Abstract
Oxytocin has traditionally been known for its physiological effects on muscle contraction associated with birth and lactation, but in the last years is widely used as a biomarker of “positive experiences” in psychology and behavior. Different types of samples have been used for oxytocin measurements with saliva samples having the particular advantage of an easy and non-stressful collection. However, the low concentration of oxytocin in saliva can represent a limitation for its use. For this reason, sensitive assays and even a previous sample treatment in some cases are required for saliva oxytocin quantification. In addition, the lack of standardized and generally agreed-upon approach to peripheral oxytocin measurement leads to large discrepancies between different laboratories, that use different sample treatment protocols and different assays. The main objectives of this review are to describe the current status of the use of saliva for oxytocin measurement, provide details of the different sample processing techniques that can be applied and inform about the analytical techniques and assays available in different animal species, and also in humans for comparative purposes. It is expected that this information can contribute to an increase in the knowledge about the measurements of oxytocin in saliva and to its wider use in the future.
Similar content being viewed by others
Background
Structure and synthesis
Oxytocin is a hormone composed of nine amino acids (CYIQNCPLG) which in many biological samples can have a cyclic chemical structure by the join of disulfide bonds between cysteine residues 1 and 6, resulting in a cyclic core comprising six amino acids with a flexible three-residue amidated tail [1,2,3] (Fig. 1). The oxytocin molecule was discovered in 1950 [4] and synthesized for the first time in 1953 [5]. Mammals produce oxytocin, while fish produce isotocin, and amphibians, reptiles and birds have mesotocin, which are oxytocin-like peptides [6]. In addition, oxytocin like peptides are found in a large number of invertebrate species such as mollusks, nematodes, and arthropods with some differences in their structure [7].
The chemical structure of oxytocin (C43H66N12O12S2) MW = 1007,19 g/mol [2]
Oxytocin is synthesized in the supraoptic and paraventricular nucleus of the hypothalamus[8]. It is transported along with the neurophysins to the posterior lobe of the pituitary gland and then it is released to the blood [8, 9]. From the pituitary gland, oxytocin pathways are distributed in many brain regions associated with the stress response, including the bed nucleus of the stria terminalis and the amygdala [10]. Moreover, oxytocin receptors have been found in some limbic structures, including the bed nucleus of the stria terminalis, central nucleus of the amygdala, septum, and hippocampus [11]. In addition to brain, oxytocin is produced in several peripheral tissues and organs, such as the uterus, ovaries, testis, vascular endothelium, and heart [12].
Oxytocin has some similarities with arginine-vasopressin, another neuropeptide synthesized in the hypothalamus, which shares with oxytocin seven of nine amino acid sequences, differing only in two amino acids [3]. However, several of the known effects of arginine-vasopressin are opposite to those of oxytocin [3] and from the analytical point of view, oxytocin assays should not have cross-reactivity with vasopressin.
Function and biological relevance
Traditionally, oxytocin is known for its physiological effects on muscle contraction associated with labor (uterine contraction) and lactation (milk ejection) [13]. In addition, some reports stated that oxytocin acts as a stress responsive hormone [14,15,16]. However, in the last years, oxytocin has been widely studied in the field of psychology and behavior, being considered as a biomarker of positive emotions since it increases in calming and relaxing situations [17]. This hormone plays an important role in social bonding[18] with both short-term and long-term effects[19] and also in birth, lactation, and maternal-filial relationship [20], being in general released during positive interaction situations [21]. Moreover, it improves social memory, social recognition and attention[22] and it also has a strong anxiolytic and stress-reducing effects [23, 24]. Overall, contrarily to most biomarkers used for evaluating welfare, such as cortisol, catecholamines or alpha-amylase that usually are associated with stress and negative situations, the oxytocin has the particularity of being associated with positive experiences. However, while oxytocin was first described as a pro-social hormone, more recent research suggests that oxytocin also increases in non-pro-social behaviors [25]. In addition, oxytocin has an important role in regulation of the immune system[26, 27] and anti-inflammatory effects.
Several mechanisms have been postulated to explain the effects of oxytocin, such as the potentiation of neuronal activity in areas of the brain related to cognitive processes, and the reduction of activity in areas that control the autonomic nervous system [28] as well, the modulation of serotonin activity leading to a reduction of anxiety [29] and interactions with the dopaminergic system and endogenous opioid system [30]. Oxytocin also plays important roles in peripheral tissues, which can feed-back to the central nervous system with routes of connection described [31, 32]. For example, inhaled oxytocin can reach the central nervous system [33], and a route for active transport from blood circulating oxytocin into the brain has recently been discovered in mice [34].
Analytical issues
There are many studies about peripheral oxytocin measurements that use blood plasma samples [3, 35, 36]. However, saliva has gained a high interest for the measurement of oxytocin in trials related with behavior and stress, since it is an ideal sample due to its non-invasive nature and its easy collection, although the difficulties for assay development and its usually low concentration at these samples can represent a limitation for its use [37].
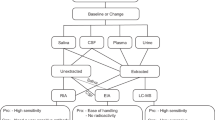
In general, there is little consensus on how to manage the saliva samples, the assays that should be used to measure oxytocin and how to interpret the often-discordant results of various methods [38]. In this line, an issue that it is known is a matter of controversy is what the different assays are measuring. Oxytocin can be present in a cyclic or linear form (Fig. 2) that can be differently recognized by the diverse antibodies used in the assays; and also some assays involve sample treatments, such as a reduction-alkylation process that irreversibly breaks disulfide bridges and produces the linear form of oxytocin [39]. These forms can have different psychophysiological effects [37, 40]: the cyclic molecule may act to initiate social interaction, whereas linear oxytocin and C-terminal fragments may induce relaxation and anti-stress effects following social interaction, and anti-inflammatory and antioxidant effects [40]. The linear form can also be formed by an enzymatic degradation[41, 42]. In addition, the oxytocin can bind to proteins but also be in free form, and there can be oxytocin metabolites that can be measured in variable degree by the different assays [38, 43].
