analysis showed that ZmpC in CZ130302 is closely related to ZmpC (H020_RS0100375) in S. pneumoniae TIGR4 (Fig. 1B), suggesting that ZmpC in CZ130302 may be functionally similar to ZmpC in TIGR4. Next, we further predicted the three-dimensional structure of ZmpC in S. pneumoniae TIGR4 and CZ130302 using SWISS-MODEL (https://swissmodel.expasy.org). The results show that the three-dimensional structure of ZmpC in TIGR4 is highly similar to that of ZmpC in CZ130302, and the conserved HEXXH…E motifs are located on the α-helix (red box) (Fig. 4A). Furthermore, our protein structural analysis of ZmpC in CZ130302 found that ZmpC has YSIRK_Signal, G5 (named after its conserved glycine residue), and M26 (contains proteolytic activity) domains (Fig. 4B). To explore whether ZmpC can cleave human MMP-9, we expressed and purified ZmpC-M26 (M26 domain of ZmpC protein) for gelatin zymography analysis. As Fig. 4C shows, a bright band at approximately 52 kDa can be detected after incubation of ZmpC-M26 with MMP-9, indicating that ZmpC can cleave human MMP-9. However, no band could be detected at 52 kDa after incubation of ZmpE-M26 with MMP-9, indicating that ZmpE cannot cleave MMP-9 (Fig. 4D). These data suggest that cleavage of MMP-9 by ZmpC is specific and the cleavage site is different from that reported in previous studies [18]. Cleavage of MMP-9 by ZmpC in S. suis Chz type strain CZ130302 may play an important role in the development of meningitis.
ZmpC in CZ130302 cleaves human MMP-9. (A) The three-dimensional structures of ZmpC in S. pneumoniae TIGR4 (left) and S. suis CZ130302 (right) were predicted using the online site SWISS-MODEL. Conserved HEXXH…E motifs in α-helix are shown in red boxes. (B) Schematic representation of the structure of ZmpC in S. suis CZ130302. ZmpC in CZ130302 contains complete M26 N-terminal and C-terminal domains. (C) The purified recombinant protein ZmpC-M26 was incubated with human MMP-9 for gelatin zymography analysis. The first lane indicates that the sample has only 2 µg human MMP-9, the second to sixth lanes indicate that 2 µg human MMP-9 was incubated with different concentrations of ZmpC-M26 (900 ng to 1300 ng), and the eighth lane indicates that the sample has only ZmpC-M26. The red arrow indicates the detection of a bright band of approximately 52 kDa. The results showed that ZmpC cleaves human MMP-9. (D) The purified recombinant protein ZmpE-M26 (900 ng to 1300 ng) was incubated with human MMP-9 for gelatin zymography analysis. No other bright bands were detected in the incubation group, indicating that ZmpE cannot cleave human MMP-9. The original gel is presented in Supplementary material 4
ZmpC is a critical protective antigen localized to the cell surface
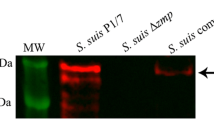
To determine the localization of ZmpC in CZ130302, we prepared a highly specific polyclonal antibody against ZmpC using ZmpC-M26 protein, and performed Western blot analysis. Western blot analysis of the cell wall proteins showed that a band consistent with the size of ZmpC was detected in the wild strain group, but not in the ΔzmpC group (Fig. 5A). Indirect immunofluorescence analysis was performed with anti-ZmpC-M26 serum and negative serum at the same time. The results showed that specific green fluorescence was detected on the cell surfaces of CZ130302 in the anti-ZmpC-M26 serum group, but not in the negative serum group (Fig. 5B). These data suggest that ZmpC in CZ130302 localizes on the bacterial cell surface.
ZmpC is a protective antigen that localizes to the cell surface. (A) Western blot analysis identified the localization of ZmpC in CZ130302. The cell wall proteins in CZ130302 and ΔzmpC were extracted and Western blot was performed. A band consistent with the size of ZmpC was detected only in CZ130302 lane. The original blot is presented in Supplementary material 4. (B) Immunofluorescence assays were used to identify ZmpC anchored to the cell wall with anti-ZmpC-M26 and negative serum, respectively. It should be noted that intracellular proteins are not recognized by extracellular antisera. White bars represent 10 μm. To explore the protective potential of ZmpC, mice immunized with recombinant protein ZmpC-M26 were challenged with Chz type CZ130302 strain (C) and serotype 2 ZY05719 strain (D), respectively. ZmpC-M26 can provide effective protection against CZ130302 and ZY05719 infection
Since ZmpC is located on the cell surface, we determined the immunogenicity and protective potential of recombinant protein ZmpC-M26 in BALB/c mice. We measured the antibody response after the fourth week of vaccinating mice with ZmpC-M26 and found that the antibody titer reached 5 × 105 (data not shown), indicating that ZmpC induced a strong antibody binding response. To determine the protective potential, 24 days after vaccinating mice with ZmpC-M26, we challenged mice with CZ130302 (Chz type) and ZY05719 (serotype 2) respectively, and observed the symptoms and survival of mice daily. The results show that ZmpC-M26 can provide effective protection against CZ130302 (Chz type) and ZY05719 (serotype 2) strains, which significantly improved the survival rate of mice and alleviated the symptoms of meningitis in mice (Fig. 5C and D). Taken together, ZmpC is an important protective antigen against CZ130302 (Chz type) infection.
Discussion
As an emerging zoonotic pathogen, S. suis not only causes huge economic losses to the swine industry but also causes public health problems [31]. To survive in the host, S. suis has evolved multiple mechanisms to counteract the host immune system, including the production of various virulence factors. The capsular polysaccharide, a crucial virulence marker, has been extensively studied in S. suis [32,33,34,35]. In addition, some important virulence factors such as MRP, EF, and SLY have been identified [8, 9]. Although more and more virulence factors have been characterized, the pathogenic molecular mechanism of S. suis still needs further exploration. Although Zmps have been identified as required for virulence of S. pneumoniae, the biological roles of Zmps in S. suis remain poorly understood. Previous study have found that ZmpC of S. suis serotype 2 P1/7 strain could not cleave IgA1 and PSGL-1, and could not activate MMP-9, and the inactivation of ZmpC did not affect the virulence [36]. However, the IgA1 protease in S. suis serotype 2 05ZYS strain can cleave human IgA and promote the virulence of 05ZYS [37]. These studies suggest that the biological roles of different Zmps in S. suis may be different. In the present study, our data showed that the deletion of ZmpC in the Chz type CZ130302 strain resulted in a significant attenuation of virulence in a mouse model. These results provide evidence for the role of Zmp in S. suis CZ130302 virulence in the mouse model.