Objectives of this review
The main objectives of this review are to report the current status of the use of saliva for oxytocin measurement, describe the options of sample management and processing and provide an update about the analytical techniques described for the measurements of oxytocin in saliva, in different animal species and also in humans for comparative purposes.
Use of saliva for oxytocin measurement
Although blood plasma is also frequently used to measure peripheral oxytocin concentrations [3, 35, 36], this review will be focused on saliva samples. The secretion of oxytocin to saliva from central nervous system could be influenced and controlled by autonomic nerves [44] and the circulating molecules in blood plasma are thought to transfer to salivary glands via surrounding capillaries [45], since lipid insoluble molecules such as oxytocin, could enter into saliva mainly via the tight junctions between acinar cells through ultrafiltration [46]. However, more studies should be needed to clearly elucidate the mechanisms and sources of the presence of oxytocin in saliva. In this line, to our knowledge, there are no studies that have evaluated if oxytocin can be synthesized directly in salivary glands.
Saliva has various advantages compared to other sample types that have been used for oxytocin measurements such as cerebrospinal fluid[47] or serum [48,49,50], such as its easy, non-invasive and quick collection. In addition, saliva sampling usually reduces various sources of stress that are likely to interfere with oxytocin system activity, since it does not require medical care or a laboratory setting.
Table 1 shows different correlations between peripheral (blood and saliva) and central (cerebrospinal fluid) oxytocin concentrations. This table shows a large divergence of correlations between different samples depending on the study. This indicates that, in general, the relationships between oxytocin concentrations in saliva, blood, and cerebrospinal fluid and the possibility of use of peripheral oxytocin quantification to evaluate cerebrospinal fluid concentrations and central oxytocin bioavailability are not yet fully well-understood [33]. Martin et al. [51] found that oxytocin in cerebrospinal fluid showed a high correlation with saliva but a low correlation with plasma, so saliva oxytocin could help to assess cerebrospinal oxytocin levels in patients in neurocritical care. In this line, other authors have also reported that plasma oxytocin levels do not predict central concentrations [51, 52].
In addition, there is in general no high correlation between plasma and saliva concentrations. Quintana et al.[33] concluded that salivary oxytocin concentrations do not accurately reflects plasma oxytocin concentrations after intranasal or intravenous oxytocin administration in men, and Javor et al.[53] did not found correlation between salivary and plasma oxytocin concentrations in healthy young men. Also, according to Martins et al. [54], salivary oxytocin is a weak surrogate for plasmatic oxytocin in humans (r = 0.10). However, also here, there is some degree of divergence between studies since authors found low to moderate correlation between salivary and plasma oxytocin, such as Grewen et al.[55] (r = 0.59) in mothers in response to infant contact and stress and Feldman et al.[56] (r = 0.41) in mothers and fathers interacting with their infants. Much further research is required to detail the mechanisms through which changes in brain oxytocin can be reflected in various peripheral systems.
Maybe one of the reasons for the divergences found in the relation between oxytocin in cerebrospinal fluid and other sample types, can be the different assays used. As an example, plasma and cerebrospinal oxytocin concentrations were not correlated (r = 0.00, P = 0.99) when radioimmunoassay (RIA) was used but showed a significant correlation (r = 0.80, P < 0.01) when an enzyme immunoassay (EIA) was used [57]. Also, these relations could be influenced by the concentration of oxytocin. For example, there is evidence for a positive association between central and peripheral oxytocin concentrations after intranasal oxytocin administration and after experimental stress induction; but this association did not appear under baseline conditions [65].
Initially, some authors did not recommend oxytocin measurement in saliva because its concentrations were very low and therefore, difficult to detect with the assays developed at that time [66]. The low values of oxytocin in saliva have been confirmed with studies reporting a range between 2 and 10 pg/mL in human saliva without extraction, that is more than 10-fold lower than the values in plasma (150–250 pg/mL) [3]. However, Carter et al. [3] used an EIA and concentrated samples, and accurately detected salivary oxytocin in humans, suggesting that measurements of biologically relevant changes in salivary oxytocin are possible. Some years later, other authors measured oxytocin in saliva with sensitive assays [55, 62] and demonstrated that oxytocin concentrations in saliva can change in stressful situations.
In animals, dogs and pigs have higher values of oxytocin in saliva than humans, with values usually higher than 200 pg/mL [43, 67]. However, different values can be obtained depending on the assay used. For example, in canine saliva a median value of 258 pg/mL (range = 207–471 pg/mL) was obtained with Arbor Assays kit (K048) and a median value of 679 pg/mL (range = 356–1073 pg/mL) with Cayman Chemical kit (Item #500,440) [67], while in case of an assay developed with AlphaLISA technology, the median value in dogs was 335 pg/mL ([68]. Also, oxytocin can be measured in saliva of goats (around 500 pg/mL) and cattle (around 200 pg/mL) [69]. In pig species, salivary oxytocin concentrations showed higher values in young individuals [70].
From the applicative point of view, changes in salivary oxytocin concentrations have been observed in both humans [71, 72] and animals [43, 67, 68] in different conditions. In human species, oxytocin can increase in saliva as after intranasal oxytocin administration; although part of this increase could result from a direct movement of mucus or it could be a residue of the spray administered present in the pharynx and oral cavity [73, 74]. In addition, it can increase in saliva in an affiliation relationship [56], lactating women [72], physiological relaxation and emotional excitation status [75], and after physical effort, psychological stress and sexual self-stimulation [71]. Oxytocin also has described to have an anti-stress function[76] showing higher values in saliva in patients with depressive symptoms [62]. In animals, oxytocin in saliva has been studied in human-animal interactions [69, 77], stroking in dogs[68, 78] and stress or situations of positive welfare [79, 80].
Sample processing
It should be point out the importance of using standardized collection conditions and sampling devices. For example, in dogs, Sarstedt Salivette® yields oxytocin concentrations significantly lower than a SalivaBio Children’s Swab, and citric acid and eating immediately prior to the sample tended to increase oxytocin values [67].
Oxytocin measurements can be made on unprocessed samples or by performing a sample extraction. In addition, reduction/alkylation has been applied for sample processing. Also, a concentration can be done in cases that the limit of the assay detection is higher than the oxytocin levels of the sample to be measured [81]. Table 2 shows examples of the different sample treatment applied. Overall, there is a huge variation in the methods for sample treatment that researchers have used.
Sample extraction
The purpose of sample extraction is to obtain a higher purity of the analyte of interest from the sample prior to analysis with the elimination of potentially interfering molecules and the reduction of sample matrix effects [87]. In the particular case of oxytocin, the sample extraction removes the oxytocin bound to proteins and other molecules. Therefore, with this procedure the “free fraction” of oxytocin is measured. In general, lower values of oxytocin are obtained after this process, possibly due to the elimination of interfering molecules, that could artificially increase oxytocin values, and the matrix effect [87] and/or because extraction procedure eliminates oxytocin bound to other molecules, and only “free” oxytocin is measured [83].
There are different methods for sample oxytocin extraction. One of them is solid phase extraction (SPE) that uses different types of columns [67, 82, 88]. This procedure usually treats the sample with 0.1% trifluoroacetic acid (TFA-H2O), and after a centrifugation the supernatant is applied to the extraction column (equilibrated with acetonitrile and TFA- H2O). The eluate is evaporated, and the samples are reconstituted with assay buffer [57]. Alternatively, to SPE, a solvent extraction, using for example acetone, can be used [89]. The type of extraction can influence the final values, since the recovery of oxytocin values in plasma samples using SPE is higher than when using a solvent [82]. SPE columns are more efficient and accurate than acetone and are the preferred method for extracting oxytocin from serum or plasma samples [88].
There is controvery in the literature over whether oxytocin extraction is or not necessary. Some authors such as McCollough et al. [50], Leng and Sabatier[87] and Christensen et al.[90] recommended extraction, because non-extracted samples contain substances that are tagged as oxytocin in assays but are not oxytocin [87]. Also, it is described a high risk of matrix interference if samples are not extracted [90]. Considering these issues, analyses of unextracted plasma have been described as “no more than a random number generator” [87]. On the other hand, some studies report direct relationship between oxytocin concentrations determined in extracted and non-extracted samples, suggesting the no need of extraction. In rhesus monkeys, there was a high correlation (r = 0.89) between oxytocin levels in non-extracted and extracted serum samples [91]. Also, in some species such as dog, the high correlation (Arbor assays: r = 0.80, Cayman kit: r = 0.61) between the extracted and non-extracted saliva samples and the high spiking recoveries found in non-extracted saliva samples, make the extraction procedure not necessary [67]. Similarly, this has been recently described in saliva of pigs [86]. This could indicate that probably if the assay is adequate, sample extraction is not necessary, and this would avoid some risks and limitations associated to the extraction procedure such as:
-
The lower oxytocin values that are obtained with extracted samples, could be due to the elimination of interfering substances but also due to the loss of the oxytocin of the sample during the procedure [66].
-
The loss of correlation between analytical values and biological effects that can be produced after the process of extraction [92].
-
Analytical extraction is expensive and time consuming.
However, there are some available commercial kits that recommend the extraction of saliva samples for its measurement, like Arbor assay kit [93] or Cayman kit [69]. Despite to this, it is demonstrated the validity of measuring oxytocin using the Arbor Assays and the Cayman kit without extraction in plasma of mice (r = 0.95 between extracted and non-extracted samples with Arbor Assays) [94] and in saliva of dogs [67] (r = 0.80 in case of Arbor Assays and r = 0.61 in case of Cayman between extracted and non-extracted samples). However, these results are not so clear with other assays, such as in the case of Enzo kit, which shows less correlation than in case of Arbor Assays between extracted and non-extracted plasma sample of mice (r = 0.676), so there are differences between kits and it would be important to evaluate the need of sample extraction in each assay, as a part of its validation. before its use [94].
Reduction/alkylation
Oxytocin in blood can bind to larger proteins, but also there is a fraction which is free. Brandtzaeg et al. [83] developed a reduction/alkylation procedure that broke the bonds between oxytocin and plasma proteins enabling the detection of total oxytocin, not only the oxytocin that is “free”. When they applied reduction/alkylation to plasma samples, obtained large increases in detectable oxytocin of human plasma compared with non-treated plasma. In addition, reduction/alkylation plasma samples yielded excellent linearity and parallelism when measured with commercial EIA kits. They indicated that total oxytocin may in many cases be better suited as a biomarker than the free fraction of oxytocin. This reduction and alkylation process could also affect to the molecular conformation of oxytocin by breaking the disulfide bridge and producing a linear form [39, 83].
López-Arjona et al.[43] developed two immunoassays with polyclonal and monoclonal antibodies for salivary oxytocin measurement. When they tested the assays after the reduction/alkylation treatment to saliva samples, the monoclonal antibody showed a high affinity by the total oxytocin (without significant differences before and after the treatment) and the polyclonal antibody showed a high affinity by bound oxytocin and other forms or metabolites of oxytocin. These results were obtained in saliva of porcine [43], canine[68] and bovine[85] species.
The performance of a reduction/alkylation procedure would be recommended at the initial stage of the analytical validation of any assay to determine which forms of oxytocin can recognize.