S. suis is also the main pathogen causing human meningitis in Vietnam, and it is particularly urgent to study the mechanism of meningitis [38]. The S. suis Chz type CZ130302 strain has the ability to cause classic bacterial meningitis in piglets and mice, making it a pattern strain for studying the mechanism of meningitis [28]. Investigating the mechanism by which CZ130302 causes meningitis will help us to understand the molecular mechanism of bacterial meningitis and provide a theoretical basis for the prevention and control of bacterial meningitis disease. Previous studies have identified a genomic island (50 K GI) that encodes the SecY2/A2 secretion system and a secreted protein, SssP1, on the CZ130302 genome [39]. The N-terminus of SssP1, which is secreted by the SecY2/A2 system, contains a specific KXYKXGKXW signal peptide and a serine-rich repeat adhesion glycoprotein AST domain. Deletion of either SecY2/A2 or SssP1 led to a significant decrease in bacterial virulence. In addition, SssP1 plays a key facilitating role in the development of meningitis [40]. SssP1 promotes CZ130302 adhesion to and invasion into host cells through interaction with vimentin, and the salivation of vimentin is necessary for the binding of SssP1 to vimentin. However, the mechanism of CZ130302 penetrating the BBB remains to be studied. Matrix metalloproteinases (MMPs) are a family of Zn+-dependent endopeptidases that degrade the subendothelial basement membrane, which forms the BBB around cerebral capillaries [41,42,43]. MMP-9 acts as a 92 kDa type IV collagenase (gelatinase B) that specifically degrades type IV collagen, which is a crucial structural component of the perivascular basement membrane [44]. MMP-9 has been shown to induce BBB disruption and promote leukocyte extravasation in experimental bacterial meningitis and other models of neuroinflammation [21, 42, 43, 45,46,47]. In this study, we demonstrated that ZmpC in CZ130302 can cleave human MMP-9, which is consistent with S. pneumoniae ZmpC [18]. These results suggest that ZmpC may also cleave porcine MMP-9, but further validation is required. The expression, secretion, and activity levels of MMPs are tightly regulated [48, 49]. All MMPs have a pro-domain that remains enzymatically inactive until protease activity is required [50]. The pro-domain serves as an internal inhibitor of MMP activity, and activation occurs when cleavage of the pro-domain leads to a conformational change into the active form [50]. This activation model has been confirmed in the resolution of the crystal structure of the MMP-1 catalytic domain [51]. In addition, cleavage of MMP-9 may substantially affect MMP-9 activity by either activating the pro-protease or cleaving the inhibitor binding domain [50, 52]. In this study, ZmpC-mediated cleavage in S. suis CZ130302 may also activate MMP-9, thereby promoting the development of meningitis. Furthermore, our data indicated that ZmpC significantly promoted the expression of MMP-9 in mouse brain tissue. In conclusion, ZmpC in CZ130302 plays an important role in damaging the BBB, which will provide a new perspective for studying the mechanism of BBB impairment.
With indiscriminate use of antibiotics causing the emergence of ever more antibiotic resistant pathogens, vaccine research is particularly important. At present, inactivated vaccines of S. suis serotypes 2 and 9 have been developed. These inactivated vaccines have the advantages of short preparation cycle, safety, and easy storage. However, these inactivated vaccines often require large dose for immunization and are only protective against infection with the same serotype of S. suis. In addition, study has reported a subunit vaccine composed of MRP and EF, which confers significant protection from S. suis serotype 2 infection [53]. There are few studies on vaccines against multiple serotypes, and our study found that ZmpC can be used to effectively resist S. suis serotype 2 and Chz type strains infections. These data provide a rationale for developing S. suis vaccine.
In summary, inactivation of ZmpC significantly attenuated the virulence of CZ130302 in a mouse model, suggesting that ZmpC might be part of the S. suis Chz type strains virulence factor arsenal. Moreover, ZmpC promotes the expression of inflammatory factors and cleaves human MMP-9, suggesting that ZmpC may be a key factor in the meningitis caused by S. suis Chz type CZ130302. The characterization of ZmpC provides several important insights for both research on pathogenic mechanisms and vaccine development in S. suis CZ130302.
Data availability
All data and materials are available at OIE Reference Lab for Swine Streptococcosis, College of Veterinary Medicine, Nanjing Agricultural University, Nanjing 210095, China.
References
Goyette-Desjardins G, Auger J-P, Xu J, Segura M, Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect. 2014;3(6):e45.
Feng Y, Zhang H, Wu Z, Wang S, Cao M, Hu D, et al. Streptococcus suis infection: an emerging/reemerging challenge of bacterial infectious diseases? Virulence. 2014;5(4):477–97.
Hill JE, Gottschalk M, Brousseau R, Harel J, Hemmingsen SM, Goh SH. Biochemical analysis, cpn60 and 16S rDNA sequence data indicate that Streptococcus suis serotypes 32 and 34, isolated from pigs, are Streptococcus orisratti. Vet Microbiol. 2005;107(1–2):63–9.
Pan Z, Ma J, Dong W, Song W, Wang K, Lu C, et al. Novel variant serotype of streptococcus suis isolated from piglets with meningitis. Appl Environ Microbiol. 2015;81(3):976–85.
Zheng H, Qiu X, Roy D, Segura M, Du P, Xu J, et al. Genotyping and investigating capsular polysaccharide synthesis gene loci of non-serotypeable Streptococcus suis isolated from diseased pigs in Canada. Vet Res. 2017;48(1):10.
Huang J, Liu X, Chen H, Chen L, Gao X, Pan Z, et al. Identification of six novel capsular polysaccharide loci (NCL) from Streptococcus suis multidrug resistant non-typeable strains and the pathogenic characteristic of strains carrying new NCLs. Transbound Emerg Dis. 2019;66(2):995–1003.
Bojarska A, Janas K, Pejsak Z, Otulak-Kozieł K, Garbaczewska G, Hryniewicz W, et al. Diversity of serotypes and new cps loci variants among Streptococcus suis isolates from pigs in Poland and Belarus. Vet Microbiol. 2020;240:108534.
Vecht U, Wisselink HJ, Jellema ML, Smith HE. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect Immun. 1991;59(9):3156–62.
Tenenbaum T, Asmat TM, Seitz M, Schroten H, Schwerk C. Biological activities of suilysin: role in Streptococcus suis pathogenesis. Future Microbiol. 2016;11:941–54.
Hooper NM. Families of zinc metalloproteases. FEBS Lett. 1994;354(1):1–6.
Bromberg-White J, Lee C-S, Duesbery N. Consequences and utility of the zinc-dependent metalloprotease activity of anthrax lethal toxin. Toxins (Basel). 2010;2(5):1038–53.
Bitar AP, Cao M, Marquis H. The metalloprotease of Listeria monocytogenes is activated by intramolecular autocatalysis. J Bacteriol. 2008;190(1):107–11.
Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen EV. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996;104(5):321–38.
Senior BW, Dunlop JI, Batten MR, Kilian M, Woof JM. Cleavage of a recombinant human immunoglobulin A2 (IgA2)-IgA1 hybrid antibody by certain bacterial IgA1 proteases. Infect Immun. 2000;68(2):463–9.
Janoff EN, Rubins JB, Fasching C, Charboneau D, Rahkola JT, Plaut AG, et al. Pneumococcal IgA1 protease subverts specific protection by human IgA1. Mucosal Immunol. 2014;7(2):249–56.
Batten MR, Senior BW, Kilian M, Woof JM. Amino acid sequence requirements in the hinge of human immunoglobulin A1 (IgA1) for cleavage by streptococcal IgA1 proteases. Infect Immun. 2003;71(3):1462–9.
Bek-Thomsen M, Poulsen K, Kilian M. Occurrence and evolution of the paralogous zinc metalloproteases IgA1 protease, ZmpB, ZmpC, and ZmpD in Streptococcus pneumoniae and related commensal species. mBio. 2012;3(5):e00303–12.
Oggioni MR, Memmi G, Maggi T, Chiavolini D, Iannelli F, Pozzi G. Pneumococcal zinc metalloproteinase ZmpC cleaves human matrix metalloproteinase 9 and is a virulence factor in experimental pneumonia. Mol Microbiol. 2003;49(3):795–805.
Leppert D, Leib SL, Grygar C, Miller KM, Schaad UB, Holländer GA. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin Infect Dis. 2000;31(1):80–4.
Kieseier BC, Paul R, Koedel U, Seifert T, Clements JM, Gearing AJ, et al. Differential expression of matrix metalloproteinases in bacterial meningitis. Brain. 1999;122:1579–87.
Leib SL, Clements JM, Lindberg RL, Heimgartner C, Loeffler JM, Pfister LA, et al. Inhibition of matrix metalloproteinases and tumour necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain. 2001;124:1734–42.