Sample concentration
Due to the low oxytocin levels in human saliva, it has been described that for some methods, a sample concentration of at least 4-fold is necessary [3]. With this procedure, the oxytocin concentration in saliva would be in a reliable part of the standard curve of some commercial kits [72]. The most common procedure to do the sample concentration is by lyophilization. As an example, for doing a concentration of 4-fold by this method, a procedure that can be followed is to dry 1 ml of supernatant from each sample at 4 °C in a lyophilizer. Then the lyophilized material is reconstituted in 250 µL, resulting in a sample with a salivary concentration 4 times higher than the original [72]. Other authors chose to evaporate saliva samples and suspended with the assay buffer for oxytocin measurements [71].
In veterinary medicine, a previous concentration for the measurement of oxytocin in plasma has been recommended for some methods and species. For example, 10-fold in sheep, goat and pigs, 15-fold in horses and 20-fold in the cow has been recommended for the Enzo Life Sciences kit [81]. However, with other methods like Arbor Assays kit, Cayman kit or AlphaLISA technology it is not necessary to concentrate the dog saliva [77], pig saliva [43] and plasma samples of dog and cat [81].
Storage
Although no reports in saliva have been found, it is described that the storage of samples in a range of temperatures from 0 to 37ºC until 18 h or several freeze/thaw cycles have minimal effects on oxytocin stability in plasma samples [82]. In urine samples, stability tests revealed that oxytocin concentrations degrade over time when stored at 4 °C but are little affected by repeated thawing [95].
Assays for oxytocin measurement
The oxytocin sequence structure is highly conserved among mammals [96], so the different methods used for oxytocin measurement could recognized the oxytocin in all the species of mammals, however, it should be taken in consideration that different species have diverse oxytocin concentrations in biological fluids.
The main types of assays used for oxytocin measurements in saliva are:
RIA
Although it was the method that was firstly used for the measurement of oxytocin [97, 98], it has some limitations such as:
-
Low sensitivity in some cases that limits the detection of low oxytocin concentrations, existing various reports in which RIAs were not sensitive enough for detecting oxytocin in human plasma [47, 55, 89, 99], although some RIAs have very low detection limits such as 0.5 pg/sample for human saliva [71].
-
Use of radioactive material and need of special conditions in the laboratory for its performance.
In some cases, the low sensitivity can be observed even after extraction and 10-fold concentration of human plasma samples, with 90% of human plasma samples showing values below the limit of detection of the RIA [82]. This has been also described in other species such as rat where Vecsernyés et al. [100] reported basal oxytocin for male rats to be 9.6 pg/ml, but the minimum detection limit for that assay was near 10 pg/ml.
EIA
EIA assays have some advantages over available RIA kits, such as a longer shelf life of the reagents, the lack of radioactive materials and a wider detection range. Moreover, EIA requires sample volumes much smaller than those necessary for the RIA, although it depends primarily on whether an extraction is performed prior to assaying. The first EIA suitable for measuring oxytocin in plasma was developed by Prakash et al. [101], showing very close agreement with RIA method in the same samples after extraction.
In a study by MacLean et al. (2018), oxytocin concentrations in dog saliva using mass spectrometry (HPLC-MS) and EIA techniques were determined, demonstrating a high correlation (Arbor assays: r = 0.80, Cayman kit: r = 0.61) of oxytocin levels in the same samples analyzed with and without extraction. However, the oxytocin concentrations detected by EIA were much higher than those for mass spectrometry. This could be probably because the HPLC-MS detects the specific compound based on a known mass-to-charge ratio. By contrast, EIA may recognize not only the primary form of the target analyte, but also structurally related molecules, including precursor forms and biologically related metabolites [38].
Table 3 provides information about EIA kits that have been used for oxytocin measurements in saliva and other kind of samples. As it can be observed there is a variability in the detection limit of the different kits; with the EIA kit developed by Assay Designs, Inc. reporting a minimum detection limit of 4.68 pg/mL [92], whereas the kit of Cayman has a detection limit of 20 pg/mL. In general, it is a lack of knowledge about the form of oxytocin and possible oxytocin metabolites that the different assays detect.
HPLC-MS
HPLC is a technique that separates molecules based on their physical properties. It has been used for oxytocin quantification in saliva of dogs [67]. They found that 9 of 20 total samples were below the limit of detection of the assay (~ 2 pg/mL) and the remaining samples had a median concentration of 18 pg/ml (range = 8–49 pg/mL). This assay showed a positive correlation with extracted and non-extracted samples measured by EIA.
Recently, various researchers have used liquid chromatography coupled to tandem mass spectrometry to assess oxytocin levels in rat, human or mice [109, 110]. Also, others reported plasma oxytocin levels, following extraction, within the range of 1–10 pg/mL [83, 111]. However, Wang et al. [84] used a LC-MS method for oxytocin measurement in dog saliva with higher values ranged between 0 and 5000 pg/ml.
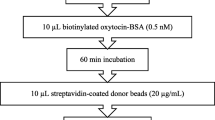
AlphaLISA
AlphaLISA technology (PerkinElmer) is an amplified luminescent proximity homogenous assay that uses two bead types, donor and acceptor. The AlphaLISA assay is based on the principle that, when a laser beam is shone on a donor bead, a single oxygen molecule is generated, producing excitation at an adjacent acceptor bead and amplification of a fluorescent signal, which is detected and quantified. Some advantages of this type of immunoassay in comparison with conventional EIA are the small sample volume and requires fewer steps than EIA, with no need for washings. There are few studies that have measured oxytocin with AlphaLISA technology in saliva or other samples. In saliva of pig, canine and bovine species, AlphaLISA methods have shown a good linearity, precision and recovery [43, 68, 85, 86]. In addition, they can measure different oxytocin forms, such as free, bound, or other metabolites of oxytocin, depending on the antibody against oxytocin used in the immunoassay. Moreover, the AlphaLISA was sensitive for detecting changes in oxytocin measurements in different situations of stress or welfare in these species.
Conclusions
Based in the current knowledge, it can be stated that (a) measurements in saliva samples can detect variations in oxytocin concentrations reflecting changes in physiological conditions and, in some cases, correlation between values of salivary oxytocin and cerebrospinal fluid concentrations have been found. (b) In cases of use of assays with a limit of detection not able to quantify oxytocin in saliva, the use of concentration procedures would be recommended, and controversy exists about the need of extraction, although last reports do not support this procedure. (c) There is a wide range of different assays for oxytocin measurements and in any case, it is important to use assays fully validated showing an adequate accuracy and precision to measure oxytocin in saliva in the species planned to be studied. In addition, it would be of interest to know which form of oxytocin (free, linked to proteins and/or oxytocin metabolites) the assay is measuring. Overall, there is a major need of more studies, knowledge, and assay standardization efforts in order to progress in the adequate and proper use of saliva for the measurement of oxytocin and also to determine all its possible applications.
Data Availability
The data generated and analyzed during the current study are available from the corresponding authors on reasonable request.
References
Beard R, Stucki A, Schmitt M, Py G, Grundschober C, Gee AD, et al. Building bridges for highly selective, potent and stable oxytocin and vasopressin analogs. Bioorg Med Chem. 2018;26:3039–45.
Kocyigit ÜM. The Effects of oxytocin and oxytocin receptor antagonist atosiban on the Carbonic anhydrase and acetylcholinesterase enzymes from lung tissues of rats. Cumhuriyet Sci J. 2017;38:450–60.
Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D et al. Oxytocin: behavioral associations and potential as a salivary biomarker. Annals of the New York Academy of Sciences. Blackwell Publishing Inc.; 2007. 312–22.
Pierce JG, Vigneaud V, Du. Studies on high pontency oxytocic material from beef posterior pituitary lobes. J Biol Chem. 1950;186:77–84.
du Vigneaud V, Ressler C, Trippett S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem. 1953;205:949–57.
Lee AG, Cool DR, Grunwald WC, Neal DE, Buckmaster CL, Cheng MY, et al. A novel form of oxytocin in new world monkeys. Biol Lett. 2011;7:584–7.
Gruber CW. Physiology of invertebrate oxytocin and vasopressin neuropeptides. Exp Physiol. 2014;99:55–61.
Hruby VJ, Chow M-S, Smith DD. Conformational and structural considerations in oxytocin-receptor binding and biological activity. Annu Rev Pharmacol Toxicol. 1990;30:501–34.
Buijs RM. Vasopressin and oxytocin innervation of the rat brain. Amsterdam; 1980.
Condés-Lara M, Veinante P, Rabai M, Freund-Mercier MJ. Correlation between oxytocin neuronal sensitivity and oxytocin-binding sites in the amygdala of the rat: electrophysiological and histoautoradiographic study. Brain Res. 1994;637:277–86.
Krémarik P, Freund-Mercier M-J, Stoeckel M-E. Histoautoradiographic detection of oxytocin-and vasopressin-binding Sites in the Telencephalon of the rat. J Comp Neurol. 1993;333:343–59.
Nishimori K, Takayanagi Y, Yoshida M, Kasahara Y, Young LJ, Kawamata M. New aspects of oxytocin receptor function revealed by knockout mice: sociosexual behaviour and control of energy balance. Prog Brain Res. 2008;170:79–90.
Russell JA, Leng G. Sex, parturition and motherhood without oxytocin? J Endocrinol. 1998;157:343–59.
King MG, Brown R, Kusnecov A. An increase in Startle response in rats administered oxytocin. Peptides (NY). 1985;6:567–8.
Nishioka T, Anselmo-Franci JA, Li P, Callahan MF, Morris M. Stress increases oxytocin release within the hypothalamic paraventricular nucleus. Brain Res. 1998;781:57–61.
Lang RE, Heil JWE, Ganten D, Hermann K, Unger T, Rascher W. Oxytocin unlike Vasopressin is a stress hormone in the rat. Neuroendocrinology. 1983;37:314–6.
Yeates JW, Main DCJ. Assessment of positive welfare: a review. Vet J. 2008;175:293–300.
Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–47.
Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–8.
Carter CS, Altemus M. Integrative Functions of Lactational Hormones in Social Behavior and Stress Management. 1997.
Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–35.
Insel TR. The challenge of translation in Social Neuroscience: a review of Oxytocin, Vasopressin, and affiliative behavior. Neuron. 2010;65:768–79.
Churchland PS, Winkielman P. Modulating social behavior with oxytocin: how does it work? What does it mean? Horm Behav. 2012;61:392–9.
Veening JG, Olivier B. Intranasal administration of oxytocin: behavioral and clinical effects, a review. Neurosci Biobehav Rev. 2013;37:1445–65.
Kemp AH, Guastella AJ. The role of oxytocin in human affect: a novel hypothesis. Curr Dir Psychol Sci. 2011;20:222–31.
Li T, Wang P, Wang SC, Wang YF. Approaches mediating oxytocin regulation of the immune system. Front Immunol. 2017;7.
Kingsbury MA, Bilbo SD. The inflammatory event of birth: how oxytocin signaling may guide the development of the brain and gastrointestinal system. Front Neuroendocrinol. 2019;55:100794.
Febo M, Shields J, Ferris CF, King JA. Oxytocin modulates unconditioned fear response in lactating dams: an fMRI study. Brain Res. 2009;1302:183–93.
Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–71.