Zaccaria E, van Baarlen P, de Greeff A, Morrison DA, Smith H, Wells JM. Control of competence for DNA transformation in streptococcus suis by genetically transferable pherotypes. PLoS ONE. 2014;9(6):e99394.
Zhang Y, Zhong X, Lu P, Zhu Y, Dong W, Roy S, et al. A novel autolysin AtlASS mediates bacterial cell separation during cell division and contributes to full virulence in Streptococcus suis. Vet Microbiol. 2019;234:92–100.
Liu J, Zhong X, He Z, Zhang J, Bai J, Liu G, Liang Y, Ya L, Qin X. Erythromycin suppresses the cigarette smoke extract-exposed dendritic cell-mediated polarization of CD4+ T cells into Th17 cells. J Immunol Res. 2020;2020:1387952.
Ju CX, Gu HW, Lu CP. Characterization and functional analysis of atl, a novel gene encoding autolysin in Streptococcus suis. J Bacteriol. 2012;194(6):1464–73.
Snoek-van Beurden PAM, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques. 2005;38(1):73–83.
Li Q, Liu H, Du D, Yu Y, Ma C, Jiao F, et al. Corrigendum: identification of Novel laminin- and fibronectin-binding proteins by Far-Western blot: capturing the adhesins of Streptococcus suis type 2. Front Cell Infect Microbiol. 2020;10:593413.
Pan Z, Ma Y, Ma J, Dong W, Yao H. Acute meningitis of piglets and mice caused by co-infected with Streptococcus suis and Aerococcus viridans. Microb Pathog. 2017;106:60–4.
Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG. Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood-brain barrier: an expanded therapeutic window. Brain Res. 1995;703(1–2):151–5.
Leib SL, Leppert D, Clements J, Täuber MG. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immun. 2000;68(2):615–20.
Segura M, Zheng H, de Greeff A, Gao GF, Grenier D, Jiang Y, et al. Latest developments on Streptococcus suis: an emerging zoonotic pathogen: part 2. Future Microbiol. 2014;9(5):587–91.
Wu Z, Wu C, Shao J, Zhu Z, Wang W, Zhang W, et al. The Streptococcus suis transcriptional landscape reveals adaptation mechanisms in pig blood and cerebrospinal fluid. RNA. 2014;20(6):882–98.
Zhang Y, Ding D, Liu M, Yang X, Zong B, Wang X, Chen H, Bei W, Tan C. Effect of the glycosyltransferases on the capsular polysaccharide synthesis of Streptococcus suis serotype 2. Microbiol Res. 2016;185:45–54.
Auger JP, Dolbec D, Roy D, Segura M, Gottschalk M. Role of the Streptococcus suis serotype 2 capsular polysaccharide in the interactions with dendritic cells is strain-dependent but remains critical for virulence. PLoS ONE. 2018;13(7):e0200453.
Tang J, Guo M, Chen M, Xu B, Ran T, Wang W, Ma Z, Lin H, Fan H. A link between STK signalling and capsular polysaccharide synthesis in Streptococcus suis. Nat Commun. 2023;14(1):2480.
Dumesnil A, Auger J-P, Roy D, Vötsch D, Willenborg M, Valentin-Weigand P, et al. Characterization of the zinc metalloprotease of Streptococcus suis serotype 2. Vet Res. 2018;49(1):109.
Zhang A, Mu X, Chen B, Han L, Chen H, Jin M. IgA1 protease contributes to the virulence of Streptococcus suis. Vet Microbiol. 2011;148(2–4):436–9.
Dat VQ, Long NT, Hieu VN, Phuc NDH, Kinh NV, Trung NV, et al. Clinical characteristics, organ failure, inflammatory markers and prediction of mortality in patients with community acquired bloodstream infection. BMC Infect Dis. 2018;18(1):535.