Putnam PT, Chang SWC. Interplay between the oxytocin and opioid systems in regulating social behaviour. Philosophical Trans Royal Soc B: Biol Sci. 2022;377.
Iwasaki Y, Maejima Y, Suyama S, Yoshida M, Arai T, Katsurada K, et al. Peripheral oxytocin activates vagal afferent neurons to suppress feeding in normal and leptin-resistant mice: a route for ameliorating hyperphagia and obesity. Am J Physiol Regul Integr Comp Physiol. 2015;308:360–9.
Tabbaa M, Hammock EAD. Orally administered oxytocin alters brain activation and behaviors of pre-weaning mice. Horm Behav. 2020;118:104613.
Quintana DS, Westlye LT, Smerud KT, Mahmoud RA, Andreassen OA, Djupesland PG. Saliva oxytocin measures do not reflect peripheral plasma concentrations after intranasal oxytocin administration in men. Horm Behav. 2018;102:85–92.
Yamamoto Y, Liang M, Munesue S, Deguchi K, Harashima A, Furuhara K, et al. Vascular RAGE transports oxytocin into the brain to elicit its maternal bonding behaviour in mice. Commun Biol. 2019;2:76.
Feldman R, Gordon I, Zagoory-Sharon O. The cross-generation transmission of oxytocin in humans. Horm Behav. 2010;58:669–76.
Bello D, White-Traut R, Schwertz D, Pournajafi-Nazarloo H, Carter CS. An exploratory study of neurohormonal responses of healthy men to massage. J Altern Complement Med. 2008;14:387–94.
Sue Carter C, Kenkel WM, Maclean EL, Wilson SR, Perkeybile AM, Yee JR, et al. Is oxytocin “nature’s medicine”? Pharmacol Rev. 2020;72:829–61.
MacLean EL, Wilson SR, Martin WL, Davis JM, Nazarloo HP, Carter CS. Challenges for measuring oxytocin: the blind men and the elephant? Psychoneuroendocrinology. 2019;107:225–31.
Fraenkel-Conrat H, Mohammad A, Ducay FD, Mecham DK. The Molecular Weight of Lysozyme after Reduction and Alkylation of the Disulfide Bonds. J Am Chem Soc. 1951;73:625–7.
Uvnäs Moberg K, Handlin L, Kendall-Tackett K, Petersson M. Oxytocin is a principal hormone that exerts part of its effects by active fragments. Med Hypotheses. 2019;133:109394.
Peter J, Burbach H, Bohus B, Cs GLK, van Nispen JW, Greven HM, et al. Oxytocin is a precursor of the potent behaviourally active neuropeptides. Eur J Pharmacol. 1983;94:125–31.
Burbach JPH, Lebouille JLM. Proteolytic conversion of arginine-vasopressin and oxytocin by brain synaptic membranes: characterization of formed peptides and mechanisms of proteolysis. J Biol Chem. 1983;258:1487–94.
López-Arjona M, Mateo S, Escribano D, Tecles F, Cerón JJ, Martínez-Subiela S. Effect of reduction and alkylation treatment in three different assays used for the measurement of oxytocin in saliva of pigs. Domest Anim Endocrinol. 2021;74:106498.
Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neuroscience: Basic Clin. 2007;133:3–18.
Gröschl M. Données actuelles sur l’analyse hormonale salivaire. Ann Biol Clin (Paris). 2009;67:493–504.
Vining RF, Mcginley RA, Symons RG. Hormones in Saliva: Mode of Entry and Consequent Implicationsfor Clinical Interpretation. ClinChem. 1983;29:1752.
Jokinen J, Chatzittofis A, Hellström C, Nordström P, Uvnäs-Moberg K, Åsberg M. Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology. 2012;37:482–90.
Nunes LAS, Mussavira S, Bindhu OS. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: a systematic review. Biochemia Med. 2015;25:177–92.
Crockford C, Deschner T, Ziegler TE, Wittig RM. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: a review. Front Behav Neurosci. 2014;8.
McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev. 2013;37:1485–92.
Martin J, Kagerbauer SM, Gempt J, Podtschaske A, Hapfelmeier A, Schneider G. Oxytocin levels in saliva correlate better than plasma levels with concentrations in the cerebrospinal fluid of patients in neurocritical care. J Neuroendocrinol. 2018;30:e12596.
Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J Neuroendocrinol. 2013;25:668–73.
Javor A, Kindermann H, Ransmayr G, Krankenhaus Linz A. Correlation of plasma and salivary oxytocin in healthy young men-experimental evidence Auswirkungen der Mediennutzung auf Jugendlichen View project Progressive supranuclear palsy view project. Neuroendocrinol Lett. 2014;35:470–3.
Martins D, Gabay AS, Mehta M, Paloyelis Y. Salivary and plasmatic oxytocin are not reliable trait markers of the physiology of the oxytocin system in humans. Elife. 2020;9:1–19.
Grewen KM, Davenport RE, Light KC. An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiology. 2010;47:625–32.
Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Dev Sci. 2011;14:752–61.
Lefevre A, Mottolese R, Dirheimer M, Mottolese C, Duhamel JR, Sirigu A. A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Sci Rep. 2017;7:17222.
Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440.
Carson DS, Berquist SW, Trujillo TH, Garner JP, Hannah SL, Hyde SA, et al. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol Psychiatry. 2015;20:1085–90.
Wang YL, Yuan Y, Yang J, Wang CH, Pan YJ, Lu L, et al. The interaction between the oxytocin and pain modulation in headache patients. Neuropeptides. 2013;47:93–7.
Chen Q, Zhuang J, Zuo R, Zheng H, Dang J, Wang Z. Exploring associations between postpartum depression and oxytocin levels in cerebrospinal fluid, plasma and saliva. J Affect Disord. 2022;315:198–205.
Holt-Lunstad J, Birmingham W, Light KC. The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology. 2011;36:1249–56.
Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–93.
Kojima S, Stewart RA, Demas GE, Alberts JR. Maternal contact differentially modulates Central and Peripheral Oxytocin in Rat Pups during a brief Regime of Mother-Pup Interaction that induces a filial huddling preference. J Neuroendocrinol. 2012;24:831–40.
Valstad M, Alvares GA, Egknud M, Matziorinis AM, Andreassen OA, Westlye LT, et al. The correlation between central and peripheral oxytocin concentrations: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;78:117–24.
Horvat-Gordon M, Granger DA, Schwartz EB, Nelson VJ, Kivlighan KT. Oxytocin is not a valid biomarker when measured in saliva by immunoassay. Physiol Behav. 2005;84:445–8.
MacLean EL, Gesquiere LR, Gee N, Levy K, Martin WL, Carter CS. Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. J Neurosci Methods. 2018;293:67–76.
López-Arjona M, Mateo SV, Cerón JJ, Martínez-Subiela S. Changes in salivary oxytocin after stroking in dogs: validation of two assays for its assessment. Res Vet Sci. 2021;136:527–34.
Lürzel S, Bückendorf L, Waiblinger S, Rault JL. Salivary oxytocin in pigs, cattle, and goats during positive human-animal interactions. Psychoneuroendocrinology. 2020;115:104636.
Ortín-Bustillo A, Escribano D, López-Arjona M, Botia M, Fuentes P, Martínez-Miró S, et al. Changes in a Comprehensive Profile of Saliva Analytes in Fattening Pigs during a complete productive cycle: a longitudinal study. Animals. 2022;12:1865.
de Jong TR, Menon R, Bludau A, Grund T, Biermeier V, Klampfl SM, et al. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: the Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology. 2015;62:381–8.
White-Traut R, Watanabe K, Pournajafi-Nazarloo H, Schwertz D, Bell A, Carter CS. Detection of salivary oxytocin levels in lactating women. Dev Psychobiol. 2009;51:367–73.
Weisman O, Zagoory-Sharon O, Feldman R. Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology. 2012;37:1582–6.
van IJzendoorn MH, Bhandari R, van der Veen R, Grewen KM, Bakermans-Kranenburg MJ. Elevated Salivary Levels of Oxytocin Persist More than 7 h after Intranasal Administration. Front Neurosci. 2012;6.
Ooishi Y, Mukai H, Watanabe K, Kawato S, Kashino M. Increase in salivary oxytocin and decrease in salivary cortisol after listening to relaxing slow-tempo and exciting fast-tempo music. PLoS ONE. 2017;12:e0189075.
Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–98.
MacLean EL, Gesquiere LR, Gee NR, Levy K, Martin WL, Carter CS. Effects of affiliative human-animal interaction on dog salivary and plasma oxytocin and vasopressin. Front Psychol. 2017;8:1606.
Ogi A, Mariti C, Baragli P, Sergi V, Gazzano A. Effects of stroking on salivary oxytocin and cortisol in guide dogs: preliminary results. Animals. 2020;10:708.
López-Arjona M, Escribano D, Mateo S, Contreras-Aguilar MD, Rubio CP, Tecles F, et al. Changes in oxytocin concentrations in saliva of pigs after a transport and during lairage at slaughterhouse. Res Vet Sci. 2020;133:26–30.
López-Arjona M, Padilla L, Roca J, Cerón JJ, Martínez-Subiela S. Ejaculate collection influences the salivary oxytocin concentrations in breeding male pigs. Animals. 2020;10:1–12.
Bienboire-Frosini C, Chabaud C, Cozzi A, Codecasa E, Pageat P. Validation of a commercially available enzyme immunoassay for the determination of oxytocin in plasma samples from seven domestic animal species. Front Neurosci. 2017;11:524.
Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med. 2011;73:393–400.
Brandtzaeg OK, Johnsen E, Roberg-Larsen H, Seip KF, MacLean EL, Gesquiere LR, et al. Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Sci Rep. 2016;6:31693.
Wang L, Marti DW, Anderson RE. Development and validation of a simple LC-MS method for the quantification of oxytocin in dog saliva. Molecules. 2019;24:3079.
López-Arjona M, Mainau E, Navarro E, Contreras-Aguilar MD, Escribano D, Mateo SV, et al. Oxytocin in bovine saliva: validation of two assays and changes in parturition and at weaning. BMC Vet Res. 2021;17:140.
López-Arjona M, Mateo S, Manteca X, Escribano D, Cerón JJ, Martínez-Subiela S. Oxytocin in saliva of pigs: an assay for its measurement and changes after farrowing. Domest Anim Endocrinol. 2020;70:106384.
Leng G, Sabatier N. Measuring oxytocin and vasopressin: Bioassays, Immunoassays and Random numbers. J Neuroendocrinol. 2016;28:1–13.
Cool DR, DeBrosse D. Extraction of oxytocin and arginine-vasopressin from serum and plasma for radioimmunoassay and surface-enhanced laser desorption-ionization time-of-flight mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;792:375–80.
Amico JA, Seif SM, Robinsonf AG. Oxytocin in Human plasma: correlation with Neurophysin and Stimulation with Estrogen. J Clin Endocrinol Metab. 1981;52:988–93.
Christensen JC, Shiyanov PA, Estepp JR, Schlager JJ. Lack of association between human plasma oxytocin and interpersonal trust in a prisoner’s dilemma paradigm. PLoS ONE. 2014;9:e116172.
Michopoulos V, Checchi M, Sharpe D, Wilson ME. Estradiol effects on behavior and serum oxytocin are modified by social status and polymorphisms in the serotonin transporter gene in female rhesus monkeys. Horm Behav. 2011;59:528–35.