Zhang Y, Lu P, Pan Z, Zhu Y, Ma J, Zhong X, et al. SssP1, a Streptococcus suis Fimbria-Like protein transported by the SecY2/A2 system, contributes to bacterial virulence. Appl Environ Microbiol. 2018;84(18):e01385–18.
Pan Z, He P, Zhang Y, Gu Q, Chen S, Yu Y, et al. SssP1, a Fimbria-like component of Streptococcus suis, binds to the vimentin of host cells and contributes to bacterial meningitis. PLoS Pathog. 2022;18(7):e1010710.
Gijbels K, Galardy RE, Steinman L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J Clin Invest. 1994;94(6):2177–82.
Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol. 1998;274(5):R1203–11.
Paul R, Lorenzl S, Koedel U, Sporer B, Vogel U, Frosch M, Pfister HW. Matrix metalloproteinases contribute to the blood-brain barrier disruption during bacterial meningitis. Ann Neurol. 1998;44(4):592–600.
Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6(4):121–5.
Liedtke W, Cannella B, Mazzaccaro RJ, Clements JM, Miller KM, Wucherpfennig KW, Gearing AJ, Raine CS. Effective treatment of models of multiple sclerosis by matrix metalloproteinase inhibitors. Ann Neurol. 1998;44(1):35–46.
Redford EJ, Smith KJ, Gregson NA, Davies M, Hughes P, Gearing AJ, Miller K, Hughes RA. A combined inhibitor of matrix metalloproteinase activity and tumour necrosis factor-alpha processing attenuates experimental autoimmune neuritis. Brain. 1997;120(Pt 10):1895–905.
Anthony DC, Miller KM, Fearn S, Townsend MJ, Opdenakker G, Wells GM, Clements JM, Chandler S, Gearing AJ, Perry VH. Matrix metalloproteinase expression in an experimentally-induced DTH model of multiple sclerosis in the rat CNS. J Neuroimmunol. 1998;87(1–2):62–72.
Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–4.
Massova I, Kotra LP, Fridman R, Mobashery S. Matrix metalloproteinases: structures, evolution, and diversification. FASEB J. 1998;12(12):1075–95.
McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today. 2000;6(4):149–56.
Lovejoy B, Cleasby A, Hassell AM, Longley K, Luther MA, Weigl D, McGeehan G, McElroy AB, Drewry D, Lambert MH, et al. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science. 1994;263(5145):375–7.
Potempa J, Banbula A, Travis J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol 2000. 2000;24:153–92.
Wisselink HJ, Vecht U, Stockhofe-Zurwieden N, Smith HE. Protection of pigs against challenge with virulent Streptococcus suis serotype 2 strains by a muramidase-released protein and extracellular factor vaccine. Vet Rec. 2001;148(15):473–7.
Acknowledgements
We would like to thank all the staff of the OIE Reference Laboratory for Swine Streptococcosis in Nanjing Agricultural University.
Funding
This study was supported by Natural Science Foundation General Project of Jiangsu Province, China (BK20191309) and China Postdoctoral Science Foundation (2020M682297).
Author information
Authors and Affiliations
Contributions
ZP conceived the idea and designed the experiments. QG performed S. suis microbiology experiments. QG and PH performed animal experiments. QB performed growth experiments. JM and XZ performed data analyses. YZ and QG performed protein purification. HY and ZP supervised the experiments. ZP and QG wrote the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental protocols were approved by the Animal Ethics Committee of Nanjing Agricultural University.
All methods were carried out in strict accordance with the animal welfare standards of the Guidelines of the Jiangsu Provincial Animal Research Committee (License Number: SYXK (SU) 2017–0007).
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gu, Q., He, P., Bai, Q. et al. Insight into the role of Streptococcus suis zinc metalloprotease C from the new serotype causing meningitis in piglets. BMC Vet Res 20, 337 (2024). https://doi.org/10.1186/s12917-024-03893-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-03893-4