Kramer KM, Cushing BS, Carter CS, Wu J, Ottinger MA. Sex and species differences in plasma oxytocin using an enzyme immunoassay. Can J Zool. 2004;82:1194–200.
Leeds A, Dennis PM, Lukas KE, Stoinski TS, Willis MA, Schook MW. Biologically validating the measurement of oxytocin in western lowland gorilla (Gorilla gorilla gorilla) urine and saliva using a commercial enzyme immunoassay. Primates. 2018;59:575.
Gnanadesikan GE, Hammock EAD, Tecot SR, Carter CS, MacLean EL. Specificity of plasma oxytocin immunoassays: a comparison of commercial assays and sample preparation techniques using oxytocin knockout and wildtype mice. Psychoneuroendocrinology. 2021;132:105368.
Schaebs FS, Marshall-Pescini S, Range F, Deschner T. Analytical validation of an enzyme Immunoassay for the measurement of urinary oxytocin in dogs and wolves. Gen Comp Endocrinol. 2019;281:73–82.
Mustoe A, Taylor JH, French JA. Oxytocin structure and function in New World monkeys: from pharmacology to behavior. Integr Zool. 2018;13:634–54.
Chard T, Boyd NRH, Forsling ML, Mcneilly AS, Landon J. The development of a radioimmunoassay for oxytocin:the extraction of oxytocin from plasma, and its measurement during parturition in human and goat blood. J Endocr. 1970;48:223–34.
Boyd NRH, Jackson DB, Hollingsworth S, Forsling ML, Chard Summary T. The development of a radioimmunoassay for oxytocin: the extraction of oxytocin from urine and determination of the excretion rate for exogenous and endogenous oxytocin in human urine. J Endocr. 1972;52:59–67.
Lee R, Garcia F, van de Kar LD, Hauger RD, Coccaro EF. Plasma oxytocin in response to pharmaco-challenge to D-fenfluramine and placebo in healthy men. Psychiatry Res. 2003;118:129–36.
Vecsernyés M, Török A, Jójárt I, Laczi F, Penke B, Julesz J. Specific radioimmunoassay of oxytocin in rat plasma. Endocr Regul. 1994;28:145–50.
Prakash BS, Metten M, Schams D, Wuttke W. Development of a sensitive enzymeimmunoassay for oxytocin determination in bovine plasma. Anim Reprod Sci. 1998;51:185–94.
Martínez-Lorenzana G, Espinosa-López L, Carranza M, Aramburo C, Paz-Tres C, Rojas-Piloni G, et al. PVN electrical stimulation prolongs withdrawal latencies and releases oxytocin in cerebrospinal fluid, plasma, and spinal cord tissue in intact and neuropathic rats. Pain. 2008;140:265–73.
Bick J, Dozier M. Mothers’ concentrations of oxytocin following close, physical interactions with biological and nonbiological children. Dev Psychobiol. 2010;52:100–7.
Pekkin AM, Hänninen L, Tiira K, Koskela A, Pöytäkangas M, Lohi H, et al. The effect of a pressure vest on the behaviour, salivary cortisol and urine oxytocin of noise phobic dogs in a controlled test. Appl Anim Behav Sci. 2016;185:86–94.
Pisansky MT, Hanson LR, Gottesman II, Gewirtz JC. Oxytocin enhances observational fear in mice. Nat Commun. 2017;8:2102.
Rault JL. Effects of positive and negative human contacts and intranasal oxytocin on cerebrospinal fluid oxytocin. Psychoneuroendocrinology. 2016;69:60–6.
Wittig RM, Crockford C, Deschner T, Langergraber KE, Ziegler TE, Zuberbühler K. Food sharing is linked to urinary oxytocin levels and bonding in related and unrelated wild chimpanzees. Proc Royal Soc B: Biol Sci. 2014;281:20133096.
Lebowitz ER, Leckman JF, Feldman R, Zagoory-Sharon O, McDonald N, Silverman WK. Salivary oxytocin in clinically anxious youth: Associations with separation anxiety and family accommodation. Psychoneuroendocrinology. 2016;65:35–43.
Meziane H, Schaller F, Bauer S, Villard C, Matarazzo V, Riet F, et al. An early postnatal oxytocin treatment prevents Social and Learning deficits in adult mice deficient for Magel2, a gene involved in Prader-Willi Syndrome and Autism. Biol Psychiatry. 2015;78:85–94.
Zhang G, Zhang Y, Fast DM, Lin Z, Steenwyk R. Ultra sensitive quantitation of endogenous oxytocin in rat and human plasma using a two-dimensional liquid chromatography-tandem mass spectrometry assay. Anal Biochem. 2011;416:45–52.
Johnsen E, Leknes S, Wilson SR, Lundanes E. Liquid chromatography-mass spectrometry platform for both small neurotransmitters and neuropeptides in blood, with automatic and robust solid phase extraction. Sci Rep. 2015;5:1–8.
Acknowledgements
Not applicable.
Funding
This study has been supported by the Spanish “Agencia Estatal de investigación” (Grant Reference PDC2021-121291-I00 / AEI / https://doi.org/10.13039/501100011033) and the European Union – NextGenerationEU. ML-A has a post-doctoral fellowship “Juan de la Cierva Formación” supported by the Spanish Ministry of Science and Innovation (FJC2021-047105-I), Spain.
Author information
Authors and Affiliations
Contributions
Conceived and designed the review: JJC and SMS. Performed the review: MLA, MB, SMS. Wrote and revised the paper: MLA, MB, JJC, SMS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval and Consent to Participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
López-Arjona, M., Botía, M., Martínez-Subiela, S. et al. Oxytocin measurements in saliva: an analytical perspective. BMC Vet Res 19, 96 (2023). https://doi.org/10.1186/s12917-023-03661-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-023-03661-w